Inhibitory effects of melatonin on the lipopolysaccharide-induced CC chemokine expression in BV2 murine microglial cells are mediated by suppression of Akt-induced NF-κB and STAT/GAS activity
Abstract
Abstract: Melatonin influences sleep and circadian rhythm, and it has anti-inflammatory functions. However, the mechanism of its anti-inflammatory roles is not well understood. In our studies, we show that melatonin blocked lipopolysaccharide (LPS)-induced CCL2 (monocyte chemotactic protein-1; MCP-1), CCL5 (Regulated upon Activation, Normal T-cell Expressed, and Secreted), and CCL9 (macrophage inflammatory protein-1γ) chemokine mRNA expression in BV2 murine microglial cells. Melatonin markedly inhibited LPS-induced Akt phosphorylation and NF-κB activation. Furthermore, melatonin inhibited LPS-induced STAT1/3 phosphorylation and interferon-gamma activated sequence (GAS)-driven transcriptional activity. Interestingly, these effects were not associated with reactive oxygen species scavenging effects of melatonin or melatonin receptor signal pathways. Taken together, our results suggested that melatonin has anti-inflammatory functions through down-regulation of chemokine expression by inhibition of NF-κB and STAT/GAS activation in LPS-stimulated BV2 murine microglial cell line.
Introduction
In the central nervous system, microglia are important immune cells. In normal states, microglia scan their vicinity and maintain homeostasis of their environment. Microglia are activated when the brain is infected or injured [1–4]. Activated microglia produce pro-inflammatory mediators, such as cytokines and chemokines [5–8]. This microglial activation affects the progression of severity of neurodegenerative diseases, for example, Alzheimer’s disease and Parkinson’s disease, and stroke [9–11].
N-acetyl-5-methoxytryptamine (melatonin) is released primarily from pineal gland, and also produced in the retina, gastrointestinal tract, skin, leukocytes, etc. [12–16]. Melatonin has multiple functional roles, including circadian rhythm regulation [13] blood pressure modulation [17], immune response regulation [18, 19], anti-depression [20], oncostatic action [21], antioxidant [22], etc. Melatonin has potent anti- inflammatory actions. In vivo melatonin inhibits proinflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and oxidative stress in striatum, cortex, hippocampus, and hypothalamus by intracerebroventricular injection of LPS, and blocks IL-1β and complement 1q expression in beta-amyloid-injected hippocampus [23, 24]. In addition to a reduction of pro-inflammatory cytokine expression, melatonin inhibits brain damage via inhibition of neutrophils and macrophage/monocyte infiltration in transient focal cerebral ischemia [25]. Melatonin likely reduces brain damage via multiple actions [26].
Chemotactic cytokines are divided into four families on the basis of the position of the cysteine residues; C, CC, CXC, and CX3C [27]. Chemokines bind to their receptors, which are G-protein coupled receptors, and then transduced intracellular signaling molecules [28]. A primary role of chemokines is as an attractant of several inflammatory cells, such as neutrophils, monocytes, and lymphocytes. In the brain, microglia and astrocytes produce chemokines, and then induced inflammatory cell infiltration to amplify inflammatory responses [29]. Interestingly, chemokines also have other function. For examples, CXCL12 regulates glutamate release through production of TNF-α in astrocytes, and then increases neuronal toxicity [30]. In hippocampal neurons, CCL2 and CXCL-2 induced neuronal apoptosis [31]. In addition, CCL2 induces microglial proliferation [32]. Therefore, the modulation of chemokine expression is important, not only for infiltration of blood inflammatory cells, but also for inflammation-mediated neuronal damage and glia activation.
In this study, we report that melatonin attenuated LPS-induced CC chemokines (CCL2, CCL5, and CCL9) expression through the inhibition of PI3K/Akt-induced NF-κB and STAT-gamma-activated sequence (GAS) signaling pathways in a BV2 microglial cell line.
Materials and methods
Materials
Anti-phospho-Akt, anti-phospho-STAT1, and anti-STAT1 were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-phospho-STAT3, and anti-STAT3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). BV2 cells were provided by Dr. Joe EH (Ajou University, Suwon, Korea) and cultured in Dulbecco’s Modified Eagle Medium (Gibco, Grand Island, NY, USA) supplemented with 5% fetal bovine serum (Hyclone, Logan, UT, USA). PCR primers were purchased from Bioneer (Daejeon, Korea), and other chemicals were from Sigma (St. Louis, MO, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted from cells using TriZol (Life Technologies, Gaithersburg, MD, USA), and cDNA was prepared using M-MLV reverse transcriptase (Gibco-BRL, Gaithersburg, MD, USA), according to the manufacturer’s instructions. The primers used for the amplification of mouse CCL2, CCL5, CCL9 and actin were the following: CCL2 (sense) 5′-GCTTCAGATTTACGGGTC-3′ and (antisense) 5′-CTCAGCCAGATGCAGTTA-3′; CCL5 (sense) 5′-TCGTGCC CACGTCAAGGAGT-3′ and (antisense) 5′-GCTGTGCAGAGCCTCGGAGC-3′; CCL9 (sense) 5′- GCAAGCAG TCTGAAGGCCAGCT -3′ and (anti-sense) 5′- TCTCCGATC ACTGGGGTTGGCA -3′; actin (sense) 5′-CATGTTTGAGTCCTT CAACACCCC-3′ and (anti-sense) 5′- GCCAT CTCCTGCTCGAAGTCTAG-3′. PCR amplification was carried out using the following cycling conditions: 94°C for 3 min followed by 17 (actin)/22 (CCL2)/27 (CCL5) and 32 (CCL9) cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 1 min, and with final extension at 72°C for 10 min. The amplified products were separated by electrophoresis on a 1.5% agarose gel and detected under UV light.
MCP-1 ELISA
BV2 cells were seeded in 12-well plates at a density of 1.5 × 105 cells/well. The cells were pretreated with melatonin and then added 1 μg/mL LPS for 12 hr. Concentrations of MCP-1 in culture medium were measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
Western blot analysis
Cells were washed with cold PBS, and lysed on ice in modified RIPA buffer (50 mm Tris–HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mm NaCl, 1 mm Na3VO4, and 1 mm NaF) containing protease inhibitors (2 mm phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 2 mm EDTA). Lysates were centrifuged at 10,000 g for 10 min at 4°C, and the supernatant fractions were collected. Proteins were separated by SDS–PAGE, and transferred to a nitrocellulose membrane. Specific proteins were detected using an enhanced chemiluminescence Western blotting kit according to the manufacturer’s instructions.
Measurement of reactive oxygen species (ROS)
Intracellular accumulation of ROS was determined using the fluorescent probes 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCFDA). H2DCFDA is commonly used to measure ROS generation [33]. Cells were seeded on a six-well plate for 24 hr and treated with 5 μm H2DCFDA for 30 min prior to treatment with 1 μg/mL LPS in the presence or absence of 5 mmN-acetylcystein (NAC) and 2 mm glutathione ethyl ester (GEE). Then, cells were trypsinized and resuspended in PBS. Fluorescence was measured at specific time intervals with a flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA).
Assessment of NF-κB-p65-EGFP nuclear translocation
BV2 cells were seeded and transfected with NF-κB-p65-EGFP vector (provided by Dr. Jun CD, GIST, Korea). After 24 hr of transfection, BV2 cells were pretreated with 1 mm melatonin for 30 min at 37°C and then exposed to 1 μg/mL LPS for 1 hr. The cells were fixed with 1% paraformaldehyde on slide glass for 5 min at room temperature. After washing with PBS, 300 nm DAPI (4′-6′-diamidino-2-phenylindole; Roche, Mannheim, Germany) was added to the fixed cells for 5 min, after which they were examined by fluorescence microscopy. Fluorescence images were observed under the Zeiss microscopy (Zeiss, Goettingen, Germany).
DNA transfection and luciferase assay
Transient transfection was performed on six-well plates. One day before transfection, BV2 cells were plated to maintain approximately 60–80% confluence. NF-κB and GAS plasmid was transfected into BV2 cells using the Lipofectamine™ 2000 reagent (Invitrogen, Carlsbad, CA, USA). To assess promoters driven expression of the luciferase gene, cells were collected and disrupted by sonication in lysis buffer (25 mm Tris-phosphate, pH 7.8, 2 mm EDTA, 1% Triton X-100, and 10% glycerol), and aliquots of the supernatants were analyzed by measuring luciferase activity as described by the manufacturer’s instructions (Promega, Madison, WI, USA).
Statistical analysis
Data are analyzed by one-way ANOVA, followed by post-hoc comparisons (Student–Newman–Keuls) using the Statistical Package for Social Sciences 8.0 (SPSS Inc., Chicago, IL, USA).
Results
Previous studies have reported that lipopolysaccharide (LPS) can induce expression and production of chemokines in primary microglia or microglial cell line [34, 35]. Here in, LPS also induced CCL2 (MCP-1), CCL5 (Regulated upon Activation, Normal T-cell Expressed, and Secreted; RANTES), and CCL9 (macrophage inflammatory protein-1γ) expression within 1 hr in BV2 cells. CCL2 expression was sustained up to 12 hr, while CCL5 and CCL9 expression gradually increased (Fig. 1A). To investigate the effect of melatonin on LPS-induced chemokine expression, BV2 cells were pretreated with the indicated concentrations of melatonin for 30 min, and then cells were treated with 1 μg/mL LPS for 12 hr. As shown in Fig. 1B, melatonin inhibited LPS-induced CCL2, CCL5, and CCL9 expression in a dose-dependent manner. Furthermore, melatonin also blocked MCP-1 production in LPS-treated BV2 cells (Fig. 1C). We found that melatonin suppressed chemokine expression and/or secretion by LPS in BV2 cells.
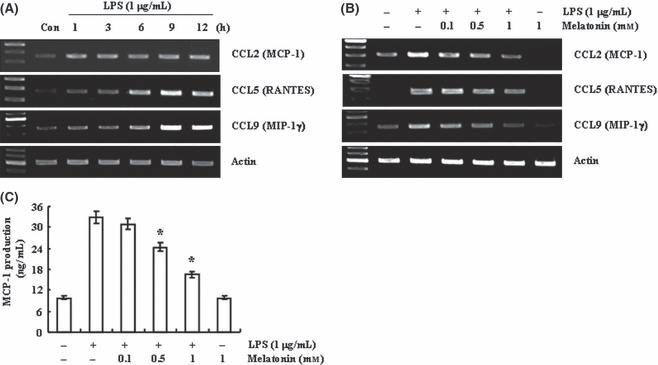
Melatonin inhibits lipopolysaccharide (LPS)-induced CC chemokine expression in BV2 murine microglial cell. (A) BV2 cells were treated with 1 μg/mL LPS for the indicated time periods. (B,C) BV2 cells were pretreated with the indicated concentrations of melatonin for 30 min, and then treated with 1 μg/mL LPS for 24 hr. Expression levels of chemokine mRNA were determined using RT-PCR (A,B) and levels of MCP were detected using ELISA (C). Values are presented as means ± S.E.M. of the three separate samples. *P < 0.01, compared with LPS. The data are representative of three independent experiments.
Previous studies reported that the effects of melatonin are mediated via its function as an antioxidant or the interaction of melatonin with its receptors, MT1 and MT2 [36–38]. Therefore, we tested whether the antioxidant action is associated with the inhibition of LPS-induced chemokine expression by melatonin. First, we checked whether ROS production by NADPH oxidase and mitochondria complex is involved in LPS-induced chemokine expression. BV2 cell were pre-treated with NADPH oxidase inhibitors (diphenylene iodonium [DPI] and apocynin) and mitochondria respiratory chain complex I inhibitor (rotenone) for 30 min, and then treated with 1 μg/mL LPS for 12 hr. As shown in Fig. 2A, both enzyme inhibitors had no effect on LPS-induced CCL2, CCL5 and CCL9 expression. Thus, we investigated whether general antioxidants, NAC and GEE, could down-regulate LPS-induced chemokine expression. Interestingly, although NAC and GEE inhibited LPS-induced ROS production (Fig. 2B), LPS-induced CCL2, CCL5, and CCL9 expression were insignificantly reduced in NAC and GEE-pretreated cells (Fig. 2C). These data indicate that although LPS increased intracellular ROS levels, ROS signaling was not involved in LPS-induced chemokine expression.
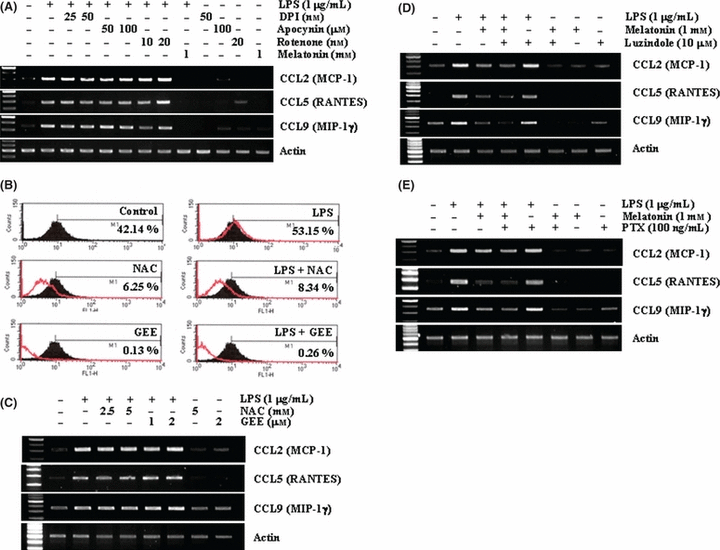
Effect of reactive oxygen species (ROS) and melatonin receptor-mediated signaling on lipopolysaccharide (LPS)-induced chemokine expression. (A) BV2 cells were pretreated with the indicated concentrations of NADPH oxidase inhibitors (diphenylene iodonium and Apocynin), mitochondria respiratory chain complex I inhibitor (rotenone), or melatonin for 30 min and then stimulated with 1 μg/mL LPS for 12 hr. (B) BV2 cells were pretreated with fluorescence dye, H2DCFDA for 30 min, and then treated with 5 mmN-acetylcystein (NAC) and 2 mm glutathione ethyl ester (GEE), for 30 min. After pretreatment, cells were stimulated with 1 μg/mL LPS for 60 min. H2DCFDA intensity was quantified by flow cytometry. (C) BV2 cells were pretreated with the indicated concentrations of ROS scavengers, NAC and GEE, and then stimulated with 1 μg/mL LPS for 12 hr. (D,E) BV2 cells were pretreated with 10 μm luzindole (D) or pertussis toxin for 30 min, and then added with 1 μg/mL LPS for 12 hr. Expression levels of chemokine mRNA were determined using RT-PCR (A,C,D, and E). The data are representative of three independent experiments.
Next, we examined whether interaction of melatonin with its receptor is involved in melatonin-mediated inhibition of chemokine expression in LPS-treated cells. BV2 cells were treated with melatonin in the presence or absence of 10 μm luzindole, which is a nonspecific melatonin receptor antagonist, in LPS-treated cells. As shown in Fig. 2D, luzindole did not overcome the inhibitory effect of melatonin. As melatonin receptor is a G-protein coupled receptor, we confirmed receptor-independent effect of melatonin using pertussis toxin (PTX), a general G-protein inhibitor [39]. As shown in Fig. 2E, BV2 cells were treated with melatonin in the presence or absence of 100 ng/mL PTX in LPS-treated cells. PTX also did not reverse the effect of melatonin (Fig. 2E). Therefore, these results suggest that the effect of melatonin on inhibition of LPS-induced chemokine expression is not associated with antioxidant function of melatonin or with receptor-mediated signaling.
Previous studies reported that PI3K/Akt signaling pathway is associated with chemokine expression [40, 41] and is inhibited by melatonin at high concentration (1 mm) [42]. Therefore, to study the mechanism of melatonin inhibition of LPS-induced chemokine expression, we examined the effect of melatonin on PI3K/Akt signaling pathway by LPS. First of all, we investigated whether PI3K/Akt signaling pathway is associated with LPS-induced chemokine expression in BV2 cells. BV2 cells were pre-treated with PI3K/Akt inhibitors, LY294002 and wortmannin, for 30 min, and then stimulated with 1 μg/mL LPS for 12 hr. Both inhibitors significantly reduced LPS-induced CCL2, CCL5, and CCL9 expression (Fig. 3A). These data indicate that LPS induced chemokine expression via activation of PI3K/Akt signaling pathway. Next, we tested whether melatonin could regulate LPS-induced Akt phosphorylation in BV2 cells. LPS induced Akt phosphorylation within 30 min, which was sustained up to 60 min. Akt phosphorylation in LPS and melatonin combined-treated cells was markedly blocked, compared with LPS-treated cells (Fig. 3B). Therefore, we suggest that melatonin inhibited LPS-induced chemokine expression via inactivation of PI3K/Akt signaling.
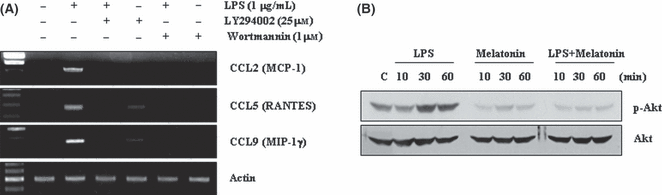
PI3K/Akt signaling is involved in lipopolysaccharide (LPS)-induced CC chemokine expression. (A) BV2 cell were pretreated with 25 μm LY294002 and 1 μm wortmannin for 30 min, and then added 1 μg/mL LPS for 12 hr. (B) BV2 cells were treated with 1 μg/mL LPS for the indicated time period in the presence or absence of 1 mm melatonin. Expression levels of chemokine mRNA were determined using RT-PCR and Akt phosphorylation was detected by Western blotting. The data are representative of three independent experiments.
As NF-κB is one of the major components of LPS-induced signaling pathway and NF-κB is involved in chemokine expression [40, 43], we studied the effect of melatonin on LPS-induced NF-κB activation. BV2 cells were pre-treated with NF-κB inhibitors, pyrrolidine dithiocarbamate (PDTC) and Bay 11-7082, for 30 min, and then treated with 1 μg/mL LPS for 12 hr. Both the NF-κB inhibitors inhibited LPS-induced CCL2, CCL3, and CCL9 expression (Fig. 4A). Melatonin suppressed NF-κB DNA binding activity in the rat spleen [44, 45]; therefore, we investigated whether melatonin could modulate NF-κB transcriptional activation in LPS-treated BV2 cells. BV2 cells were transiently transfected with luciferase reporter plasmids containing NF-κB element, and then treated with 1 μg/mL LPS in the presence or absence of melatonin. As shown in Fig. 4B, LPS-induced NF-κB luciferase activity was blocked by melatonin in a dose-dependent manner. To confirm these data, we examined nuclear translocation of p65 using NF-κB-p65-EGFP vector. Expectedly, melatonin blocked p65 nuclear translocation by LPS (Fig. 4C).
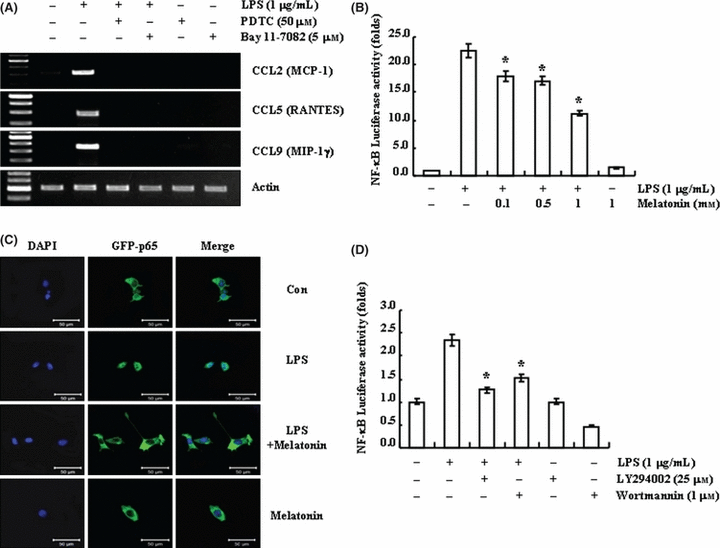
Effect of melatonin on lipopolysaccharide (LPS)-induced NF-κB activation. (A) BV2 cells were pretreated with NF-κB inhibitors (50 μm PDTC and 5 μm Bay 11-7082) for 30 min and then stimulated with 1 μg/mL LPS for 24 hr. (B) BV2 cells were transiently transfected with a NF-κB-luciferase construct, and then treated with 1 μg/mL LPS for 12 hr in the presence or absence of the indicated concentrations of melatonin. (C) BV2 cells were transiently transfected with NF-κB-p65-EGFP vector, and exposed to 1 μg/mL LPS for 1 hr in the presence or absence of 1 mm melatonin. Scale bar = 50 μm. (D) BV2 cells were transiently transfected with a NF-κB-luciferase construct. Cells were pretreated with 25 μM LY294002 and 1 μm wortmannin for 30 min, and then stimulated with 1 μg/mL LPS for 12 hr. Expression levels of chemokine mRNA were determined using RT-PCR (A), and cells were lysed and assayed for luciferase activity (B,D). Fluorescence images were observed under the Zeiss microscopy (C). The data are representative of three independent experiments.
Next, we tested whether PI3K/Akt could be up-stream signals responsible for NF-κB signaling. PI3K/Akt inhibitors, LY294002 and wortmannin, pretreatment markedly inhibited LPS-induced NF-κB transcriptional activity (Fig. 4D). Therefore, we suggested that melatonin reduced LPS-induced chemokine expression via inhibition of PI3K/Akt-NF-κB signaling pathway.
In addition to activation of NF-κB signaling in LPS-treated cells, LPS also activates JAK/signal transducer and activator of transcription (STAT) signaling pathway [46, 47], and JAK/STAT signaling is important on chemokine expression [48, 49]. To investigate the relationship of JAK-STAT signaling pathway on LPS-induced chemokine expression, BV2 cells were pre-treated with JAK/STAT inhibitor, AG490, for 30 min, and then stimulated with 1 μg/mL LPS for 12 hr. AG490 significantly inhibited LPS-induced CCL2, CCL5, and CCL9 expression in BV2 cells (Fig. 5A). These data indicate that JAK/STAT signaling is associated with LPS-induced chemokine expression in BV2 cell. Melatonin could down-regulate contusion-induced STAT1 activation in traumatic brain injury [50]. Thus, we examined whether melatonin could modulate JAK/STAT signaling in LPS-treated BV2 cells. LPS induced STAT1/3 phosphorylation within 2 hr, and gradually increased it for up to 6 hr. In LPS and melatonin co-treated BV2 cells, phosphorylation of STAT1 and STAT3 were reduced, compared with that of LPS-treated cells (Fig. 5B). Phosphorylated STAT forms dimers and then translocates into the nucleus. Nuclear STAT dimer binds the specific DNA elements, such as interferon-gamma-activated sequence and interferon-sensitive response element [51]. To confirm that melatonin could modulate JAK/STAT activation by LPS, BV2 cells were transiently transfected with luciferase reporter plasmids containing eight- (GAS) elements and then treated with 1 μg/mL LPS in the presence or absence of melatonin for 12 hr. Melatonin inhibited LPS-induced GAS transcriptional activity in dose-dependent manner (Fig. 5C).
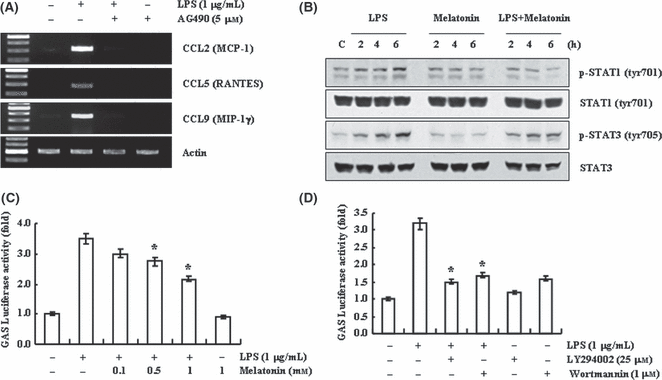
Effect of melatonin on lipopolysaccharide (LPS)-induced STAT-GAS activation. (A) BV2 cells were pretreated with JAK/STAT inhibitor (5 μm AG490) for 30 min, and then treated with 1 μg/mL LPS for 12 hr. (B) BV2 cells were treated with 1 μg/mL LPS for the indicated time periods in the presence or absence of 1 mm melatonin. (C,D) BV2 cells were transiently transfected with a GAS-luciferase construct. Cells were pretreated with 1 mm melatonin (C), or 25 μm LY 294002 or 1 μm wortmannin for 30 min, and then stimulated with 1 μg/mL LPS for 12 hr, and cells were lysed and assayed for luciferase activity. Values are presented as means ± S.E.M. of the three separate samples. *P < 0.01, compared with LPS. Expression levels of chemokine mRNA were determined using RT-PCR (A) and STAT phosphorylation was detected by Western blotting. The data are representative of three independent experiments. GAS, gamma-activated sequence.
Finally, we investigated whether PI3K/Akt could also regulate LPS-induced GAS transcriptional activity. LPS-induced GAS-transcriptional activity blocked in PI3K/Akt inhibitors-treated BV2 cells (Fig. 5D). These data indicate that PI3K/Akt signaling pathway is an upstream regulator of the STAT-GAS signal. Taken together, we suggest that melatonin inhibits LPS-induced CC chemokine expression through inhibition of NF-κB and STAT/GAS, which are downstream of PI3K/Akt signaling, in LPS-treated BV2 cells.
Discussion
In this study, we show that melatonin inhibits LPS-induced CC chemokine expression and/or secretion in BV2 cells. This effect of melatonin is independent of its antioxidant function or receptor-mediated signaling, while melatonin significantly inhibited Akt phosphorylation in LPS-treated cells. Furthermore, melatonin blocked LPS-induced NF-κB transcriptional activity and p65 nuclear translocation, and STAT1/3 phosphorylation and GAS-transcriptional activity were also blocked in LPS and melatonin-treated BV2 cells. Therefore, melatonin has an anti-inflammatory functions through inhibition of chemokines, and these anti-inflammatory functions are related with the inhibition of NF-κB and STAT/GAS signaling via down-regulation of PI3K/Akt activation (Fig. 6).
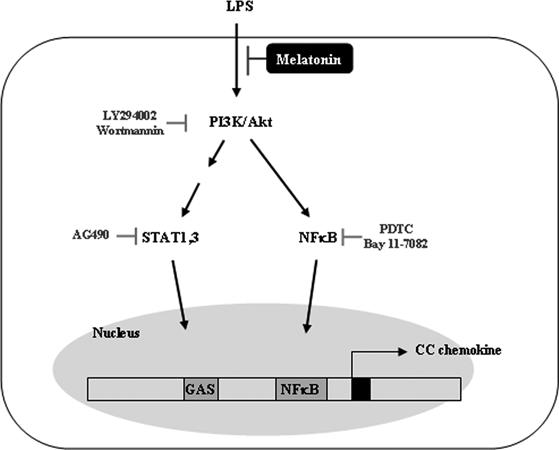
Model for down-regulated mechanisms of melatonin on lipopolysaccharide (LPS)-induced CC chemokines in BV2 cells. LPS activates PI3K/Akt signaling pathway, and then signaling is transmitted into NF-κB and STAT/GAS pathways. NF-κB and STAT binds to the NF-κB and GAS binding sites of CC chemokine promoter and then increases chemokine expression. Melatonin blocks LPS-induced PI3K/Akt signaling and then reduces CC chemokine expression through the inhibition of NF-κB and STAT/GAS signaling pathway. GAS, gamma-activated sequence.
Melatonin has important anti-inflammatory functions. In the brain, melatonin inhibits inflammatory cytokines in microglia, which is an important immune cell compartment. For example, melatonin inhibits pro-inflammatory mediator expression, such as IL-1β, IL-6, TNF-α, and inducible nitric oxide synthase, and ROS in highly aggressively proliferating immortalized cells [52, 53]. Furthermore, melatonin inhibited beta-amyloid-induced ROS production in primary rat microglial cells [54]. In our studies, we investigated whether melatonin could modulate LPS-induced chemokine expression in BV2 cells. As shown in Fig. 1B, melatonin significantly suppressed CC chemokine expression in LPS-treated cells. Lee et al. [25] demonstrated that melatonin blocked infiltration of blood immune cells, such as neutrophils and macrophages/monocytes in transient focal cerebral ischemia. Therefore, we suggest that melatonin could be an effective down-regulator of inflammation via modulation of not only cytokine expression but also chemokine expression in brain.
The mechanism of anti-inflammatory function by melatonin is mainly associated with roles as an antioxidant directly or in directly [55]. However, ROS signaling was not associated with LPS-induced CC chemokine expression in the current study (Fig. 2). While melatonin significantly reduced CC chemokine expression in LPS-treated cells, ROS scavengers insignificantly inhibited LPS-induced CC chemokine expression (Fig. 2). Furthermore, interaction of melatonin with its receptors also is not involved in the inhibition of LPS-induced chemokine expression (Fig. 2D,E). We found that melatonin-mediated modulation of chemokine expression is associated with inhibition of PI3K/Akt signaling pathway (Fig. 3), but not reduction of ROS or receptor-mediated signaling. PI3K/Akt signaling events linked to melatonin also probably vary among cell types and concentrations of the melatonin. Melatonin induced Akt phosphorylation in primary cortical neuron, SH-SY5Y neuroblastoma and astrocytes [56–58], in contrast to melatonin-inhibited Akt phosphorylation in human umbilical vein endothelial cells [42]. Low doses of melatonin (<10 μm) increased Akt phosphorylation, but high concentration of melatonin (>1 mm) decreased Akt phosphorylation [42, 56–58]. We also used a high concentration of melatonin (>0.1 mm), and it significantly blocked LPS-induced Akt phosphorylation (Fig. 3B). However, additional further studies are needed to identify melatonin-mediated Akt regulatory mechanisms.
In the previous study, Mohan et al. [44] and Chuang et al. [45] reported that melatonin decreases NF-κB DNA binding activity in Hela S3 cells and the rat spleen, respectively. Mohan et al. [44] reported that melatonin inhibits NF-κB binding activity in tumor necrosis factor-α or phorbol 12-myristate 13-acetate-treated cells. Chuang et al. [45] investigated the relationship between melatonin concentration and NF-κB activation. Interestingly, NF-κB DNA binding activity is lower at night, compared with that at day in the rat spleen [45]. In our study, we also checked whether melatonin decreased LPS-induced NF-κB promoter activity and p65 nuclear translocation (Fig. 4). Therefore, inhibition of NF-κB activation could be one of the mechanisms to inhibit inflammation by melatonin.
Taken together, we suggest that melatonin could down regulate CC chemokine expression and/or secretion in microglia, and PI3K/Akt-dependent inhibition of NF-κB and STAT/GAS signaling are involved in melatonin-induced anti-inflammation. Our studies provide information about regulatory mechanism of melatonin on chemokine expression.
Acknowledgement
This work was supported by research program of dual regulation mechanisms of aging and cancer from KOSEF(M1075604000107N560400110).




