Munc 18-1 and Granuphilin Collaborate During Insulin Granule Exocytosis
Abstract
Munc 18-1 is a member of the Sec/Munc family of syntaxin-binding proteins known to bind to the plasma membrane Q-SNARE syntaxin1 and whose precise role in regulated exocytosis remains controversial. Here, we show that Munc 18-1 plays a positive role in regulated insulin secretion from pancreatic beta cells. Munc 18-1 depletion caused a loss in the secretory capacity of both transiently transfected INS 1E cells and a stable clone with tetracycline-regulated Munc 18-1 RNA interference. In addition, Munc 18-1-depleted cells exhibited defective docking of insulin granules to the plasma membrane and accumulated insulin in the trans Golgi network. Furthermore, glucose stimulation after Munc 18-1 depletion resulted in the rapid formation of autophagosomes. In contrast, overexpression of Munc 18-1 had no effect on insulin secretion. Although there was no detectable interaction between Munc 18-1 and Munc-18-interacting protein 1 or calcium/calmodulin-dependent serine protein kinase, Munc 18-1 associated with the granular protein granuphilin. This association was regulated by glucose and was required for the specific interaction of insulin granules with syntaxin1. We conclude that Munc 18-1 and granuphilin collaborate in the docking of insulin granules to the plasma membrane in an initial fusion-incompetent state, with Munc 18-1 subsequently playing a positive role in a later stage of insulin granule exocytosis.
Insulin is packaged and stored in large dense-core vesicles (LDCVs) of pancreatic beta cells from where it is released by exocytosis in response to an elevation of glycaemia (1). Docking and fusion of LDCVs to the plasma membrane are key aspects of insulin granule exocytosis and are mediated, as in synaptic neurotransmitter release, by a core machinery of SNARE proteins (2). In the beta cell, the vesicular membrane protein VAMP-2 is an R-SNARE that specifically interacts with the plasma membrane-associated Q-SNARE proteins syntaxin1 and synaptosome associated protein of 25 kDa (SNAP-25) that form the core SNARE complex (3–5). SNARE core complex assembly is necessary but not sufficient for membrane fusion in vivo, and other protein families, such as Rabs and members of the Sec1/Munc18 (SM) protein family, are known to play essential roles in the regulation of vesicle exocytosis (6).
SM proteins are a highly conserved family with essential roles in vesicle trafficking and fusion that display high binding affinity for cognate syntaxins (for reviews, see 7,8). The SM protein Munc 18-1 is essential for regulated exocytosis in neurons and neuroendocrine cells (9) and binds with high affinity to the N-terminal Habc domain and a C-terminal region of syntaxin1 (10,11). This interaction generates a closed conformation state of syntaxin1 that prevents assembly of the core SNARE complex in vitro (12). Given that SNARE complex formation is essential for exocytosis, it could be expected that Munc 18-1 plays a negative role in regulated secretion. However, a large body of evidence points to an essential positive function for Munc 18-1 in exocytosis. For example, Munc 18-1-null mutant embryos are paralyzed and stillborn and show no synaptic neurotransmitter release at embryonic day 18 (13). In addition, a number of studies have shown that overexpression of mammalian Munc 18-1 does not have a negative impact on exocytosis (14–16).
Recent studies have shed new light upon the physiological function of Munc 18-1 in regulated exocytosis. An important role for Munc 18-1 in LDCV docking (17) and tethering (18) has been revealed in adrenal chromaffin cells. Additionally, Munc 18-1 has been found to bind to assembled SNARE complexes as well as to syntaxin1 (19) and to be directly displaced by VAMP-2 after binding to syntaxin1 in intact fusion-competent membranes (20). Munc 18-1 has also been found to accelerate the vesicle fusion reaction in a reconstituted system (21), suggesting that Munc 18-1 binding to syntaxin1 might enhance SNARE complex formation in vivo.
In addition to its interaction with syntaxin1, Munc 18-1 also binds to other proteins involved in vesicular secretion. In particular, Munc 18-1 can interact with DOC2, a double C2 domain family of proteins that copurify with synaptic vesicles (22) and translocate to the plasma membrane upon elevation of intracellular calcium (23). Munc 18-1 is also known to interact with the multi-modular adaptor Munc-18-interacting (Mint) proteins Mint1 and Mint2 in the brain (24). Mint1 has been proposed to act upon the recruitment of Munc 18-1 to neurexin-rich regions of the plasma membrane by its interaction with the membrane-associated guanylate kinase domain protein calcium/calmodulin-dependent serine protein kinase (CASK) (25). Finally, an interaction between Munc 18-1 and the Rab3/Rab27a effector granuphilin, which is specific for pancreatic beta cells and pituitary gland, has been observed using a two-hybrid assay in the insulin-secreting HIT-T15 cell line (26) and in neuroendocrine PC12 cells (27,28).
In the context of the pancreatic beta cell, Munc 18-1 expression was initially detected in pancreatic islets (29) and in the mouse MIN6 beta cell line (30) and was found to be associated with syntaxin1 in insulin-secreting HIT-T15 cells, where it was suggested to act as a negative regulator of the secretion process (31). Subsequently, Munc 18-1 overexpression was shown to have no effect on the number of exocytotic events in MIN6 cells (15). In addition, Munc 18-1 expression has been found to be decreased in pancreatic islets of insulin-resistant Goto–Kakizaki rats (32) and human type 2 diabetic patients (33).
In this study, we re-evaluate the role of Munc 18-1 in insulin secretion in view of the recent new data emerging about this SM protein and its interactions with other factors involved in regulated exocytosis.
Results
The pattern of expression of Munc 18-1 in INS 1E cells was first evaluated by immunofluorescence and confocal microscopy (Figure 1A). As expected, Munc 18-1 partially localized to the plasma membrane of cells, where it colocalized with syntaxin1. In addition, we could detect Munc 18-1 immunostaining in the cytoplasm, supporting the view that SM proteins might cycle on and off membranes during vesicle transport (34). To confirm Munc 18-1 immunolocalization to the plasma membrane, we isolated plasma membrane lawns of stimulated INS 1E cells and submitted these to immunofluorescence analysis (Figure 1B). Here again we could detect a Munc 18-1-specific signal, most of which was associated with both syntaxin1 and insulin staining, this latter representing the secretory granules associated to the internal face of the plasma membrane.
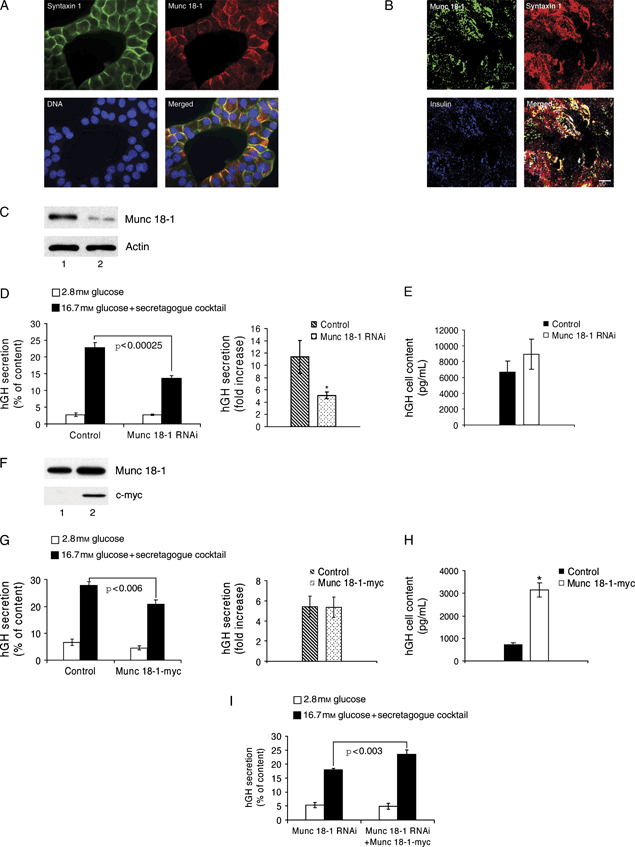
Munc 18-1 stimulates the exocytosis and growth hormone biosynthesis of INS 1E cells. A) Subcellular localization of syntaxin1 (green) and Munc 18-1 (red) at the plasma membrane of INS 1E cells. B) Plasma membrane lawn preparation of INS 1E cells showing colocalization of Munc 18-1 (green), syntaxin1 (red) and insulin (blue). Bar, 5 μm. C) Western blot showing the effect of RNAi on Munc 18-1 protein levels: INS 1E cells were transfected with either pSUPER-EGFP control (lane 1) or pSUPER-EGFP Munc 18-1 RNAi (lane 2), and the EGFP-positive cell population was selected by FACS and used for immunoblotting. A decrease of 77% in the level of Munc 18-1 relative to actin was obtained after Munc 18-1 RNAi. D) INS 1E cells were cotransfected with pSUPER-control RNAi or pSUPER-Munc 18-1 RNAi plasmids and an hGH-coding vector, and growth hormone secretion was measured after stimulation with 16.7 mm glucose supplemented with a secretagogue cocktail. Data are mean ± SEM, and n = 8 from three independent experiments (*p < 0.04). E) INS 1E cells cotransfected with pSUPER-control RNAi or pSUPER-Munc 18-1 RNAi and an hGH-coding vector show no significant difference in cellular hGH content. F) Western blot showing the exogenous expression of c-myc-tagged Munc 18-1 in the INS 1E cell line: cells were transfected with either a control empty vector (lane 1) or a vector expressing rat Munc 18-1 fused to c-myc (lane 2). Exogenous Munc 18-1-myc was specifically detected with an anti-c-myc-tag antibody (10 μg total protein/lane). An increase of 66% in the level of Munc 18-1 in the total (transfected plus non-transfected) population compared with the control was obtained after Munc 18-1-myc transfection. G) No significant difference in growth hormone secretion (fold stimulation) was measured in INS 1E cells cotransfected with either a control empty vector or the Munc 18-1-myc expression plasmid and an hGH-coding vector. H) hGH content of INS 1E cells cotransfected with an hGH-coding vector and either a control empty vector or the Munc 18-1-myc plasmid. Data are mean ± SEM, and n = 9 from three independent experiments (*p < 0.00001). I) INS 1E cells were cotransfected with an hGH-coding vector and the pSUPER-Munc 18-1 RNAi plasmid or a combination of pSUPER-Munc 18-1 RNAi and Munc 18-1-myc plasmids, and growth hormone secretion was measured after stimulation with 16.7 mm glucose supplemented with a secretagogue cocktail. Data are mean ± SEM, and n = 8 from three independent experiments.
To readdress the issue of the regulation of insulin exocytosis by Munc 18-1, we employed RNA interference (RNAi) to knock down Munc 18-1 in INS 1E cells (Figure 1C–E). To assess the efficacy of Munc 18-1 downregulation, INS 1E cells were transiently transfected with a plasmid coding for a short hairpin RNA (shRNA) sequence specific for rat Munc 18-1 messenger RNA (mRNA) and for enhanced green fluorescent protein (EGFP), which was used to mark the transfected cells. For a negative control, cells were transfected with the same type of plasmid coding for an unrelated non-mammalian shRNA sequence. Three days after transfection, Munc 18-1 protein levels were determined in an EGFP-positive cell population obtained by fluorescence-activated cell sorting (FACS). Western blot analysis showed that the shRNA against Munc 18-1 knocked down this protein in INS 1E cells compared with the level observed in cells expressing the negative control shRNA (Figure 1C).
To determine the involvement of Munc 18-1 in regulated insulin secretion, INS 1E cells were cotransfected with the plasmid coding for the Munc 18-1 shRNA and with a vector coding for human growth hormone (hGH). This hormone was used as a reporter of secretion from the small proportion (approximately 5%) of transfected cells as it can be stored (35) and cosecreted with insulin after transfection of pancreatic beta cells (36–38). In order to achieve adequate stimulation of insulin secretion from parental INS 1E cells, it was necessary to expose them to a cocktail of secretagogues (see Materials and Methods). INS 1E cells transfected with the control non-specific shRNA displayed an 11.35-fold increase in growth hormone secretion after stimulation, whereas cells transfected with the Munc 18-1-specific shRNA reached only a 5.11-fold increase in growth hormone secretion (Figure 1D). Because both cell types had a similar growth hormone content (Figure 1E), Munc 18-1-depleted INS 1E cells lost 55% of their secretory response compared with that of control cells.
To investigate the effect of overexpressing Munc 18-1, INS 1E cells were transfected with either a plasmid coding for rat Munc 18-1 fused to the c-myc tag under control of the cytomegalovirus (CMV) promoter or an empty vector. Under the conditions of our experiments, the level of Munc 18-1 was increased in the total, non-sorted cell population (Figure 1F), indicating that Munc 18-1 was highly overexpressed in the small proportion of cells that were transfected. To determine the secretion of these cells, we cotransfected the Munc 18-1-myc plasmid with the hGH-coding vector and analyzed hGH secretion. We measured a small but significant decrease in stimulated hGH secretion in cells overexpressing Munc 18-1, which was accompanied by a concomitant decrease of basal secretion and did not negatively impact on the fold increase induced by stimulation (Figure 1G). In addition, we measured a 4.35-fold increase in the hGH content of the cells overexpressing Munc 18-1, reflecting an effect of Munc 18-1 overexpression on the synthesis and/or storage of this hormone (Figure 1H). Last, and in order to evaluate the specificity of the Munc 18-1 RNAi secretory phenotype, hGH secretion was determined in INS 1E cells cotransfected with the hGH-coding vector and either Munc 18-1 shRNA or a mixture of this last plasmid and the Munc 18-1-myc vector (Figure 1I). These latter cells increased their secretory response by 30.6% compared with cells transfected with the Munc 18-1-specific shRNA alone, demonstrating that the reduction in the secretory capacity observed after Munc 18-1 shRNA plasmid transfection is specific for the depletion of Munc 18-1.
In view of previous reports indicating marked defects in the tethering and/or docking of LDCVs to the plasma membrane of adrenal chromaffin cells (18,39) and pituitary somatotrophs (17) of Munc 18-1-null mutant mice, we investigated whether Munc 18-1 is involved in the tethering/docking of insulin granules to the plasma membrane. For this purpose, we cotransfected INS 1E cells with either the Munc 18-1 shRNA or the control non-specific shRNA and a fusion made of phogrin, an LDCV transmembrane protein, and EGFP (40,41). Cotransfected cells were then stimulated for insulin secretion as in the experiments shown in Figure 1 and observed by total internal reflection fluorescence (TIRF) microscopy. Under these conditions, we measured a significant reduction in the number of granules adjacent to the plasma membrane of Munc 18-1-depleted cells (Figure 2), indicating that Munc 18-1 is involved in the tethering and/or docking of insulin granules to the plasma membrane.
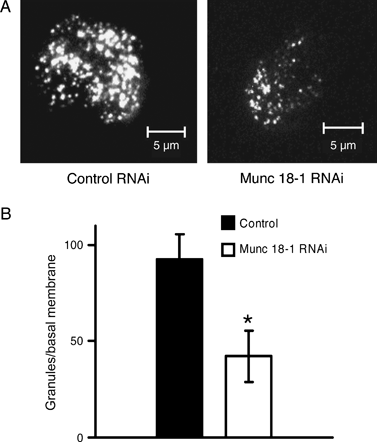
Number of secretory vesicles at the plasma membrane of control versus Munc 18-1-depleted INS 1E cells. A) Representative TIRF images of the plasma membrane of INS 1E cells cotransfected with either pSUPER-control RNAi or pSUPER-Munc 18-1 RNAi and a phogrin–EGFP plasmid. Bars, 5 μm. B) The number of secretory vesicles detected by TIRF at the plasma membrane of Munc 18-1-depleted INS 1E cells is reduced compared with that of control cells. Data are mean ± SEM, and n = 10 cells of comparable size for each experimental condition (*p < 0.003).
Next, we examined endogenous insulin secretion from a newly generated line of INS 1E cells, hereafter referred to as clone 2 cells, which features a Tet-inducible control of Munc 18-1 shRNA expression. This particular subclone of INS 1E cells is also exquisitely sensitive to glucose, with a much more robust stimulation of insulin secretion than the parental INS 1E cells. We observed that the levels of Munc 18-1 were markedly reduced after 3 days of shRNA expression in the presence of doxocyclin (Figure 3A). Munc 18-1 downregulation was confirmed by immunofluorescence and confocal microscopy and was not associated with changes in syntaxin1 immunolocalization (Figure 3B). Thus, in disagreement with a previous report (42) but in agreement with results obtained in Munc 18-1-null mutant neurons (43), Munc 18-1 does not act as a chaperone-like protein required for the targeting of syntaxin1 to the plasma membrane of insulin-secreting cells.
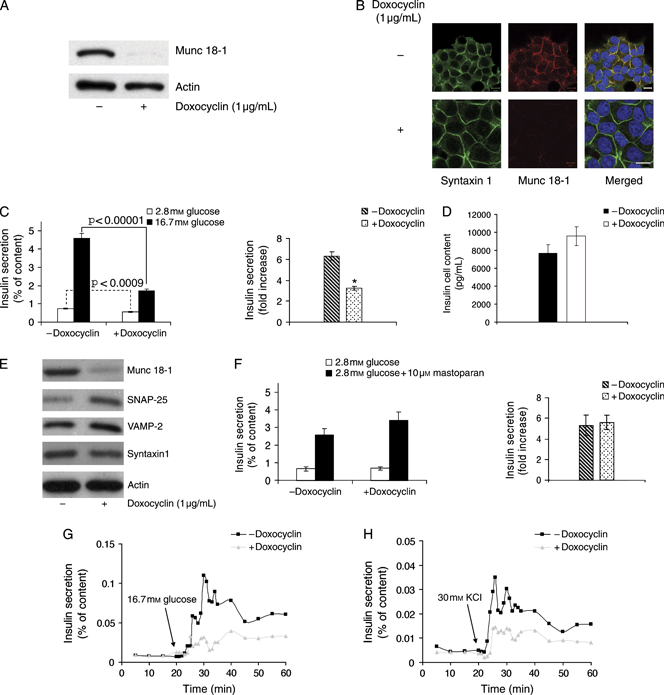
Effects of the Tet-controlled change in Munc 18-1 expression on insulin secretion. A) Analysis of the level of Munc 18-1 in INS 1E-Tr1 pSUPERIOR Munc 18-1 (clone 2) cells ± doxocyclin induction of Munc 18-1 RNAi: clone 2 cells were cultured for 3 days in the presence or absence of 1 μg/mL doxocyclin, and cell lysates were analyzed by Western blot. The level of Munc 18-1 drops 96% relative to actin when Munc 18-1 shRNA expression is induced by the presence of the Tet analogue in the culture medium. B) Immunofluorescence analysis of syntaxin1 (green) and Munc 18-1 (red) in clone 2 cells ± doxocyclin. Nuclei are labelled blue in the merged image. Note the near-complete depletion of Munc 18-1-specific signal after doxocyclin induction of Munc 18-1 RNAi. Bars, 10 μm. C) Glucose-stimulated insulin secretion was measured on clone 2 cells ± doxocyclin induction of Munc 18-1 RNAi. Data are mean ± SEM, and n = 12 from four independent experiments (*p < 0.00001). D) No significant difference in cellular insulin content was detected in clone 2 cells ± doxocyclin induction of Munc 18-1 RNAi. E) Western blot analysis of the level of the SNARE proteins SNAP-25, VAMP-2 and syntaxin1 in clone 2 cells ± doxocyclin induction of Munc 18-1 RNAi. The levels of SNAP-25 and VAMP-2 increased by 2.3- and 1.4-fold, respectively, in Munc 18-1-depleted cells, while the level of syntaxin1 relative to actin did not change (n = 3 independent experiments). F) Mastoparan-stimulated insulin secretion measured on clone 2 cells ± doxocyclin induction of Munc 18-1 RNAi. Data are mean ± SEM, and n = 9 from three independent experiments. G and H) Perifusion experiments performed to evaluate the time–course of insulin secretion in clone 2 cells ± doxocyclin treatment for 3 days: cells were perifused with 2.8 mm glucose and challenged with 16.7 mm glucose (G) or 2.8 mm glucose + 30 mm KCl (H) for 40 min at 37°C. The data correspond to a single experiment representative of three independent ones.
The doxocyclin-induced Munc 18-1 depletion resulted in a small but significant reduction in basal secretion as well as a marked decrease in glucose-stimulated insulin secretion of clone 2 cells (Figure 3C). This was not the reflection of a change in the insulin content of the cells (Figure 3D), confirming a positive role of Munc 18-1 in glucose-regulated insulin secretion. In addition, we observed an increase in the level of expression of the SNARE proteins SNAP-25 and VAMP-2 after doxocyclin-induced Munc 18-1 depletion (Figure 3E). This could represent a compensatory mechanism for the loss of glucose-stimulated insulin secretion in Munc 18-1-depleted cells. By contrast, mastoparan, a wasp venom toxin that induces secretion by activating trimeric G proteins (44), was still effective in stimulating insulin secretion of clone 2 cells after depletion of Munc 18-1 (Figure 3F).
By perifusing the cell cultures, we observed that clone 2 cells depleted of Munc 18-1 featured decreased insulin secretion at all time-points during both glucose (Figure 3G) and KCl stimulation (Figure 3H), showing that Munc 18-1 affects steps of the insulin-releasing machinery that follow ATP production and membrane depolarization and that its role is not restrained to any particular secretory phase.
Using coimmunolocalization with the trans Golgi network (TGN) marker TGN38 (45), we observed that insulin immunoreactivity accumulated in aggregates in the TGN of clone 2 cells cultured in the presence of doxocyclin in both basal (2.8 mm glucose) and stimulated (16.7 mm glucose) conditions (white arrows, Figure 4A,B), while the insulin signal at the plasma membrane was diminished after antibiotic treatment in glucose-stimulated cells (compare with arrowheads, Figure 4B). Such changes were not observed in the doxocyclin-unresponsive INS 1E cell line from which clone 2 cells were derived (Figure 4C), suggesting that they are unrelated to doxocyclin and because of Munc 18-1 depletion.
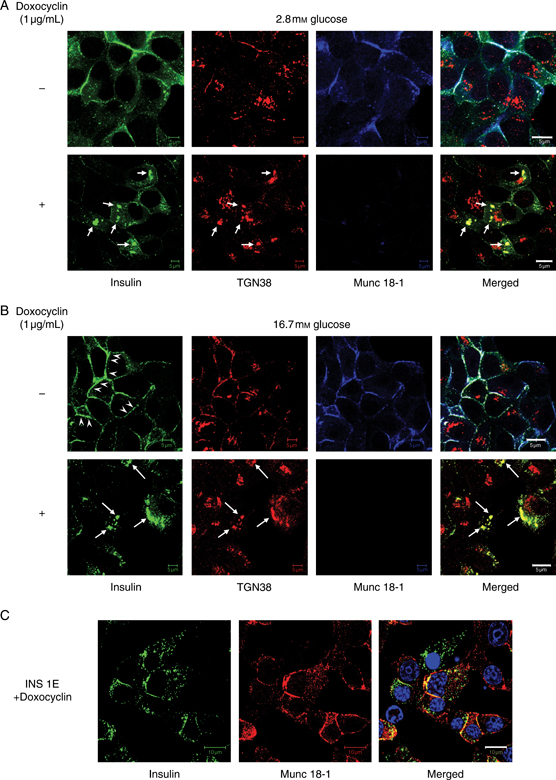
Insulin accumulates in the TGN of clone 2 cells following Munc 18-1 depletion. A and B) Immunolocalization of insulin (green), the TGN marker TGN38 (red) and Munc 18-1 (blue) in clone 2 cells incubated in 2.8 mm glucose (A) or stimulated with 16.7 mm glucose (B) ± Munc 18-1 depletion by doxocyclin treatment. Note the presence of insulin aggregates positive for TGN38 in Munc 18-1-depleted cells (arrows) and the decrease in plasma membrane insulin staining after glucose stimulation of doxocyclin-treated compared with untreated cells (arrowheads). C) Immunolocalization of insulin (green) and Munc 18-1 (red) in the parental INS 1E cell line after 3 days of treatment with doxocyclin. Bars, 10 μm.
By electron microscopy, the ultrastructure of clone 2 cells cultured in the presence of doxocyclin in basal glucose conditions (Figure 5A) was similar to that of the control parental INS 1E cells (not shown) and of clone 2 cells not exposed to doxocyclin (Figure 5B). By contrast, 30 min of glucose stimulation in the doxocyclin-treated clone 2 cells resulted in the appearance of numerous circular areas of electron-lucent cytoplasm limited by multiple membranes (Figure 5C). These structures had the typical characteristics of autophagosomes and occasionally contained secretory granules (Figure 5D). The presence of insulin-containing autophagosomes in glucose-stimulated doxocyclin-treated clone 2 cells was confirmed by coimmunolocalization of insulin with the autophagic vacuole marker microtubule-associated protein light chain 3 (LC3) (Figure 5E).
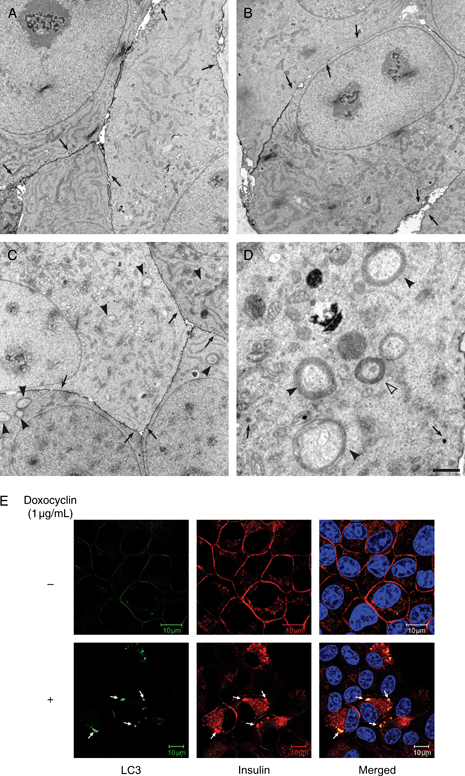
Munc 18-1 depletion induces autophagic vacuoles in glucose-stimulated clone 2 cells. A) Clone 2 cells were exposed to doxocyclin for 3 days and incubated for 2 h in 2.8 mm glucose. Electron microscopy of these cells showed an ultrastructural organization similar to that of wild-type controls with secretory granules (arrows) distributed along the cell membrane. B) The same granule distribution (arrows) was observed in clone 2 cells not exposed to doxocyclin and stimulated for 30 min in 16.7 mm glucose. C) By contrast, clone 2 cells exposed to doxocyclin for 3 days and stimulated for 30 min in 16.7 mm glucose featured unusually numerous, round areas of electron-lucent cytoplasm (arrowheads); secretory granules (arrows). D) At higher magnification, these structures (arrowheads) were identified as autophagic vacuoles by their multi-membrane boundary and their cytoplasmic content, including occasional secretory granules (open arrowhead). Bars, 1.2 μm (A–C) and 270 nm (D). E) Immunofluorescence analysis of the autophagosomal marker LC3 (green) and insulin (red) in clone 2 cells stimulated for 30 min in 16.7 mm glucose ± doxocyclin exposure. Nuclei are labelled blue in the merged image. Note that LC3 aggregates are absent in doxocyclin-untreated cells, while they are present and positive for insulin in doxocyclin-treated cells (arrows). Bars, 10 μm.
We next set out to determine the molecular partners interacting with Munc 18-1 to regulate insulin secretion. The multidomain adaptor protein Mint1 is known to interact with Munc 18-1 in synaptic neurons (24) and complexes with CASK, another adaptor protein that, in turn, interacts with the neuron-specific cell surface proteins neurexins (46). As Mint1 is known to be expressed in pancreatic islets (47), we investigated a possible interaction of Munc 18-1 with Mint1 and CASK in INS 1E cells. Both Mint1 and CASK were found in total cell extracts of INS 1E cells, with the latter protein concentrated in the membrane fraction together with Munc 18-1, syntaxin1 and SNAP-25 (Figure 6A). Mint1 was only detected intracellularly by immunofluorescence and confocal microscopy, although CASK colocalized well with Munc 18-1 at the plasma membrane of untreated clone 2 cells (Figure 6B; note that we could not colocalize Mint1 and Munc 18-1 because the available primary antibodies for these proteins are both raised in rabbits). Moreover, we were unable to coimmunoprecipitate Munc 18-1 with either Mint1 or CASK in extracts of untreated clone 2 cells, indicating that either Munc 18-1 does not complex with these two proteins in INS 1E cells or it does so with a binding affinity too low to allow for coimmunoprecipitation.
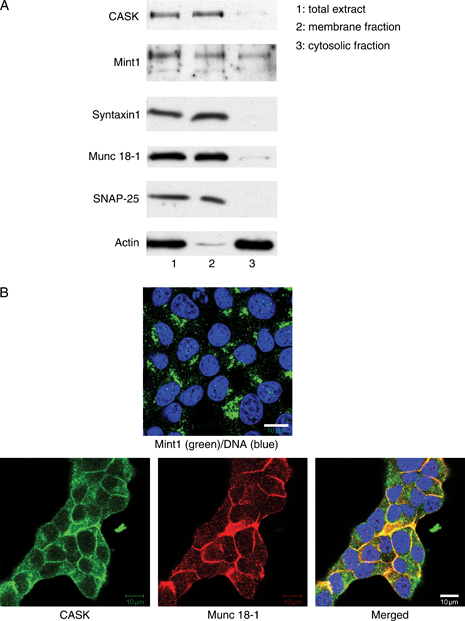
Munc 18-1 does not interact with Mint1 and CASK to regulate insulin secretion in INS 1E cells. A) Western blot showing the distribution of the proteins CASK, Mint1, syntaxin1, Munc 18-1, SNAP-25 and actin in the membrane and cytosolic fractions of clone 2 cells. B) Immunofluorescence analysis of Mint1 (top panel, green), CASK (bottom panels, green) and Munc 18-1 (bottom panels, red) showing colocalization of Munc 18-1 and CASK at the plasma membrane of clone 2 cells, while Mint1-specific signal localizes intracellularly. Nuclei are labelled in blue. Bars, 10 μm.
Another possible candidate for interaction with Munc 18-1 is the rabphilin-3-like protein granuphilin, also referred to as synaptotagmin-like protein-4 (48), that was originally identified in pancreatic beta cells, where it associates with mature insulin LDCVs (26,49). Granuphilin, which exists in two alternatively spliced isoforms (49), interacts with the GTP-bound form of the small guanosine triphosphatase (GTPase) Rab3a (26) and with both the GDP- and the GTP-bound forms of Rab27a (27,50–52) in LDCVs and also with the Munc 18-1/syntaxin1 complex in both PC12 (28) and insulin-secreting HIT-T15 cells (26).
Using a previously characterized granuphilin-a/b-specific antibody (26), we observed a granular immunostaining for granuphilin-a/b in the cytoplasm and at the plasma membrane of clone 2 cells, where it colocalized with the Q-SNARE syntaxin1, with a reduced cytoplasmic signal after stimulation with 16.7 mm glucose (Figure 7A). We could not test directly whether granuphilin and Munc 18-1 colocalized because the available primary antibodies were generated in the same animal species. We therefore transfected the Munc 18-1-myc fusion protein in clone 2 cells not treated with doxocyclin. Munc 18-1-myc was seen both intracellularly and at the plasma membrane, where it colocalized with granuphilin (Figure 7B). After immunoprecipitation of Munc 18-1 in doxocyclin-untreated clone 2 cells, we detected a band corresponding to granuphilin-b (together with a faint band for granuphilin-a) and syntaxin1 in the membrane, but not in the cytosolic fraction (Figure 7C), providing evidence for a direct interaction between granuphilin, Munc 18-1 and syntaxin1 in clone 2 cell membranes.
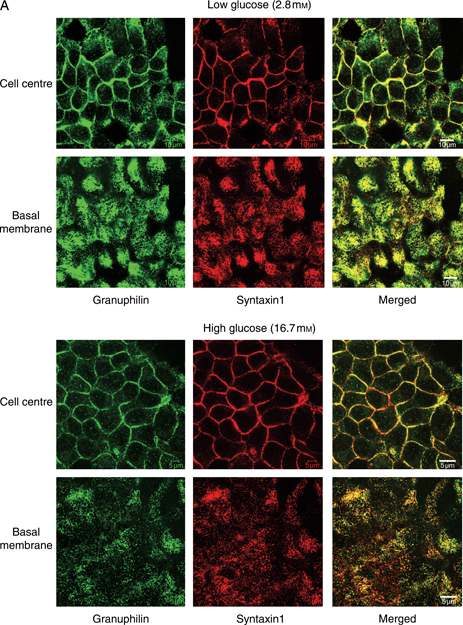
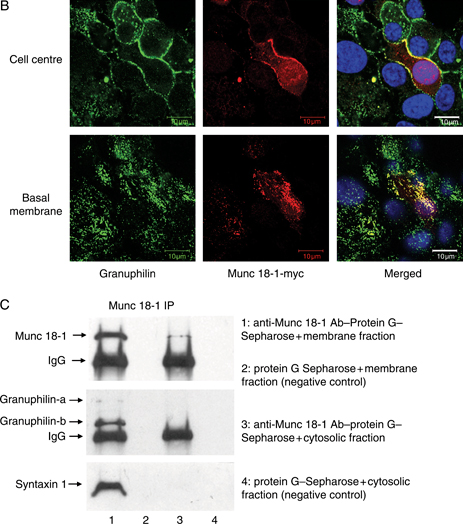
Munc 18-1 interacts with granuphilin and syntaxin1 at the plasma membrane of INS 1E cells. A) Immunofluorescence analysis of doxocyclin-untreated clone 2 cells showing colocalization of granuphilin (green) and syntaxin1 (red) at the plasma membrane, both in cells exposed to 2.8 mm glucose (top panels) or stimulated with 16.7 mm glucose (bottom panels). Bars, 10 μm. B) Immunolocalization of granuphilin (green), Munc 18-1-myc (red) and nuclei (blue in the merged image) in untreated clone 2 cells transfected with the Munc 18-1-myc plasmid. The signals for granuphilin and c-myc tag colocalize at the plasma membrane of transfected cells. Bars, 10 μm. C) Immunoprecipitation of Munc 18-1 from membrane and cytosolic fractions of untreated clone 2 cells. Note how both granuphilin and syntaxin1 coimmunoprecipitate with Munc 18-1 in the membrane fraction but are absent from the immunoprecipitate in both the cytosolic fraction and the negative controls. IgG, immunoglobulin G; IP, immunoprecipitation.
Next, we immunoprecipitated syntaxin1 to determine if the interaction between granuphilin and Munc 18-1 was mediated by this Q-SNARE. We observed that granuphilin-b coimmunoprecipitated with syntaxin1 in untreated but not in doxocyclin-treated Munc 18-1-depleted cells (Figure 8A), demonstrating that Munc 18-1 is required for the association of the vesicle-bound granuphilin with the syntaxin1 of the plasma membrane.
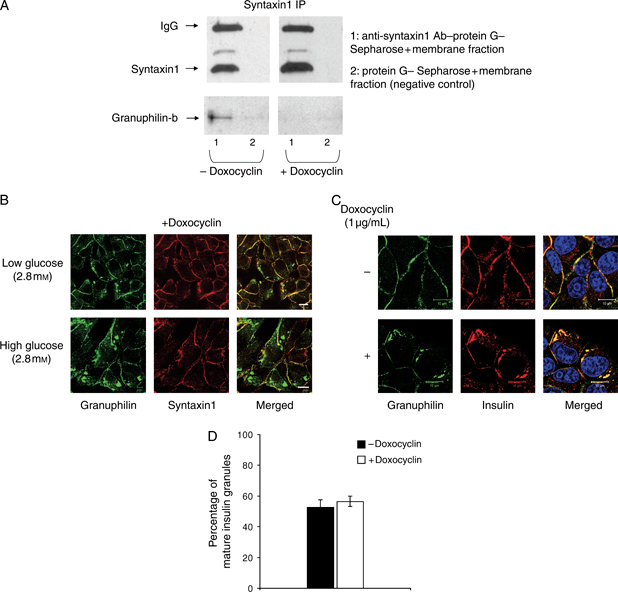
The interaction of Munc 18-1 with granuphilin is required for the association of granuphilin-positive LDCVs with plasma membrane syntaxin1. A) Immunoprecipitation of syntaxin1 from membrane fractions of clone 2 cells ± doxocyclin induction of Munc 18-1 depletion. Note that granuphilin coimmunoprecipitates with syntaxin1 only in cells not exposed to doxocyclin. B) Immunolocalization of granuphilin (green) and syntaxin1 (red) after doxocyclin induction of Munc 18-1 depletion in clone 2 cells at 2.8 mm glucose (top panels) and 16.7 mm glucose (bottom panels) showing the presence of granuphilin aggregates in the cytoplasm of Munc 18-1-depleted cells. Bars, 10 μm. C) Immunofluorescence analysis of granuphilin (green), insulin (red) and nuclei (blue in merged images) in clone 2 cells in the absence (top panels) or presence (bottom panels) of doxocyclin. Note that the granuphilin aggregates found in the cytoplasm of Munc 18-1-depleted cells are positive for insulin. Bars, 10 μm. D) Quantification of the percentage of mature insulin granules (expressed as a percentage of granuphilin-positive insulin signal) in clone 2 cells ± doxocyclin induction of Munc 18-1 RNAi. Data are mean ± SEM, and n = 7 cells of comparable size for each experimental condition. IgG, immunoglobulin G; IP, immunoprecipitation.
Accordingly, immunofluorescence analysis of clone 2 cells treated with doxocyclin revealed the presence of granuphilin in intracellular aggregates under both basal and glucose-stimulated conditions (Figure 8B). These accumulations did not stain for syntaxin1 but were positive for insulin (Figure 8C), suggesting that depletion of Munc 18-1 causes an alteration of the traffic of granuphilin-positive mature insulin granules towards the plasma membrane without impacting the granule maturation process as there was no change in the percentage of insulin associated with granuphilin (Figure 8D), this latter being thought to localize specifically to mature granules (53).
To determine the role of the granuphilin/Munc 18-1 complex in regulated secretion, we knocked down granuphilin-a and -b and/or Munc 18-1. Transient transfection of INS 1E cells with three plasmids coding for three different shRNA sequences specific for rat granuphilin-a/b and EGFP as a marker for the FACS selection of the transfected cells revealed that the protein levels of granuphilin-a/b were effectively knocked down by two of the three shRNAs tested (Figure 9A; data not shown for the ineffective granuphilin RNAi-2).
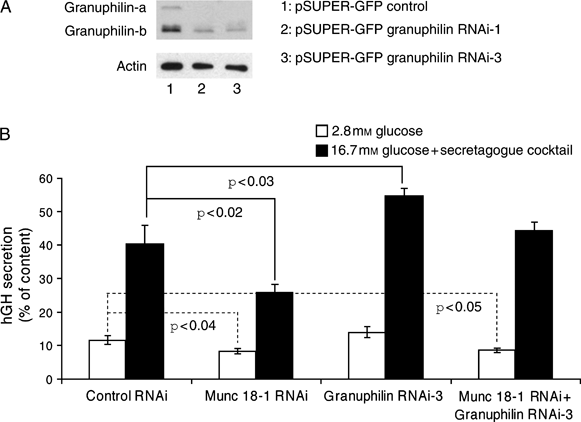
Effects of granuphilin and Munc 18-1 depletion on the exocytosis of INS 1E cells. A) Western blot showing the effect of RNAi treatment on granuphilin protein levels: INS 1E cells were transfected with pSUPER-EGFP control (lane 1), pSUPER-EGFP granuphilin RNAi-1 (lane 2) or pSUPER-EGFP granuphilin RNAi-3 (lane 3), and the EGFP-positive cell population was selected by FACS and used for immunoblotting. A decrease of 62 and 75% in the level of granuphilin-b relative to actin was obtained with granuphilin RNAi-1 and granuphilin RNAi-3, respectively, while granuphilin-a was totally depleted with both RNAi sequences. B) INS 1E cells were cotransfected with pSUPER-control RNAi, pSUPER-Munc 18-1 RNAi, pSUPER-granuphilin RNAi-3 and a mixture of pSUPER-Munc 18-1 RNAi + pSUPER-granuphilin RNAi-3 plasmids (1:1 ratio) and an hGH-coding vector. Growth hormone secretion was then measured after stimulation with 16.7 mm glucose supplemented with a secretagogue cocktail. Data are mean ± SEM, and n = 12 from four independent experiments. One-way analysis of variance: p < 0.003 (basal secretion); p < 0.0001 (stimulated secretion).
We then cotransfected either control RNAi, Munc 18-1 RNAi, granuphilin RNAi-3 or a mixture of Munc 18-1–RNAi and granuphilin RNAi-3 with hGH. Three days later and as expected, INS 1E cells transfected with the Munc 18-1-specific shRNA displayed a significant reduction in the basal and stimulated secretion of hGH compared with cells transfected with control RNAi. By contrast, granuphilin RNAi-3-transfected cells showed a significant increase in stimulated secretion, with no change in basal secretion. Cotransfection of Munc 18-1 and granuphilin shRNA returned the stimulated secretion to control levels, while significantly reducing the basal hGH secretion (Figure 9B).
Finally, to gain insight into the mechanism of action of the granuphilin/Munc 18-1 complex in insulin secretion, we investigated the regulation of this complex formation and plasma membrane localization by glucose. First, we performed coimmunofluorescence analysis of granuphilin and c-myc tag in doxocyclin-untreated clone 2 cells transfected with Munc 18-1-myc at different time-points of glucose exposure (Figure 10A). The signals specific for Munc 18-1 and granuphilin colocalized extensively at the plasma membrane of cells under basal glucose conditions (2.8 mm). However, after 15 and 30 min of 16 mm glucose exposure, we observed an accumulation of granuphilin-negative Munc 18-1 signal at the plasma membrane. Quantification of the percentage of colocalization of granuphilin and Munc 18-1-myc after different glucose incubation periods revealed that the percentage of granuphilin-positive Munc 18-1 signal at the plasma membrane gradually decreased during glucose exposure and was significantly diminished after 15 and 30 min of glucose treatment, while the majority of granuphilin remained colocalized with Munc 18-1 throughout the experiment (Figure 10B). Munc 18-1 coimmunoprecipitation studies from membrane fractions of clone 2 cells at basal (2.8 mm) glucose conditions and after 16.7 mm glucose stimulation showed a greater association of Munc 18-1 with granuphilin under basal glucose levels, with a moderate but readily apparent decrease in the amount of granuphilin that coimmunoprecipitated with Munc 18-1 after glucose stimulation of insulin secretion. As a control, there was no change in the amount of syntaxin1 that coimmunoprecipitated with membrane-associated Munc 18-1 (Figure 10C). In the same experiment, Rab27a was not detected in the immunoprecipitates at any of the glucose concentrations analyzed.
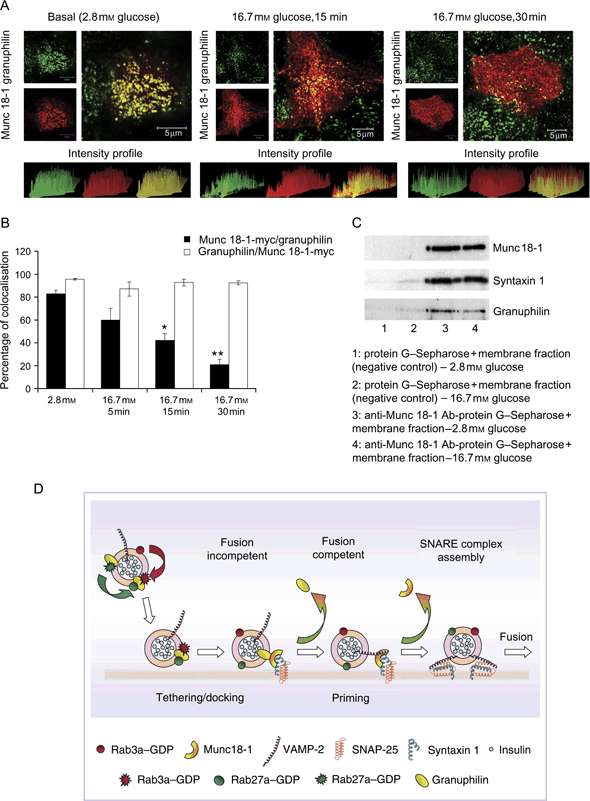
Regulation of the granuphilin/Munc 18-1 complex by glucose. A) Representative images of the plasma membrane of doxocyclin-untreated clone 2 cells transfected with the Munc 18-1-myc plasmid: coimmunolocalization of granuphilin (green) and Munc 18-1-myc (red) in basal conditions (2.8 mm glucose) or after stimulation with 16.7 mm glucose for 15 and 30 min. Histograms displaying the signal intensity profile are shown at the bottom of each immunofluorescence image. Bars, 5 μm. B) Quantification of the percentage of Munc 18-1-myc-specific signal colocalized with granuphilin and granuphilin-specific signal colocalized with Munc 18-1-myc at the plasma membrane of untreated clone 2 cells at different time-points of glucose stimulation. Data are mean ± SEM, n = 7 cells of comparable size for each experimental condition (*p < 0.0001; **p < 0.00001). C) Coimmunoprecipitation of syntaxin1 and granuphilin with Munc 18-1 from membrane fractions of doxocyclin-untreated clone 2 cells at 2.8 mm glucose and after 30 min stimulation with 16.7 mm glucose. A decrease of 32% in the amount of granuphilin associated with Munc 18-1 was observed after stimulation with 16.7 mm glucose (band densities relative to Munc 18-1: 0.42 versus 0.28, 2.8 versus 16.7 mm glucose), while no decrease was observed in the level of syntaxin1 associated with Munc 18-1 (band densities relative to Munc 18-1: 1.0 versus 1.09, 2.8 versus 16.7 mm glucose). D) Model for the function of Munc 18-1 and granuphilin during insulin exocytosis: granuphilin-positive insulin granules are retained at the plasma membrane in a fusion-incompetent state by the interaction between granuphilin, Munc 18-1 and syntaxin1. Subsequent disengagement of granuphilin from Munc 18-1 permits the incorporation of VAMP-2 to the SNARE complex. Finally, the granule becomes fusion-competent, and calcium-dependent granule fusion to the plasma membrane occurs.
Discussion
Our results show that Munc 18-1 colocalizes with syntaxin1 and insulin at sites of granule docking at the plasma membrane of INS 1E cells and is required for full stimulation of insulin release. In addition, the accumulation of insulin in aggregates at the TGN of Munc 18-1-depleted cells indicates that Munc 18-1 depletion induces defective trafficking and/or exocytosis of insulin-containing vesicles.
Using immunofluorescence and electron microscopy, we identified an abnormal accumulation of autophagosomes in the cytoplasm of Munc 18-1-depleted clone 2 cells after stimulation with 16.7 mm glucose. Autophagy is a lysosomal mechanism by which cells degrade proteins in order to produce amino acids for ongoing protein synthesis, to sustain energy production during starvation and to eliminate unwanted or damaged cell structures (54). The accumulation of autophagic vesicles has been previously observed in neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases and amyotrophic lateral sclerosis or in transmissible spongiform encephalopathies (prion diseases), syndromes that are all associated with proteins that misfold and aggregate (55).
In beta cells, endoplasmic reticulum stress is increased during diabetes, resulting in an overall augmentation of protein misfolding and aggregation (56). A recent report has pointed out the importance of autophagy in the clearance of stress-induced protein aggregates in insulin-secreting cells (57). A possible explanation for the accumulation of autophagosomes in Munc 18-1-depleted cells could be to guarantee the degradation of the excess of insulin after failure of the granules to be secreted in response to 30 min of glucose stimulation. Such rapid formation of autophagic vacuoles has previously been detected in sensitized cells in which autophagosomes accumulate after only 15–60 min of treatment (58,59). An alternative explanation would be that the accumulation of autophagosomes is caused by a disruption of the insulin autocrine effect on beta cells. Indeed, activation of the Akt/PKB signalling pathway by insulin through the insulin receptor is known to result in the activation of the protein kinase mammalian target of rapamycin, which negatively regulates autophagy (54,55). So, a sustained decrease in glucose-stimulated insulin secretion after Munc 18-1 depletion might cause an attenuation of the autocrine activation of the Akt/PKB pathway, which could in turn result in the stimulation of autophagy. Further experiments outside the scope of this study would be needed to validate this last hypothesis, which opens an attractive possibility to explain deleterious autocrine effects of insulin secretion defects in beta cells.
In contrast to Munc 18-1 depletion, overexpression in either INS 1E or clone 2 cells did not lead to an inhibition of growth hormone release measured as the fold increase between stimulated and basal secretion. Our results agree with previous work in neuronal and neuroendocrine systems (14,16,60) and reinforce the idea that Munc 18-1 plays a positive role during exocytosis. This is in contrast with the previously proposed negative regulatory function of Munc 18-1 on insulin exocytosis by controlling syntaxin1 availability for SNARE complex formation (31,61). One explanation for these apparently contradictory findings lies in our hypothesis for a dual action of Munc 18-1 (see model below), with both negative and positive actions on secretion depending upon the binding partners at each specific stage of exocytosis.
However, and in agreement with a previous report in PC12 cells (14), overexpression of Munc 18-1 resulted in a marked increase in the rate of growth hormone synthesis. As hGH expression is driven in our system by the non-physiological CMV promoter, the regulation of its synthesis by Munc 18-1 is unlikely to be at the transcriptional level but rather at a post-transcriptional stage (i.e. at the level of translation or consequent to increased residence time of secretory granules). Whether this effect has physiological consequences in somatotrophs from the anterior pituitary gland, which are the main producers of growth hormone, remains to be studied. Even though the fold increase of hGH secretion was unchanged in cells overexpressing Munc 18-1, we did detect lower levels of both basal and stimulated secretion measured as a percentage of the total hGH content. This might simply represent a saturation of the secretory system given the highly increased hGH cell content under these conditions.
In this study, we failed to detect an interaction between Munc 18-1 and Mint1 or CASK, even though both adaptor proteins were expressed in INS 1E cells. As Mint1 interacts with Munc 18-1 in synaptic neurons (24) and RNAi against Mint1 decreases the secretion of INS 1E cells (62), it has been suggested that Mint1 might localize Munc 18-1 to plasma membrane sites defined by neurexins in the vicinity of exocytosis sites through its interaction with CASK (25). However, our results indicate that this is not likely the case for insulin-secreting cells.
However, and as shown for chromaffin (18) and anterior pituitary cells (17), we have identified a positive role for Munc 18-1 in the tethering/docking of insulin granules to the plasma membrane in insulin-secreting cells. In parallel, we have established the existence of a physiological interaction in INS 1E cells between Munc 18-1 and granuphilin, a Rab effector that localizes to insulin granules (26,49). We also establish that the interaction between Munc 18-1 and granuphilin is responsible for the link between granuphilin and syntaxin1 at the plasma membrane. Moreover, depletion of Munc 18-1 causes the accumulation of granuphilin-positive mature insulin granules in intracellular aggregates without affecting the process of granule maturation.
Granuphilin is thought to play an important role in promoting the tethering/docking of LDCVs to the plasma membrane as its overexpression results in increased numbers of plasma membrane-associated vesicles in MIN6 (53) and PC12 cells (28), whereas granule docking is markedly reduced in pancreatic beta cells of granuphilin-null mice (63). Granuphilin has also been suggested to inhibit regulated secretion because its over-expression markedly reduces the stimulated secretion of PC12 (51) and insulin-secreting cell lines (26), whereas insulin secretion is enhanced in islets of granuphilin-null mice (63). Our data suggest that Munc 18-1 and granuphilin collaborate in the tethering/docking of insulin granules in an initial fusion-incompetent state that could represent a temporal brake for exocytosis by inhibiting constitutive fusion of incoming vesicles. This stage should precede the granule priming and fusion and could account for a precursor pool of granules of importance for the refilling of the rapidly releasable pool during insulin secretion (64,65).
As expected, RNAi-mediated depletion of granuphilin in INS 1E cells causes an augmentation of their secretory response, but this effect is abolished by the concomitant depletion of Munc 18-1. Munc 18-1 must therefore play an additional positive role in the secretion process independently of its interaction with granuphilin. This role must occur during the last stages of regulated secretion, downstream of membrane depolarization, as potassium-stimulated insulin secretion, which mimics the effect of the closure of the ATP-dependent potassium channels induced by glucose metabolism (66), is decreased after Munc 18-1 depletion. Previous reports have shown no effect of the overexpression of either wild-type Munc 18-1 (15) or a mutant form of Munc 18-1 with reduced affinity for syntaxin1 (16) in LDCVs fusion kinetics, suggesting that Munc 18-1 function precedes granule fusion.
During this study, we failed to detect a decrease in mastoparan-induced insulin secretion after Munc 18-1 depletion. Mastoparan is a secretagogue thought to stimulate insulin secretion by the activation of Rac, a small GTPase of the Rho family (44). Rac is a known activator of type I phosphatidylinositol 4-phosphate 5 kinase (PIP5KI) (67), the main enzyme implicated in the production of phosphatidylinositol 4,5 bisphosphate (PIP2). A role for PIP2 during vesicle exocytosis in the ATP-dependent stage of granule priming has been previously documented (64,68), so we can speculate that the positive function of Munc 18-1 should lie in a post-docking, pre-priming stage of insulin exocytosis. The exact mechanism remains to be unravelled, but it might involve a labile, half-closed state of syntaxin1 that represents an intermediate in the formation of SNARE complexes, as suggested for PC12 cells (20). Alternatively, mastoparan-dependent secretion could involve a different mechanism that would not require vesicle docking and would bypass the Munc 18-1/granuphilin-dependent stage of insulin release (69).
Our data allow us to refine the existing model for the function of Munc 18-1 during insulin exocytosis (Figure 10D). In this model, mature granuphilin-positive insulin granules that approach the plasma membrane are captured in a fusion-incompetent tethered state by the interaction between granuphilin, Munc 18-1 and syntaxin1 in a mechanism most likely governed by the Rab3a and/or Rab27a GTPases of the granules. During glucose stimulation of insulin secretion, a further signal results in the disengagement of granuphilin from the complex, allowing the incorporation of the v-SNARE VAMP-2 to the SNARE complex in a process that is facilitated by Munc 18-1 (70). A subsequent ATP-dependent priming process by which the granule becomes fusion competent with ejection of Munc 18-1 ensues. Finally, calcium-dependent granule fusion to the plasma membrane and SNARE complex disassembly enables the liberation of the granule insulin content to the extracellular milieu.
Materials and Methods
Cell lines and culture conditions
Rat insulinoma INS 1E cells (71,72) were cultured in RPMI-1640 medium (Gibco, Invitrogen) containing 10% foetal calf serum and supplemented with 10 mm HEPES, 2 mm glutamine, 1 mm sodium pyruvate, 0.05 mm 2-mercaptoethanol, 100 U/mL penicillin and 100 mg/mL streptomycin. INS 1E-Tr1 cells were cultured in the same medium supplemented with 2 μg/mL blasticidin, and the INS 1E-Tr1 pSUPERIOR Munc 18-1 (clone 2) subline was maintained in RPMI-1640 medium supplemented with 10% Tet system approved FBS (BD Biosciences), 10 mm HEPES, 2 mm glutamine, 1 mm sodium pyruvate, 0.05 mm 2-mercaptoethanol, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 μg/mL blasticidin and 2 μg/mL puromycin. Cells were subcultured for 3 days in 35-mm diameter dishes to ∼80% confluence before the experiments. For confocal microscopy, cells were plated before fixation onto 35-mm glass bottom microwell dishes (MatTek) coated with extracellular matrix from 804G rat bladder carcinoma cells to favour cell attachment (73).
Generation of a stable tetracycline-inducible Munc 18-1 RNAi subline
INS 1E cells were transfected with the tetracycline (Tet) repressor-expressing vector pcDNA6/TR (Invitrogen) and selected in 2 μg/mL blasticidin. Resistant clones were picked and grown in separate wells without drug until cell clusters were visible, then passed onto small bottles and reselected in blasticidin. The resultant INS 1E-Tr clones were tested for the level of Tet repressor expression by transient cotransfection of an EGFP-expressing vector and the Tet-inducible shRNA-expressing pSUPERIOR.puro vector (OligoEngine, Inc.) encoding an shRNA specific for EGFP (pSUPERIOR EGFP RNAi) (1:3 DNA ratio). After 3 days of culture, the level of EGFP expression was assessed by Western blot in the presence or absence of 1 μg/mL doxocyclin. The subclone INS 1E-Tr1 was selected for its expression of shRNA in the presence of doxocyclin and subjected to a second round of transfection with pSUPERIOR Munc 18-1 RNAi, a vector consisting of pSUPERIOR.puro encoding the same shRNA specific for rat Munc 18-1 used for transient shRNA expression. Clones were selected and expanded in the presence of 2 μg/mL puromycin as explained above. One of these subclones, INS 1E-Tr1 pSUPERIOR Munc 18-1 (clone 2), was selected for future experiments after testing its capacity to secrete insulin in response to glucose and to selectively knock down Munc 18-1 after 3-days incubation in the presence of 1 μg/mL doxocyclin.
Antibodies
Primary antibodies for immunofluorescence, Western blot and immunoprecipitation were mouse anti-syntaxin1 monoclonal antibody (mAb) and goat anti-LC3 polyclonal antibody (pAb) from Santa Cruz Biotechnology, rabbit anti-Munc 18-1 pAb from Calbiochem (Merck Biosciences), mouse anti-pan actin mAb from Chemicon International, guinea-pig anti-insulin serum [D. Bosco, University of Geneva Medical School, Geneva, Switzerland, following Wright et al.’s protocol (74)], rabbit anti-granuphilin pAb (26), mouse anti-TGN38, mouse anti-Munc 18 and mouse anti-CASK mAbs (from BD Biosciences), rabbit anti-Rab27a pAb and mouse anti-VAMP-2 mAb (from Synaptic Systems GmbH), rabbit anti-Mint1 pAb (Abcam), mouse anti-SNAP-25 mAb (Sternberger Monoclonals Inc.) and mouse anti-c-myc tag mAb (Cell Signaling Technology). Secondary antibodies were goat anti-mouse AlexaFluor 488, donkey anti-mouse AlexaFluor 555, donkey anti-rabbit AlexaFluor 555, goat anti-rabbit AlexaFluor 633, donkey anti-rabbit AlexaFluor 488, goat anti-guinea-pig AlexaFluor 488, donkey anti-goat AlexaFluor 488 and goat anti-mouse AlexaFluor 633 (Molecular Probes, Invitrogen), goat anti-guinea-pig rhodamine (Jackson ImmunoResearch Laboratories), donkey anti-rabbit horseradish peroxidase (HRP) and sheep anti-mouse HRP (Amersham Biosciences).
Insulin secretion assays
INS 1E-Tr1 pSUPERIOR Munc 18-1 (clone 2) cells cultivated for 3 days in the presence or absence of 1 μg/mL doxocyclin were washed twice with a modified Krebs–Ringer bicarbonate HEPES buffer [KRBH: 125 mm NaCl, 4.74 mm KCl, 1 mm CaCl2, 1.2 mm KH2PO4, 1.2 mm MgSO4, 5 mm NaHCO3, 25 mm HEPES (pH 7.4) and 0.1% BSA] and pre-incubated with this same buffer for 2 h at 37°C. Cells were then incubated for 1 h at 37°C in KRBH supplemented with 2.8 mm glucose (basal conditions), followed by 1 h incubation with KRBH supplemented with 16.7 mm glucose (stimulated conditions). Basal and stimulated incubation buffers were recovered, and the cells were extracted with acid–ethanol. The amount of insulin in the incubation buffers and cell extracts was measured by radioimmunoassay (RIA) as described previously (75,76). Insulin secretion is expressed as a percentage of the total insulin content, which is the sum of the insulin contained in the buffers and the cell extracts.
For perifusion assays, INS 1E-Tr1 pSUPERIOR Munc 18-1 (clone 2) cells were cultivated to ∼80% confluency in adherent dishes for 3 days in the presence or absence of 1 μg/mL doxocyclin. Cell monolayers were washed twice with KRBH buffer, pre-incubated in this same buffer for 2 h at 37°C and transferred to a 37°C pre-warmed perifusion chamber connected to a peristaltic pump and a fraction collector. The basal perifusate was KRBH buffer containing 2.8 mm glucose, and the flow rate was set at 500 μL per minute. At the beginning of each experiment, the basal medium was perifused for 40 min without sample collection, followed by 20 min to obtain baseline values for hormone secretion. Stimulated medium (consisting of KRBH buffer supplemented with 16.7 or 2.8 mm glucose + 30 mm KCl) was subsequently perifused, and samples of the effluent were collected at 1-min intervals during 15 min, followed by 5-min intervals during 25 min. The immunoreactive insulin concentration in the effluent samples was determined by RIA as described above.
Growth hormone secretion assays
To study secretion from transfected cells only, cells were cotransfected with the hGH-expressing vector pcDNA3-hGH and the vector of interest (1:3 DNA ratio). Transfections were performed using Lipofectamine™ 2000 reagent (Invitrogen) following the manufacturer’s instructions.
After a 3-day culture, cells were analyzed for hGH release, which was used as a surrogate marker for insulin secretion only from cotransfected cells (36). To this end, cotransfected cells were washed twice in KRBH buffer and pre-incubated with this same buffer for 2 h at 37°C. Cells were then incubated for 1 h at 37°C in KRBH with 2.8 mm glucose (basal conditions), followed by 1 h incubation with KRBH containing 16.7 mm glucose, 0.1 mm 3-isobutyl-1-methylxanthine (IBMX), 5 μm forskolin and 0.1 μm phorbol 12-myristate 13-acetate (PMA) (stimulated conditions). Basal and stimulated incubation buffers were recovered, and cells were extracted with acid–ethanol. The amount of growth hormone in the incubation buffers and cell extracts was measured by enzyme-linked immunosorbent assay using the hGH ELISA kit from Roche Diagnostics following the manufacturer’s instructions. Growth hormone secretion is expressed as a percentage of the total growth hormone content, which is the sum of growth hormone contained in the buffers and cell extracts.
RNAi-mediated silencing of endogenous Munc 18-1 and granuphilin
The following sequence 5′-AAGGCACAGATGAAGAATCCC-3′, encoding a 21-bp-long shRNA specific for rat Munc 18-1, was cloned into both pSUPER and pSUPER-EGFP plasmids (from OligoEngine, Inc.). The same procedure was performed with three similar sequences specific for rat granuphilin-a and -b: 5′-AAGGATTCGGCGACTGAAGAA-3′ (granuphilin RNAi-1), 5′-AAAGTCCTGAGGCCTTCAGAA-3′ (granuphilin RNAi-2) and 5′-AAGTAAGCCTGTGGCAGAAGA-3′ (granuphilin RNAi-3). For negative control, a non-specific shRNA without mammalian homology was employed (77). The resulting shRNAs were tested for their capacity to downregulate Munc 18-1 or granuphilin mRNA in INS 1E cells as follows: cells were transiently transfected with the pSUPER-EGFP plasmid containing one of the shRNAs against Munc 18-1 or granuphilin-a/b or the negative control shRNA. Cells were then cultured for 72 h for RNAi expression before selection of EGFP-positive cells by FACS. Selected cells were pooled and lysed for Western blot analysis of Munc 18-1 or granuphilin-a/b levels compared with the negative control cells. The rat Munc 18-1 shRNA sequence and one of the rat granuphilin-a/b shRNA sequences tested (granuphilin RNAi-3) exhibited at least 75% silencing capacity and were selected for subsequent experiments.
Plasma membrane lawn preparation
Plasma membrane sheets were prepared from INS 1E cells as previously described (78). Briefly, cells were washed twice with KRBH buffer, pre-incubated in this same buffer for 2 h at 37°C and stimulated for 30 min in KRBH buffer supplemented with 16.7 mm glucose, 0.1 mm IBMX, 5 μm forskolin and 0.1 μm PMA. Cells were then washed in ice-cold PBS, incubated in ice-cold 0.55 mg/mL poly-l-lysine solution (Sigma-Aldrich) in PBS, washed three times in hypotonic buffer (23.3 mm KCl, 10 mm HEPES, 1.67 mm MgCl and 1 mm EGTA, pH 7.5) and sonicated directly in the culture dish for 3–5 seconds in sonication buffer [70 mm KCl, 30 mm HEPES, 5 mm MgCl, 3 mm EGTA, 1 mm DTT and 100 μm phenylmethylsulphonyl fluoride (PMSF), pH 7.5]. After sonication, the resulting membrane lawns were fixed on ice for 20 min in 2% paraformaldehyde (PFA) in sonication buffer without DTT and PMSF, incubated in quench solution (100 mm glycine in PBS) for 15 min, blocked for 30 min with 5% BSA and analyzed by immunofluorescence and confocal microscopy.
Immunofluorescence and confocal microscopy
Cells were washed in PBS, fixed/permeabilized on ice for 30 min in 2% PFA supplemented with 0.1% Triton-X-100 in PBS, washed four times in PBS and blocked for 15 min with 1% BSA. Fixed cells were incubated in a moist chamber with primary antibody in 1% BSA for 1 h, followed by four washes of 5–10 min each in PBS, before incubation with the secondary antibody in 1% BSA for a further hour. After a further three washes in PBS, cells were observed under a Zeiss LSM 510 inverted confocal microscope (Carl Zeiss MicroImaging) using a ×63 oil immersion lens. Images were acquired and processed using the lsm510 software (Carl Zeiss AG). Measures of colocalization and intensity profile histograms were obtained using metamorph v6.0 (Universal Imaging Corporation).
TIRF microscopy and quantification of the number of secretory vesicles at the plasma membrane
INS IE cells were cotransfected with a plasmid encoding a fusion construct between the secretory granule membrane glycoprotein phogrin and EGFP (40) and either pSUPER-Munc 18-1 RNAi or pSUPER-control RNAi vectors (1:3 DNA ratio). After 3 days in culture, cells were washed twice with KRBH buffer, pre-incubated in this same buffer for 2 h at 37°C and stimulated for 30 min in KRBH buffer supplemented with 16.7 mm glucose, 0.1 mm of IBMX, 5 μm forskolin and 0.1 μm PMA. Cells were then fixed and analyzed by TIRF microscopy.
TIRF images of phogrin–EGFP fluorescence were obtained with a Plan Neofluar ×100 (NA 1.45) objective mounted on an Axiovert 100M (both from Carl Zeiss MicroImaging), equipped with a 12-bit digital charge-coupled device camera (model Orca 4742–95; Hamamatsu Photonics) and controlled by the openlab software (Improvision). Intraobjective TIRF was obtained with a 488-nm laser through a TIRF adaptor (TILL Photonics). The number of secretory vesicles docked at the plasma membrane was quantified as the average number of individual fluorescent dots per cell membrane counted on 10 cells of similar size for each experimental condition.
Cell fractionation
INS 1E-Tr1 pSUPERIOR Munc 18-1 (clone 2) cells cultivated for 3 days in the presence or absence of 1 μg/mL doxocyclin were washed twice in PBS, resuspended in 500 μL of Buffer 1 [20 mm Tris–HCl, 1 mm ethylenediaminetetraacetic acid (EDTA) and 1 mm ethylene glycol tetraacetic acid (EGTA), Complete Mini protease inhibitor cocktail (Roche), pH 7.4] and incubated for 10 min on ice. Cell samples were lysed by three rounds of sonication on ice, lysates were pre-cleared by centrifugation at 420 × gfor 5 min at 4°C and then transferred to ultracentrifuge tubes. Membrane and cytosolic fractions were separated by ultracentrifugation at 100 000 × gfor 60 min at 4°C. Membrane fractions (pellets) were separated from cytosolic fractions (supernatants), washed with 500 μL of Buffer 1 and resuspended in 500 μL of Buffer 2 (Buffer 1 + 0.5% Triton-X-100). Both membrane and cytosolic fractions were subsequently used for immunoprecipitation and/or Western blot analysis.
Immunoprecipitation
Cell fractions were pre-incubated with 50 μL 50% protein G–Sepharose 4B fast flow bead slurry (Sigma-Aldrich) for 30 min at 4°C and then centrifuged at 16 000 × gfor 5 min at 4°C. In parallel, rabbit anti-nSec1 or mouse anti-syntaxin1 antibody was immobilized on protein G–Sepharose beads, added to the pre-cleared fractions for 16 h at 4°C, centrifuged and the supernatant discarded. Beads were washed five times in lysis buffer (20 mm Tris–HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA and 0.5% Triton-X-100) supplemented with Complete Mini protease inhibitor cocktail. After removal of the supernatant, the immunoprecipitates were resuspended in SDS–PAGE sample buffer, boiled for 5 min and resolved by SDS–PAGE.
SDS–PAGE and Western blotting
Cultured cells were washed in PBS and solubilized in lysis buffer supplemented with Complete Mini protease inhibitor cocktail. Lysates were pre-cleared at 16 000 × gfor 5 min at 4°C, and total protein levels were quantified with the DC protein assay kit (BioRad). Protein samples were resuspended in SDS–PAGE sample buffer, boiled for 5 min and resolved by SDS-PAGE on discontinuous mini-gels. Proteins were then electroblotted for 45 min onto a Protran nitrocellulose transfer membrane (Schleicher & Schuell GmbH). Membranes were treated with blocking solution (5% skimmed milk in tris buffered saline (TBS) + 0.05% Tween-20) for 1 h at room temperature and incubated for 16 h at 4°C with primary antibody in blocking solution. Following three TBS-Tween washes, membranes were incubated with secondary antibody for 90 min, also in blocking solution. After three more TBS-Tween washes, blots were incubated with the ECL Plus Western blotting detection system (Amersham Biosciences) and exposed onto BioMax MR films (Kodak). The films were developed in a Kodak M35 X-OMAT Processor, and the resulting bands were scanned and analyzed using scion image for Windows.
Electron microscopy
INS 1E-Tr1 pSUPERIOR Munc 18-1 (clone 2) cells were cultivated for 3 days in the presence or absence of 1 μg/mL doxocyclin. Cells were washed twice with KRBH buffer, pre-incubated in this same buffer for 2 h at 37°C and incubated for 1 h at 37°C in KRBH supplemented with 2.8 mm glucose, followed, when indicated, by a 30-min stimulation with KRBH supplemented with 16.7 mm glucose. Cells were then fixed in a 2.5% glutaraldehyde solution in 0.1 m phosphate buffer (pH 7.4), postfixed in 1% osmium tetroxide in the same buffer and dehydrated and in situ embedded in Epon (71). Thin sections were cut parallel to the culture support, stained with lead citrate and examined in a Philips CM10 electron microscope.
Statistics
Significance of differences between groups was evaluated by Student’s unpaired two-tailed t-test with p < 0.05 considered significant. One-way analysis of variance was performed using graphpad instat.
Acknowledgments
We thank Stéphane Dupuis for technical assistance, Dorothée Caille for electron microscopy technical support, Dr Andreas Wiederkehr and Professor Claes B. Wollheim for the use of the perifusion system and Dr Harald Hirling for the Munc 18-1-myc expression plasmid. This work was supported by grant numbers 5-R01-DK063332 from the National Institutes of Health (P. A. H. and J. E. P. teams) and 310000-113967/1 from the Swiss National Science Foundation (P. A. H. team). The R. R. group is supported by the Swiss National Science Foundation (grant number 3100A0-113421), and the P. M. team is supported by grants from the Swiss National Science Foundation (grant number 310000-109402), the Juvenile Diabetes Research Foundation (grant numbers 1-2005-46 and 1-2007-158) and the Geneva Program for Metabolic Disorders (GeMet).




