Rituximab Inhibits the In Vivo Primary and Secondary Antibody Response to a Neoantigen, Bacteriophage phiX174
Abstract
The response to primary immunization in patients treated with Rituximab (RIT) is not clear. We studied the in vivo antibody response of chronic renal failure (CRF) patients to the neoantigen bacteriophage phiX174 given alone or after ablation with RIT. Eighteen CRF subjects received two immunizations with phiX174 separated by 6 weeks. Nine subjects received a single dose of RIT. The intensity and immunoglobulin isotype of the antibody response (Kv) were measured post-infusion. In addition, three subjects previously immunized and treated with RIT underwent a third and fourth immunization with phiX174 and a tetanus control 2 years later. RIT significantly decreased peak Kv responses when compared to both historic non-CRF controls and to CRF subjects. CRF itself decreased peak Kv responses compared to non-CRF controls. Percent-ratio of anti-phage IgM to IgG was significantly decreased in RIT treated subjects. One of three subjects treated with RIT was found to have developed a partial B cell tolerance to phiX174 administration 2 years later. RIT decreases antibody production and isotype switching to neoantigens and might be useful to prevent antibody response to therapeutic drugs and to newly transplanted organs.
Introduction
Rituximab (Rituxan, Biogen-IDEC Pharmaceuticals) (RIT) is a chimeric mouse-human antibody that targets the human B cell antigen, CD20, present on pre-B and mature B lymphocytes. RIT eliminates CD20+ cells by complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity (ADDC) (1–4). RIT is currently approved for the treatment of B cell lymphoma. It has been successfully used in small pilot trials for the treatment of non-malignant diseases thought to be caused by autoantibodies such as immune thrombocytopenic purpura (5), rheumatoid arthritis (6) and autoimmune hemolytic anemia (7). We are currently evaluating it as a possible treatment to lower preformed anti-HLA antibodies in patients awaiting renal transplantation (8).
While it has been shown that RIT almost completely eliminates circulating B cells there is little information on the effect of RIT on in vivo humoral responsiveness. In one study, RIT decreased antibody responses of lymphoma patients to recall antigens (9). A similar report showed decreased primary and secondary antibody responses to keyhole limpet hemocyanin (KLH) in baboons (10). One established method used to assess in vivo antibody responses in humans is immunization with the T-cell dependent, neoantigen bacteriophage phiX174, a single stranded DNA phage with a dodecahedron structure of about 250 Å diameter composed of 180 identical subunits. Since bacteriophage phiX174 only grows in E. coli, human cells are not infected, and no toxic effects have been observed with human inoculation (11,12).
Bacteriophage phiX174 has been used to test immune responsiveness in patients with primary and secondary immunodeficiency disorders such as SCID (13), X-linked agammaglobulinemia (XLA) (12), X-linked hyper-IgM syndrome (14), the Wiskott-Aldrich syndrome (15), patients after bone marrow transplantation (16,17), patients with HIV infection (18,19) and patients treated with the immunosuppressive agent CTLA4-Ig (20). Normally, phage circulates for 3–4 days following primary immunization, then is rapidly neutralized by the resulting IgM antibody and is no longer detectable by day 7. In normal subjects, following primary immunization, the resulting immune response consists of predominately IgM neutralizing antibody with the titer peaking at 2 weeks. After a secondary immunization, given 6 weeks later, the resulting antibody titer peaks at 1 week, is substantially higher, and consists of approximately equal amounts of IgM and IgG (11). In summary, the response to phiX174 depends on proper antigen processing and presenting, the presence of mature, antigen specific T and B-lymphocytes and the production of certain lymphokines. Thus, measuring the immune response to phiX174 allows the assessment of various aspects of the immune system; amplification, immunologic memory and isotype switching. When the immune system is defective, the antibody responses to this antigen are reproducibly depressed. If B cells are absent, for example in patients with XLA, or in patients with the TB-SCID, phage clearance is delayed and production of phage neutralizing antibody is very low or absent. If B cell function is impaired, as in XLA patients with some residual function of Bruton's tyrosine kinase (BTK) or in a subset of patients with common variable immune deficiency, phage clearance is normal, but the quantity of phage specific antibody produced in response to immunization is reduced, occasionally being less that 0.01% of normal. If T-helper function is impaired as in some patients with CVID, or in patients with mutations in the CD40 ligand, both the quantity and quality (isotype switching) is affected (14). Thus, bacteriophage phiX174 provides useful information to characterize immune defects and has become one of the standard antigen for the assessment of the humoral immune responses in man (21).
Because chronic dialysis affects immune function, secondary immunodeficiency associated with renal failure is of concern in chronic renal failure (CRF) patients. Between 13 and 35.7% (22) of all deaths in renal failure are due to infectious causes. The exact mechanism is unknown but CRF patients are at increased risk for systemic infections with Staph. aureus, E. coli and Strep bovis (23) and have poor and often unreliable responses to protein and polysaccharide vaccines (24–27). Chronic uremia produces immunosuppression (28) by as of yet unknown mechanisms (29). Granulocytes from CRF patients have decreased glucose uptake and oxygen consumption (30). The function of phagocytes tends to be depressed both in peripheral blood and in peritoneal dialysate (31,32). Lymphocytes from uremic patients have been shown to have decreased proliferative responses to various mitogens as well as decreased antibody secretion in vitro (29). In vivo response to antigens is difficult to standardize because of unknown prior exposure, risk to subjects, and reliability and purity of antigens.
The objective of this study was to explore the B-cell-depleting activity of RIT to non-specifically eliminate B cells that we hypothesized are responsible for alloantibody production. The primary goal of the project was to determine the safety and tolerability of RIT in patients with renal failure who were on dialysis (8). Our secondary goal was to analyze the effect of RIT on the in vivo antibody responses to the neoantigen bacteriophage phiX174 of treated versus non-treated patients with end stage renal disease. We also analyzed 2 years later the tertiary and quaternary responses to phage as well as recall responses to tetanus immunization in selected patients treated with RIT, after circulating B cells had recovered.
Materials and Methods
Study design and patient selection
This is an FDA-IND and IRB approved prospective, non-randomized, controlled clinical trial in which 18 subjects with end stage renal disease were enrolled (8). Each subject underwent complete medical history and physical examination. Nine subjects awaiting renal transplant with panel reactive antibodies (PRA) >50% were selected to receive a single dose of RIT and primary and secondary phage immunization. They were compared to nine CRF control subjects who received phage immunization without RIT administration. The CRF group not treated with RIT was selected to match the RIT-treated subjects by age, race and gender. Comparisons were also made to normal healthy control subjects that were collected from prior studies.
Subjects were eligible for the study if they were at least 18-year old and on dialysis (peritoneal or hemodialysis) for at least 12 months. Subjects with significant cardiac or pulmonary disease, pregnancy, HIV infection or those receiving any immunosuppressive drugs including non-steroidal anti-inflammatory drugs or with a history of any immunosuppressive disorder, for example post-splenectomy or sickle cell disease or trait, etc. were excluded.
Rituximab administration
Nine subjects were infused intravenously with a single dose of RIT (n = 3 per group) at 50, 150 or 375 mg/m2 (8). RIT was administered in the General Clinical Research Center (GCRC) at Indiana University. Subjects on hemodialysis were infused after completion of baseline studies and 1 day after completion of the previous dialysis session. Subjects on continuous ambulatory peritoneal dialysis were infused in the morning after completion of the baseline studies. All subjects were given acetaminophen (650 mg) and diphenhydramine (25 mg), orally, 30 min prior to the infusion. RIT was administered intravenously at 50 mg/h for the first hour. Increments of 50 mg/h every 30 min, up to 400 mg/h were administered if no side effects occurred.
phiX174 formulation
phiX174 Lot No 2-96 was prepared in the laboratory of Hans D Ochs at the University of Washington. The phage preparation was sterilized by passage through a 0.22 μm millipore filter and tested for pyrogenicity and leukopenic responses in rabbits and suspended in 0.10 M ammonium acetate with 0.001 M CaCl2 at a pH of 8. The final concentration was 1 × 1011 plaque forming units (PFU)/mL. Each vial was kept frozen at −80°C until ready for use.
Immunization protocol
Subjects were immunized twice with phiX174 by intravenous injection of 2 × 109 PFU/kg of body weight. Subjects receiving RIT were immunized 14 days after RIT infusion. A secondary immunization was given 6 weeks after the primary immunization. Antibody activity to phiX174 was determined by a phage neutralization assay at 1, 2 and 4 weeks following each immunization. Serum was obtained from whole blood samples collected by standard laboratory phlebotomy techniques. Blood obtained 7 days after phage injection was assayed for clearance of circulating phage. This protocol was devised 30 years ago and used in multiple studies (11–19).
Re-immunization protocol
Three subjects (No. 1, 4 and 8) who received RIT and phiX174 as described above, were immunized with a second set of two inoculations of phiX174, an average of 819 ± 108 days after receiving RIT to determine tertiary and quaternary responses. Subjects no. 1 and 4 also received 0.5 mL of tetanus toxoid IM on the day of the third phiX174 administration. Anti-tetanus IgG antibody levels were determined by a reference lab on serum obtained prior to immunization and at weeks 1, 2 and 4 post-immunization. Each subject tested had received his or her initial tetanus immunizations during childhood. Subject no. 8 was not immunized with tetanus toxoid because of a suspected allergy.
Control subjects
Two different collections of healthy control subjects were used for comparisons. To determine antibody responses to phiX174 normal volunteers of both sexes were immunized at University of Washington using the standard protocol without rituximab infusion. For comparisons of immunophenotyping data, normal volunteers were enrolled at Indiana University to undergo a complete blood count as well as flow cytometry as described in the protocol.
Phage clearance, neutralization assay and isotype switching
Concentrations of circulating phage were measured 15 min after primary immunization to ensure intravenous delivery and at 1 week to determine phage clearance. Phage concentrations in the serum were determined by an agar overlay method using serial dilutions as described previously (11,12).
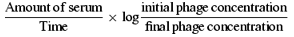
The K value (rate of phage inactivation) uses phage neutralization to measure antibody to a relatively simple surface structure of bacteriophage, a method that is highly sensitive—at least 10 times more sensitive than ELISA. Kv is calculated based on serial dilution of serum and is directly dependent on the amount of antibody since the technique uses a ‘straight line’ to measure the rate of phage inactivation over a standard period of time (60 min). Therefore, Kv is directly related to the amount of antibody that neutralizes bacteriophage. An ELISA technique was developed but it was less sensitive and less consistent.
A Kv of 0.01 represents the lower limit of the phage inactivation assay sensitivity and corresponds to less than one ng/mL of antibody (11). Those subjects who had no detectable antibody by the assay were assigned a value of 0.01 for statistical purposes. Peak Kv was defined as the highest Kv obtained by a subject for each of the immunizations regardless of time after immunization; time to peak Kv was measured in weeks post-immunization. The ratio of IgM and IgG activities against phiX174 was determined by the in vitro treatment of serum with 2-mercaptoethanol (2-ME). 2-ME is performed using the method of Grubb and Swahn (33). Incubation of diluted patient serum with 2-ME for 30 min prior to the phage neutralization assay inactivates IgM antibody but does not affect IgG antibody(11,12). Isotypes were measured 2 weeks following the primary and secondary immunization.
Immunophenotyping
Prior to each phage immunization, absolute CD3 and CD19 counts were determined by flow cytometric phenotyping. RIT-treated subjects also had baseline measurements prior to infusion of RIT. Analyses were performed on fresh whole blood using Coulter XL System II software (Coulter, Naples, FL). Normal, healthy, non-renal failure control samples were obtained from volunteers and measured for CD3 and CD19.
Statistical analysis
RIT-treated subjects were compared to CRF subjects and healthy control subjects. Statistical analyses of responses to phiX174 were performed on log-transformed data and analyzed by one-way repeated measures ANOVA. K values are expressed as geometric mean (95% confidence interval). CD19 and CD3 counts are expressed as cells/μL and are analyzed by one-way repeated measures ANOVA. Mean percent IgG was analyzed using one-way ANOVA. Median peak Kv analysis was performed using Mann-Whitney rank sum test. Statistical significance was defined as p < 0.05.
Results
Demographics
The demographics of the two groups of CRF subjects were similar (Table 1). Nine subjects (age 44 ± 10 years, 4F/5M) received RIT prior to phiX174 immunization. Nine subjects (age 45 ± 6 years, 4F/5M) were immunized with phiX174 alone. One RIT-treated subject did not receive a secondary immunization due to possible infusion-associated side effects of mild decrease in blood pressure, nausea and vomiting. One CRF subject did not receive a secondary immunization of bacteriophage after being excluded for failing to comply with the protocol. The primary antibody responses from these two subjects were included in the data analysis. Three RIT subjects, one from each dose of RIT (Nos. 1, 4 and 8), were available for a second set of immunizations with phiX174 for tertiary and quaternary responses. Each of these patients was on hemodialysis at the time of the second set of immunizations, and none had undergone isotype switching with the first set of immunizations. The demographics of the normal group consist of 52 subjects (age range 18–45, 31F/21M) all healthy without any medical conditions and without allergies to the phage components. Of those, 19 individuals (12 females, 7 males) received a tertiary immunization.
| Group | Age | Gender | Race | Type of dialyses | Time post-RIT to re-immunization |
|---|---|---|---|---|---|
| RIT no. | |||||
| 1—low | 30 | F | C | CAPD | 933 days |
| 2—low | 31 | M | H | HEME | |
| 3—low | 44 | F | C | CAPD | |
| 4—mid | 34 | M | C | HEME | 805 days |
| 5—mid | 55 | F | C | HEME | |
| 6—mid | 54 | F | B | CAPD | |
| 7—high | 52 | M | B | HEME | |
| 8—high | 46 | M | B | HEME | 719 days |
| 9—high | 49 | F | C | HEME | |
| CRF no. | |||||
| 10 | 32 | M | H | HEME | |
| 11 | 50 | M | B | HEME | |
| 12 | 47 | M | B | HEME | |
| 13 | 47 | F | C | CAPD | |
| 14 | 56 | F | C | HEME | |
| 15 | 47 | F | B | CAPD | |
| 16 | 53 | F | B | HEME | |
| 17 | 47 | M | B | HEME | |
| 18 | 42 | M | B | HEME | |
- RIT: subjects who received rituximab infusion; CRF: chronic renal failure subjects not infused with rituximab; CAPD: peritoneal dialysis; HEME: hemodialysis.
- RIT patients are separated into three groups according to dose of RIT given (low—50, mid—150 or high—375 mg/m2 of rituximab).
Immunophenotyping
Immunophenotyping results are summarized in Table 2. CD3 counts in both the CRF and RIT subjects were unchanged by the infusion of rituximab or immunization with phiX174. CD3 counts in CRF and RIT groups were not significantly lower at any time point compared to the normal controls (p = 0.29). CD19 counts of both CRF and RIT subject groups were not significantly lower at baseline than were the normal controls (p = 0.35). CRF subjects had no change in CD19 levels over the study, whereas the RIT subjects did.
| Group | CD 19 counts (cells/μL) prior to each immunization | CD3 counts (cells/μL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (before RIT) | Primary | Secondary | Tertiary | Quanternary | Baseline (before RIT) | Primary | Secondary | |
| Normal controls mean ± SD | 247 ± 118 | 1225 ± 378 | ||||||
| CRF patients no. | ||||||||
| 10 | 58 | 96 | 1604 | 2276 | ||||
| 11 | 152 | 177 | 890 | 925 | ||||
| 12 | 40 | 41 | 838 | 849 | ||||
| 13 | 203 | 270 | 2044 | 2044 | ||||
| 14 | 157 | 121 | 892 | 981 | ||||
| 15 | 60 | 76 | 1953 | 1186 | ||||
| 16 | 158 | 123 | 657 | 575 | ||||
| 17 | 587 | 374 | 1129 | 1010 | ||||
| 18 | 259 | 153 | 1305 | 905 | ||||
| Mean ± SD | 186 ± 167 | 159 ± 104 | 1257 ± 505 | 1195 ± 573 | ||||
| Paired t-test | p = 0.156 | p = 0.641 | ||||||
| RIT-infused no. | ||||||||
| 1—low | 21 | 7.8 | 8.4 | 36 | 20 | 508 | 525 | 630 |
| 2—low | 64 | 6.5 | 6.1 | 800 | 923 | 828 | ||
| 3—low | 157 | 6.7 | 11 | 1590 | 1134 | 1169 | ||
| 4—mid | 348 | 3.7 | 4.7 | 424 | 269 | 345 | 323 | 234 |
| 5—mid | 123 | 4.6 | 5.4 | 818 | 556 | 528 | ||
| 6—mid | 182 | 6.5 | 11 | 1158 | 1047 | 1013 | ||
| 7—high | 63 | 4.1 | 4.5 | 1093 | 922 | 892 | ||
| 8—high | 427 | 3.9 | 24 | 310 | 434 | 1868 | 1425 | 1164 |
| 9—high | 241 | 26 | 7.2 | 1499 | 2102 | 905 | ||
| Mean ± SD | 181 ± 137 | 7.7 ± 7.0 | 9.2 ± 6.2 | 1075 ± 510 | 995 ± 537 | 818 ± 307 | ||
| One way-repeated | p < 0.001 | |||||||
| measures ANOVA | p < 0.001 | NS | ||||||
- Controls: non-rituximab infused, non-phiX174-infused subjects with normal renal physiology; CRF: chronic renal failure subjects.
- RIT, treated subjects are tested prior to infusion of RIT, which is given 2 week prior to first immunization of phiX174.
Phage clearance and neutralizing antibody titers
All 18 patients had circulating phage at the expected concentrations (2–4 × 107 PFU/μL serum) at 15 min post-infusion and showed normal phage clearance by day 7. Phage-neutralizing antibody titers expressed as Kv are summarized in Table 3. Rituximab significantly inhibited the antibody response to phiX174 at all timepoints following both primary and secondary immunizations compared to CRF subjects (p < 0.001) and normal controls (p < 0.001) (Figure 1). Subjects with CRF have significantly reduced antibody response following both primary (p = 0.02) and secondary (p < 0.001) immunizations compared to normal controls. There were no obvious differences in Kv responses among the different RIT dosage groups.
| Day post- immunization | Primary response | Secondary response | ||||
|---|---|---|---|---|---|---|
| Rituximab N = 9 (±2 SD) | CRF N = 9 (±2 SD) | Healthy subjects N = 52 (±2 SD) | Rituximab N = 8 (±2 SD) | CRF N = 8 (±2 SD) | Healthy subjects N = 52 (±2 SD) | |
| 7 | 0.11 (<0.01–4.22) | 3.20 (0.09–112.4) | 8.71 (1.55–49) | 0.14 (<0.001–22.8) | 186 (48.15–715.6) | 550 (167–1808) |
| 14 | 0.14 (<0.01–8.27) | 22.2 (0.67–730.7) | 114 (9.13–1424) | 0.23 (<0.01–67.7) | 191 (61.12–595.2) | 357 (114–1114) |
| 28 | 0.08 (<0.01–4.54) | 16.6 (0.99–275.9) | 65.3 (7.72–553) | 0.50 (<0.01–93.6) | 105 (26.12–424.8) | 183 (61–549) |
| p-value = <0.001 | p-value = <0.001 | |||||
| p-value = <0.02 | p-value = <0.001 | |||||
| p-value = <0.001 | p-value = <0.001 | |||||
- Data presented are geometric mean of Kv with 2 SD displayed in parenthesis. p-values are comparisons between peak Kv for each group. CRF—chronic renal failure subjects.
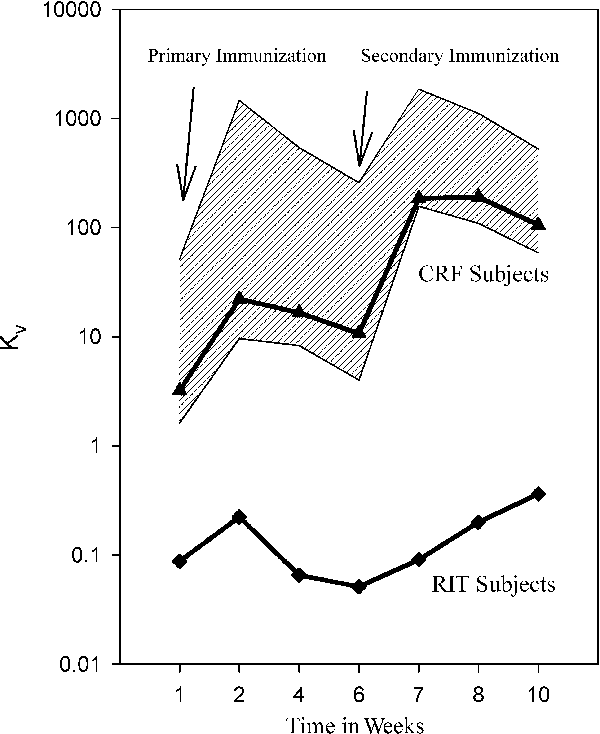
Graphical representation of antibody response to phiX174. Each line consists of the geometric mean for that group. Hatched area represents normal control responses ± 2 SD. Y-axis is the Kv. X-axis is week after primary or secondary immunization. p-values are shown in Table 3.
Peak Kv occurred at 2 weeks post-primary immunization in normal controls, in CRF subjects and in RIT-treated subjects (Table 4). Peak Kv following secondary immunization occurred at week 1 in normal controls, at 2 weeks in CRF subjects and at 4 weeks in RIT-treated subjects, which was significantly longer when compared to the normal controls (p = 0.03) (Figure 1).
| Primary response | Secondary response | ||||
|---|---|---|---|---|---|
| Rituximab N = 9 | CRF N = 9 | Healthy subjects N = 52 | Rituximab N = 8 | CRF N = 8 | Healthy subjects N = 52 |
| 0.15 (0.01–11.8) | 3.20 (1.4–182) | 128 (10.6–1168) | 0.47 (0.06–10.6) | 226 (110–548) | 540 (138–2544) |
| p-value = <0.001 | p-value = <0.001 | ||||
| p-value = <0.02 | p-value = <0.001 | ||||
| p-value = <0.001 | p-value = <0.001 | ||||
- Data presented are peak values of Kv with ranges displayed in parenthesis. p-values are comparisons between peak Kv for each group. CRF—chronic renal failure subjects.
Isotype switching
Control and CRF subjects demonstrated isotype switching during the secondary phage immunization. Percent IgG anti-phage antibody was reduced in CRF subjects (36%± 18) when compared to normal controls 53%± 32 (p = 0.11). Six of eight RIT subjects failed to produce antibody of the IgG class. Two RIT subjects (No. 7 and 9), both with very low Kv's, had very small percentage of IgG antibodies (5.8% and 15%, respectively)
Re-immunization group
Three subjects were available for re-immunization with phiX174 and tetanus toxoid. Each of the three subjects had received a different dose of RIT (Table 1). After RIT administration at the time of receiving tertiary and quaternary phage immunizations, CD19 counts had returned to baseline (Table 2). Following tertiary immunizations normal controls (n = 14) had a geometric mean Kv of 990 with peak Kvs occurring at 1 week, of which 89% was IgG. The peak Kv in the three patients that were re-immunized following the tertiary immunization was at 1 week. In the three patients immunized a fourth time, the peak Kv was also seen at 1-week post-immunization.
Subject no. 1, who received the low dose of RIT, had an undetectable primary response with a peak secondary response of 0.06 with no isotype switching. A tertiary immunization was given 933 days after RIT administration resulting in a peak Kv of 405 with 99.9% IgM. Following a quaternary immunization his peak Kv occurred at 1 week, was 778, 49% of which was of the IgG isotype. Tetanus IgG antibody titer increased from a baseline of 0.63 IU/mL to a peak of 5.13 IU/mL by week no. 4.
Subject no. 4, who received the medium dose of RIT, had markedly depressed primary and secondary responses with peak Kv of 0.045 and 0.036, respectively, without isotype switching. Tertiary immunization produced a peak Kv at 1 week of 0.52 with undetectable IgG followed by a quaternary response with a peak Kv of 3.87 and only 0.46% IgG. Tetanus IgG antibody titer increased from a baseline of 1.52 IU/mL to a peak of >20.05 IU/mL by week no. 4.
Subject no. 8, who received the high dose of RIT, had a weak primary and secondary response with peak Kv's of 0.289 and 10.6 and no detectable IgG. Tertiary immunization produced a peak Kv at 1 week of 2512 with a strong isotype switch (48% IgG) followed by a quaternary response with a peak Kv of 278 with 46% IgG.
Discussion
Observations from clinical trials involving the use of rituximab have lead investigations into the function of this drug in vivo. Drug effects include cell cycle arrest primarily through influx of Ca2+ ions through the CD20 molecule causing G0 arrest, induction of apoptosis and antibody dependent cellular cytotoxicity (34). Removal of CD20 cells by selective elimination is completed by 24 to 72 h (4). Drug effects have been seen up to 2 months after treatment (35). In this study, we observed that after treatment with rituximab, circulating CD19+ lymphocytes were nearly completely eliminated at 2 weeks, the time when the primary immunization with phiX174 was given. Since antibody production to phage occurs in secondary lymphoid tissue such as spleen and lymph nodes, it is likely that B cells in those tissues are also depleted or non-functional. In studies of lymphoma patients, administration of rituximab resulted in depletion of tissue based B cell; lymph node biopsies performed 14 days after therapy showed a decrease in the percentage of B cells in seven of eight patients who had received a single dose of rituximab (≥100 mg/m2)(2).
Reactivity to bacteriophage phiX174 enables classification of immune deficiencies compared to normal responses into five types depending on the rate of antigen clearance, amount of antibody produced and level of class switching (11). Type 0 patients show neither antigen clearance, nor antibody responses, to primary or secondary immunizations. Type I patients are able to clear antigen but fail to mount substantial antibody responses. Rituximab-treated subjects showed a Type I response that is similar to patients with XLA who have some residual B cell function and is similar to a subset of patients with common variable immune deficiency (11,12). The small amount of residual response in some patients probably reflects incomplete depletion of B cells. It is possible that early differentiating B cells with low-level expression of CD20 survived and are the source of the small amount of antibody produced. In a murine model with a human CD20 transgene, marginal B cells, despite expressing high levels of CD20 are apparently resistant to rituximab depletion (A Chan, personal communication). It is conceivable that these marginal B cells are the source of the weak primary response seen. Pharmacokinetic studies in our patient population showed that the half-life of rituximab was 10–14 days and substantial amounts of circulating rituximab were present well beyond the time of secondary immunization (8). Therefore, as these early B cells were stimulated and increased expression of CD20 or marginal B cells migrated from the marginal zone, they could have been degraded by circulating rituximab, thus preventing class switch and somatic hypermutation required for high affinity phage neutralization of the IgG isotype.
Our results are similar to previous reports that studied the effect of rituximab on humoral immune response in baboons or in humans. Baboons pretreated with rituximab showed a significant decrease in responsiveness to a primary and secondary immunization with KLH and failed to switch from IgM to IgG (10). In lymphoma patients undergoing treatment with rituximab, secondary immune responses to either KLH or an inactivated hepatitis A virus strain was impaired (9).
Because of the known effects of CRF on in vivo immune responsiveness, it was necessary to include a control group of subjects with CRF but who did not receive rituximab. In contrast to the rituximab-treated subjects, the CRF subjects demonstrate a Type IV response. Type IV patients are defined as having both a decreased primary and secondary antibody production with robust amplification but decreased percentage of phage neutralizing antibody (11). The Kv's observed in CRF patients were lower than those of the normal controls, but mostly within 2SD of the controls. Formation of memory B cells is suggested by the strong secondary response demonstrating amplification and the production of IgG anti-phage antibodies. Therefore, the profound effects seen after rituximab treatment cannot be explained simply by CRF.
Of particular interest are the three different types of responses noted in the three subjects who were re-immunized more than 2 years after exposure to rituximab. Subject no. 1, who belongs to the low-dose rituximab group, had a re-immunization pattern following the tertiary immunization that resembled a primary response and following the quaternary immunization had a pattern that resembled a secondary response. This implies that rituximab completely ablated the immune response in this patient leading to essentially no response during primary and secondary immunization suggesting a failure to generate memory B cells. Subject no. 8 who received the highest dose of rituximab had a mildly depressed primary and secondary response with no isotype switching. The tertiary response produced a brisk initial response with a peak Kv at 1 week and normal isotype switching, characteristic for a secondary (recall) response. This was followed by a weaker quaternary response indicating that the subject did not develop tolerance to the antigen and generated memory T and B cells following the secondary immunization. Of note is that subject no. 8 had this highest level of circulating B cells at the time of secondary immunization. Subject no. 4 who received the medium dose of rituximab had barely detectable primary and secondary antibody response with only minimal amplification during the tertiary and quaternary response without isotype switching suggesting a permanent defect in recognizing and responding to the neoantigen phiX174 to which he was exposed while on rituximab. At the same time subject nos. 1 and 4 were able to mount a normal antibody response to tetanus demonstrating that their immune system responded well to recall antigens to which they were repeatedly exposed prior to rituximab treatment. These findings suggest that Subject no. 4 had become tolerant to the antigen, since none of the CRF subjects responded as low as this subject during their primary and secondary response
Several currently used drugs induce anti-drug antibodies that have the potential to reduce the efficacy of the drug. Infliximab, a human-mouse chimeric antibody against tumor necrosis factor similar in structure to rituximab, has shown to be efficacious in the treatment of Crohn's disease (36), ulcerative colitis (37–39), rheumatoid arthritis (40) and plaque psoriasis (41). Studies have shown that the formation of human anti-chimeric antibodies (HACA) against infliximab can be as high as 53% of patients receiving the drug. This has been shown to decrease the efficacy of infliximab (42–45). More recently, neutralizing antibodies against interferon-beta have been reported in 34% of patients being treated for multiple sclerosis with reduction in clinical efficacy (46). Similar results have been seen in other xeno-derived antibodies such as Thymoglobulin, ATGAM and OKT3 (47–49). In this study, rituximab has been shown to reduce antibody production to antigen. This suggests that the infusion of rituximab prior to or perhaps at the time of initiation of dosing with these various drugs may decrease anti-drug antibody responses and increase efficacy. Rituximab itself induces HACA formation in about 1% of patients (35). In previous reports, rituximab has been shown to have a low incidence of side effects and cytokine production in non-lymphoma treated patients (8). Therefore rituximab might be used in these patients with only minimal increases in morbidity.
The use of bacteriophage has provided an opportunity to study the effect of rituximab on antibody production after destruction of B cells. The overall goal of this project is to provide assistance to the treatment of patients who are highly sensitized against MHC antigens and prevent sensitization after organ transplant. The development of anti-MHC antibodies directed against organ donor alloantigens has been shown to both predict and correlate with chronic rejection (50,51). Although the relationship between antibody production against phiX174 and neo-MHC antigens may not be identical, some correlation to rituximab's ability to ablate antibody production may be assumed. Rituximab when infused in patients at the time of transplantation may have the ability to ablate the neo-MHC antigen immune response. Prospective analysis of the anti-MHC response in subjects treated with rituximab at the time of transplantation will be needed to confirm this hypothesis. This may translate into reduced rates of chronic rejection and improve long-term graft survival.
Acknowledgments
This study was supported in part by Genentech and by NIH General Clinical Research Center Grant PHS M01 RR750 to Mark D Pescovitz and NIH Grant HD 17427-33 to Hans D Ochs.




