Melatonin inhibits serotonin transporter activity in intestinal epithelial cells
Abstract
Abstract: Gastrointestinal serotonin (5-HT) and melatonin are two closely related neuromodulators which are synthesised in the enterochromaffin cells of the intestinal epithelium and which have been shown to be involved in the physiopathology of the gastrointestinal tract. The effects of 5-HT depend on 5-HT availability which is, in part, modulated by the serotonin transporter (SERT). This transporter provides an efficient 5-HT uptake after release and is expressed in the membrane of the enterocytes. Although the origin and effects of 5-HT and melatonin are similar, the interrelationship between them in the gastrointestinal tract is unknown. The main aim of this study was to determine whether melatonin affects SERT activity and expression, and, if so, to elucidate the mechanisms involved. Caco-2 cell line was used to carry out the study as these cells have been shown to endogenously express SERT. The results showed that melatonin inhibits SERT activity by affecting both Vmax and kt kinetic constants although SERT synthesis or intracellular trafficking did not appear to be affected. The melatonin effect seemed to be independent of melatonin receptors MT1 and MT2 and protein kinase C and cAMP intracellular pathways. Our results suggest that the inhibition of SERT might be due to a catalytic effect of melatonin on the allosteric citalopram-sensitive site in SERT. This study shows, for the first time, that melatonin modulates SERT activity, thus demonstrating the feedback system between melatonin and the serotoninergic system in the gastrointestinal tract.
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) plays an important role in the physiology of the gastrointestinal (GI) tract. Ninety percent of the body’s 5-HT content is found in enterochromaffin cells (EC) which act as a sensory transducer that responds to mechanical or chemical GI stimulation. 5-HT activity in the GI tract contributes to controlling intestinal motility [1], the intestinal absorption of nutrients [2, 3] and intestinal secretion of water and electrolytes [4]. The effects of 5-HT are mediated by its binding to specific receptors present along the gut [5–7] and depend on 5-HT availability which is partly determined by 5-HT reuptake carried out by the serotonin transporter (SERT). SERT is a transmembrane protein located in the intestinal epithelial cells, whose molecular sequence coincides with SERT from the central nervous system [8].
Changes in SERT activity can lead to alteration in the GI function and numerous studies have concluded that the alteration of the gastrointestinal serotoninergic system may be involved in the origin and/or prevalence of chronic gastrointestinal pathologies such as irritable bowel syndrome and inflammatory bowel disease [9, 10]. Recent studies have demonstrated that SERT expression and activity was impaired in intestinal epithelial cells treated with pro-inflammatory factors [11, 12] and in animal models [13–15] and human forms [16] of intestinal inflammation.
Not only 5-HT but also other potentially important compounds are present in the GI mucosa, where they act on normal or altered GI function. One of these compounds is melatonin (5-methoxy-N-acetyltryptamine), a close derivative of 5-HT, which is a well known paracrine hormone secreted in a cyclic manner by the pineal gland. This gland is not the sole organ where melatonin is synthesised; the analysis of extrapineal sources of melatonin has identified the GI tract as a major source of this factor [17], and serotonin-rich EC as the main synthesisers of melatonin [18]. Intestinal melatonin is immediately released in the extracellular medium, the GI lumen and circulation and, as a highly lipophilic compound, melatonin easily diffuses through the biological membranes. Melatonin concentration in GI tissues exceeds the blood plasma levels by 10–100 times and it increases after food intake [19].
Many of the biological actions of melatonin are mediated by membrane receptors [20]; however, others have been shown to be receptor independent [21, 22]. Melatonin has been shown to regulate intestinal physiology by modulating transmembrane transport of ions and water [23–25] and intestinal motility [26]. Many of these effects seem to counterbalance 5-HT activity and some authors have suggested that melatonin functions as a feedback modulator of 5-HT [24]. Numerous studies have also shown that melatonin has important anti-inflammatory and immunomodulatory effects [27] and melatonin administration has been proposed as a treatment for reducing the severity of intestinal inflammatory pathologies such as colitis [28].
Although the GI origin of both 5-HT and melatonin are similar, and the involvement of the serotoninergic system and melatonin on the physiopathology of the gastrointestinal tract is well known, the interrelationship between melatonin and SERT activity in the GI tract is unexplored. The aim of this work was to study the effect of melatonin on SERT activity and molecular expression and to determine the mechanism of this effect.
The results of this study may contribute to a better understanding of the role of melatonin in the regulation of the intestinal serotoninergic system mediated by SERT activity. The work may be also useful for clarifying the possible association between 5-HT and melatonin and its application in the treatment of several pathologies in which serotoninergic activity is involved.
Material and methods
Chemicals
The following drugs and substances were used (abbreviations and respective suppliers in parentheses): Serotonin (5-HT), melatonin, fluoxetine, phorbol 12-myristate 13-acetate (PMA), dibutyryl-cAMP (d-cAMP), bisindolylmaleimide IV (BIS IV), KT 5720 (Sigma-Aldrich, St. Louis, MO, USA). Luzindole (Tocris Bioscience, Biogen Científica, Madrid, Spain). [3H]-5-HT (specific activity 20 Ci/mmol) (Perkin-Elmer, Boston, MA, USA). Mouse monoclonal antibody anti-human SERT (Abcam, Cambridge, UK); rabbit polyclonal anti-actin antibody and secondary antibodies coupled to horseradish peroxidise (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Melatonin and luzindole were dissolved in 1% ethanol and this vehicle had not significant effects on control or treated cells.
Cell culture
Caco-2/TC7 [29] cells were kindly provided by Dr Edith Brot-Laroche (INSERM, VMR S 872). Cell culture media and supplements were from Invitrogen (Carlsbad, CA, USA). The cells were cultured at 37°C in an atmosphere of 5% CO2 and maintained in high glucose DMEM supplemented with 2 mm glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% nonessential amino acids and 20% foetal bovine serum (FBS). The cells were passaged enzymatically (0.25% trypsin-1 mm EDTA) and sub-cultured in 25 or 75 cm2 plastic culture flasks. The medium was changed 48 hr after seeding and daily thereafter. The cells were always used between passages 19 and 35. For 5-HT uptake assays, cells were seeded in 24-well plates at a density of 4 × 104 cells/well and uptake measurements were carried out 14 days after seeding (9 days after confluence). Previous results have shown that Caco-2 cells express SERT and its activity reaches a plateau on the fifth day after confluence [8]. Melatonin and the different modifiers were added to the culture medium at different concentrations and periods, depending on the determination. In the experiments, the cell medium was free of FBS 24 h before using the cells. This condition did not affect either the functional differentiation status or the SERT activity of Caco-2 cells (data not shown).
5-HT uptake studies
Uptake measurements were performed on cells attached to 24-well plates, either under control or after different experimental conditions. The transport medium composition in mm was as follows: 137 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 10 HEPES pH 7.4, 4 Glutamine, 0.1% BSA and both 5-HT 2 × 10−7 m and [3H]-5-HT. Before measuring uptake, cells were pre-incubated at 37°C in an atmosphere of 5% CO2 with substrate-free transport medium for 30 min. The cells were immediately washed with substrate-free transport medium at 37°C and then incubated with transport medium at 37°C for 6 min. Transport was stopped by removing the transport medium and washing the cells twice with ice-cold substrate-free transport medium containing 2 × 10−5 m 5-HT. The cells were solubilised in 0.1 m NaOH and samples were taken for radioactivity counting. Protein was measured using the Bradford method (Bio-Rad, Hercules, CA, USA), with BSA as standard. Results were calculated in pmol 5-HT/mg protein and were expressed as a % of control value (100%).
In experiments in which 5-HT fluxes were measured, Caco-2 cells were seeded in 12-well permeable polyester (PET) membranes with porous size 0.4 μm and 1 cm2 growth area. These inserts establish apical (A) and basal (B) compartments. 5-HT transepithelial apical to basal (A–B) flux was measured by adding 10−7 m 5-HT plus [3H]-5-HT to the apical compartment. After 20-min equilibration period, samples were removed from the basal compartment every 10 min, for 90 min, and replaced with fresh medium. The results were calculated in pmol 5-HT/10 min and were expressed as a % of control (100%). Cell monolayer integrity and confluence were checked by measuring transepithelial resistance with an Epithelial Voltohmmeter (Millicell Electrical resistance system; Millipore, Billerica, MA, USA) before the beginning of each experiment.
RNA extraction, reverse transcription and semiquantitative-PCR analysis
RNA extractions were carried out with the RNeasy mini kit (QIAGEN, Hilden, Germany) following manufacturer’s instructions as previously described [30]. Briefly, total RNA was extracted from cells cultured in 25 cm2 flasks 14 days after seeding and under different experimental conditions. The extracted RNA (1 μg) was used as a template for first-strand cDNA synthesis using Oligo(dT) primers and a modified MMLV Reverse Transcriptase (Invitrogen). Negative amplification control was performed in the absence of Reverse Transcriptase. One-tenth of the resultant cDNA was used for human SERT-polymerase chain reaction (PCR) amplification with human β-actin as an internal control. The specific primers used were (5′–3′): SERT sense AAATCCAAGCACCCAGAGAT and SERT anti-sense AGACTGTGTCCCTGTGGAGA; β-actin sense AGCACGGCATCGTCACCAACT and β-actin antisense ACATGGCTGGGGTGTTGAAGG. Twenty-seven cycles were carried out for SERT-PCR amplification as follows: 94°C for 30 s, 56°C for 30 s and 68°C for 2 min 30 s. PCR amplification of β-actin was obtained after 25 cycles under the following conditions: 94°C for 30 s, 60°C for 30 s and 68°C for 30 s. These final SERT and β-actin PCR conditions were determined after screening (20–40 cycles). Final PCR amplification conditions were chosen so that none of the RNAs (cDNAs) analysed reached a plateau at the end of the amplification protocol. PCR products were electrophoresed on 1% agarose gel and visualised under UV light after ethidium bromide staining. The images from the gels were captured with Biodoc-It Imaging System (UVP Inc, Upland, CA, USA). The amplified fragment sizes were 2105 bp for SERT and 193 bp for β-actin. SERT/β-actin ratio in densitometric units was calculated with the Quantity One Analysis Software (Bio-Rad). The results were expressed as % of control value (100%).
Preparation of the brush border purified fraction from Caco-2 cells and Western blotting
Caco-2 cells were cultured in 75 cm2 flasks and were used 14 days after seeding. The cells were washed twice with phosphate buffered saline and immediately resuspended with cold Tris-manitol buffer (Tris 2 mm, Manitol 50 mm, pH 7.1) containing protease inhibitors and 0.02% sodium azide. The suspension was homogenised and the cells were disrupted by sonication (fifteen 1 s bursts, 60 W). One sample was taken from the lysate for total protein analysis and protein quantification. CaCl2 (final concentration 20 mm) was added to the cell lysate and after standing for 10 min in ice, the mixture was centrifuged for 10 min at 950 g. The supernatant was taken and centrifuged at 33,500 g for 30 min. Then the pellet (brush border purified fraction) was resuspended in phosphate buffer (KH2PO4/K2HPO4 10 mm pH 6.8) and a sample was taken for protein analysis. Protein was measured using the Bradford method.
Brush border purified samples and cell lysate (total protein 60 μg) from Caco-2 cells were electrophoresed in 9% SDS-PAGE gels, and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore) by electroblotting. The membranes were blocked with 5% nonfat dried milk plus 1% BSA and probed with mouse monoclonal anti-human SERT 1:500. The primary antibody was detected using a secondary goat anti-mouse Ig coupled to horseradish peroxidase and the ECL Plus detection kit (GE Healthcare, Buckinghamshire, UK) and was visualised with X-ray films (Hyperfilm MP, GE Healthcare). The blots were reprobed, after stripping, with a rabbit polyclonal anti-actin antibody to determine differences in the sample load. The SERT/β-actin protein ratio was calculated in densitometric units from the films with the Quantity One Analysis Software (Bio-Rad) and the results were expressed as % of de control values (100%).
Statistical analysis
All results are expressed as means ± S.E.M. Statistical comparisons were performed by one-way ANOVA followed by Bonferroni post-test with a confidence interval of 99% (P < 0.01). Kinetic study of the 5-HT transport values was performed by nonlinear regression, fitting the results to an equation containing a saturable (Michaelis–Menten) plus a nonsaturable (diffusion) component. Statistical analysis was carried out by the computer-assisted Prism GraphPad Program (Prism version 4.0, GraphPad Software, San Diego, CA, USA).
Results
The melatonin effect on SERT activity was determined by measuring 5-HT uptake in cells treated during a short-term (6 or 30 min) or long-term (1 or 2 days) period with different melatonin concentrations. The results showed that melatonin reduces 5-HT uptake and this effect is dose-dependent. In relation with the influence of the period of treatment on melatonin effects, the results showed that after 6 min of treatment, the inhibition induced by melatonin was significant at high concentrations (0.5 and 1 mm). However, at 30 min, 1 day and 2 days of treatment, inhibition was significant at a lower melatonin concentration (Fig. 1A). The reversibility of the inhibitory effect of melatonin 0.5 mm on 5-HT uptake after long-term treatment (1 day) was determined and the results showed that the cells recover 5-HT uptake to control level after 36 min in a medium without melatonin (Fig. 1B).
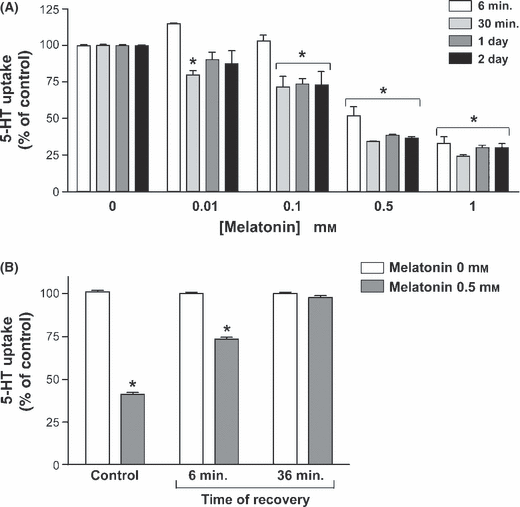
Effect of the treatment with melatonin on 5-HT uptake. (A) Uptake was measured after 6 min incubation of 2 × 10−7 m 5-HT. Melatonin concentrations assessed were 0.01, 0.1, 0.5 and 1 mm. The treatment periods were 6 and 30 min (short term), or 1 and 2 days (long term). Each time period was studied in independent cells. (B) Reversibility of the effect of melatonin on 5-HT uptake. The cells were treated with 0.5 mm melatonin for 1 day. Two periods of recovery (without treatment) were assayed (6 and 36 min). The results in the figure are indicated as the % of 5-HT uptake compared with the control (100%), and are expressed as the mean ± S.E.M of five independent experiments. *P < 0.01 compared with the corresponding control (untreated cells).
A kinetic study of the 5-HT uptake in cells treated with melatonin was carried out and the kinetic constants Vmax and kt, which reveal the capacity and affinity of the transporter, respectively, were calculated. The results showed that treatment for 30 min with melatonin 0.5 mm, significantly diminished Vmax (in pmol 5-HT/mg protein; control = 31.04 ± 1.77 and melatonin = 23.48 ± 2.64) and increased kt (in μm; control = 1.30 ± 0.16 and melatonin = 2.64 ± 0.57).
The effect of melatonin on the SERT mRNA and SERT protein expression level was determined in Caco-2 cells treated with melatonin. The results showed that melatonin treatment for 1 day did not affect SERT mRNA level (Fig. 2A). Moreover, the treatment with melatonin for 6 min, 30 min or 1 day did not modify the SERT protein expression in the cell lysate or the apical membrane (Fig. 2B).
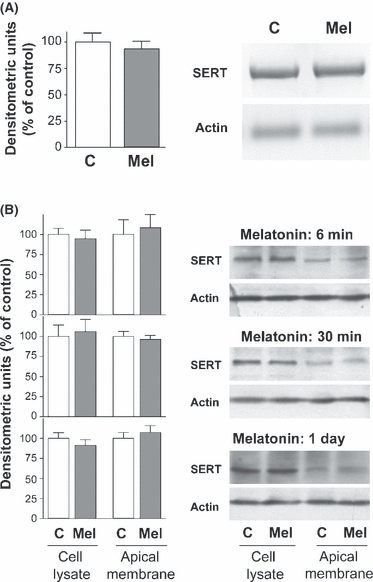
Effect of melatonin on serotonin transporter (SERT) mRNA levels and protein expression. (A) Cells were treated for 1 day with melatonin 0.5 mm and the total RNA was extracted. PCR amplification of SERT (2105 bp) and actin (193 bp) in control and melatonin treated cells was obtained under conditions described in the Material and Methods section. Results are expressed as a percentage of control densitometric units (100%) and are the mean ± S.E.M of three independent experiments. (B) Effect of melatonin treatment on SERT protein expression. Caco-2 cells were treated with 0.5 mm melatonin during 6, 30 min or 1 day. Cell lysate and apical membrane samples were electrophoresed and SERT immunodetected. The results are expressed as a percentage of control densitometric units (100%) and are the mean ± S.E.M of three independent experiments.
The 5-HT transepithelial apical–basal (A–B) flux was also measured in cells treated with melatonin 0.5 mm for 10 min, from either the apical or basal compartment. The results showed that apical melatonin significantly reduces 5-HT apical–basal flux. However, melatonin added to the basal compartment did not significantly affect the apical–basal 5-HT flux (Fig. 3).
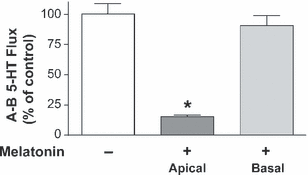
Effect of melatonin on 5-HT transepithelial apical–basal flux. Melatonin (0.5 mm) was added to apical or basal compartment and 5-HT apical to basal (A–B) flux was measured. 5-HT concentration was 10−7 m and samples were taken every 10 min. Control conditions correspond to untreated cells. The results are expressed as a percentage of the control value and are the mean ± S.E.M of four independent experiments.*P < 0.01 compared with the control value.
To clarify the mechanism involved in melatonin effect on SERT, the role of melatonin membrane receptors MT1 and MT2 was assessed. SERT activity was measured in cells treated with melatonin ± luzindole, a melatonin receptor antagonist [31]. The results showed that treatment of the cells with luzindole did not reverse the inhibition of SERT activity yielded by short- and long-term treatment with melatonin. Luzindole did not show an intrinsic effect on 5-HT uptake (Fig. 4).
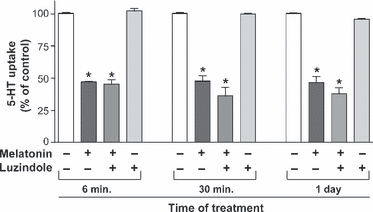
The lack of a role of melatonin receptors MT1 and MT2 in melatonin effect on serotonin transporter (SERT) activity. Cells were treated for 6, 30 min or 1 day with melatonin 0.5 mm and/or luzindole 1 μm. 5-HT uptake was measured after 6 min of incubation and 5-HT concentration was 2 × 10−7 m. The results are expressed as a percentage of the control uptake and are the mean ± S.E.M of six independent experiments. *P < 0.01 compared with the corresponding control (untreated cells).
The involvement of protein kinase C (PKC) and cAMP in the effect of melatonin on SERT was also analysed. Uptake of 5-HT was measured in Caco-2 cells treated with melatonin (0.5 mm) and either PMA, an activator of PKC, or d-cAMP, a nonmetabolisable analogue of cAMP and either BIS IV or KT 5720, inhibitors of PKC and protein kinase A (PKA) respectively. The results showed that the melatonin effect was additive to the inhibition yielded by PKC or cAMP on SERT activity, and BIS IV and KT 5720 did not reverse the effect of melatonin (Fig. 5).
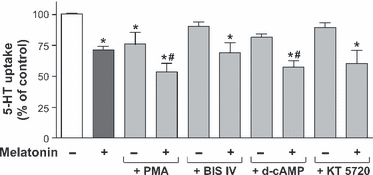
Role of protein kinase C (PKC) and cAMP signalling pathways in melatonin effects on serotonin transporter (SERT) activity. 5-HT uptake was measured after 6 min of incubation and 5-HT concentration was 2 × 10−7 m. Before starting the experiment the cells were treated during 60 min with the PKC inhibitor BIS IV, or the PKA inhibitor KT 5720 and after 30 min the cells were treated with melatonin and/or the PKC activator PMA or d-cAMP. The results are expressed as a percentage of the control uptake, and are the mean ± S.E.M of five independent experiments. *P < 0.01 compared with the control (untreated cells). #P < 0.01 compared with melatonin treated cells.
To gain an in-depth knowledge of the melatonin mechanism, the effect of melatonin on SERT activity was measured in Caco-2 cells treated with fluoxetine or citalopram, two selective serotonin reuptake inhibitors (SSRIs). Citalopram binds to both high affinity (transport) and low affinity (allosteric) binding sites in SERT. Fluoxetine binds to high affinity sites and does not possess any significant binding affinity for the allosteric site [32, 33]. The results obtained showed that melatonin inhibition was additive to the inhibition yielded by both citalopram at low concentration and fluoxetine (Fig. 6A,B). However, the effect of melatonin was not additive to the inhibition yielded by citalopram at a high concentration (Fig. 6A).
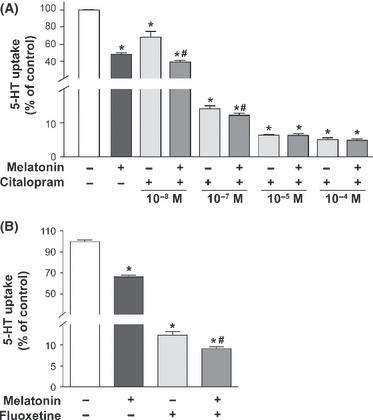
Effect of citalopram and fluoxetine treatment on 5-HT uptake in cells treated with melatonin. In control conditions, cells were not treated. In treated cells, melatonin 0.5 mm was added to the medium during 30 min. (A) Treatment with citalopram for 30 min at different concentrations. (B) Cells were treated with fluoxetine 1 μm for 30 min. 5-HT concentration in uptake determination was 2 × 10−7 m and incubation time was 6 min. Results are expressed as a percentage of the control uptake and are the mean ± S.E.M. of five independent experiments. *P < 0.01 compared with control. #P < 0.01 compared with corresponding citalopram concentration treatment (A) or fluoxetine treatment (B).
Discussion
Numerous studies have demonstrated that 5-HT and melatonin play an important role in the physiopathology of the GI tract [1–4, 9, 10, 28]. Moreover, 5-HT and melatonin are closely related; both are synthesised and released from EC cells and melatonin is a derivative of 5-HT. However, the biological association between the serotoninergic system and melatonin in the GI tract remains unexplored. Therefore, the aim of this study was to determine whether melatonin interferes with SERT and if so, to identify the mechanisms involved. The study has been carried out in Caco-2 cells, a human enterocyte-like cell line which expresses SERT endogenously [8] and that has been shown to be an excellent model for the study of SERT activity and regulation [30, 34].
The results showed that melatonin inhibited 5-HT uptake mediated by SERT. This inhibition was dose-dependent and was obvious during both short- and long-term melatonin treatments of the cells. Melatonin effect on SERT was reached at high concentrations [35]. However, 5-HT uptake in cells treated with melatonin for 1 day was returned up to the control value after 36 min of melatonin withdrawal, indicating a rapid reversibility of the effect and a lack of tissue damage.
The kinetic study of SERT activity has indicated that melatonin diminished both SERT capacity (Vmax) and affinity (kt) which suggests that melatonin may exert a mixed type of inhibition – competitive (increase of kt) and noncompetitive (decrease of Vmax). This SERT inhibition has been previously described in epithelial cells treated with high concentration of 5-HT [34]. The decrease of Vmax yielded by melatonin may indicate a reduction in SERT protein availability in the cell. However, neither the SERT mRNA nor the protein level in cell lysates or apical membranes was altered by melatonin treatment. These results suggest that melatonin may not affect either the SERT protein synthesis or the SERT protein intracellular trafficking processes.
Melatonin has been described as a highly lipophilic compound which diffuses easily through the biological membranes and recent results have also shown that some cells transport melatonin by specific mechanisms [36], therefore melatonin effect on SERT might be yielded from the extracellular or intracellular compartment. To clarify this point, 5-HT transepithelial apical to basal flux was measured when melatonin was added, either to the apical or the basal compartment. The results showed that apical melatonin significantly inhibited 5-HT flux, whereas basal melatonin did not affect this flux. This result suggests that melatonin may require a direct contact with the apical membrane to affect the activity of SERT located in this border. Different biological actions of melatonin have been described as being mediated by its binding to plasma membrane localised melatonin receptors [20]. Two distinct G-protein coupled melatonin membrane receptors have been reported to be expressed in humans, MT1 and MT2 [37] and a previous study has demonstrated MT1 and MT2 expression in rat small intestine [38]. To study whether melatonin receptors MT1 and MT2 are involved in SERT inhibition, the cells were treated with melatonin and/or luzindole, a MT1 and MT2 antagonist. The results showed that luzindole did not reverse the melatonin inhibition of SERT, indicating that the melatonin effect may be independent of its receptor mediation or the effects of melatonin were mediated by a melatonin metabolite [39]. This result seems to be reinforced by the fact that MT1 and MT2 activation led to reduced cAMP formation, and cAMP has been demonstrated to inhibit SERT activity [7, 8]. Moreover, melatonin inhibition of SERT is yielded at a much higher concentration (0.5 mm) than the Km (pm) described for MT1 and MT2.
Many studies have demonstrated that melatonin interferes with 5-HT receptors [40, 41] and different 5-HT receptor types have been described as being expressed in intestinal epithelium [5–7]. As PKC and cAMP have been described as intracellular pathways involved in 5-HT receptor effects, we have measured the effect of melatonin on SERT activity in the presence of PKC and cAMP/PKA activators and inhibitors. The results showed that melatonin inhibition of SERT did not seem to involve these intracellular pathways, and therefore, it suggests that melatonin may not interfere with 5-HT receptors to induce its effect on SERT.
A possible explanation for the melatonin effect on SERT, independent of receptors, may be a catalytic action of melatonin on the SERT protein in the membrane, which might affect both the capacity (Vmax) and the affinity (kt) of the transporter. Several studies using brain neuronal and platelet membranes have revealed that at least two binding sites are present on the SERT protein: a primary high affinity binding site that mediates 5-HT reuptake; and an allosteric low-affinity binding site that regulates the binding of ligands at the primary site [42–44]. These two sites in SERT have been characterised by the action of the SSRIs fluoxetine and citalopram. Fluoxetine has been shown to bind to the high affinity site (reuptake site) [32]; however, citalopram, at low concentrations, has been described as binding to the reuptake site, and, at high concentration, to the allosteric site [33]. Our results showed that the melatonin inhibition of SERT was additive to the inhibition yielded by fluoxetine, thus indicating that melatonin and fluoxetine may act from different sites in SERT. The effect of melatonin was also additive to the inhibition yielded by low concentrations of citalopram, which corroborates the above results. However, the inhibitory effect of melatonin was not additive to the inhibition yielded by a high concentration of citalopram, indicating that both melatonin and citalopran might affect the allosteric SERT site. This might explain the mixed type of inhibition of melatonin on SERT activity obtained in the kinetic study and the direct effect of melatonin on the apical membrane, independent of receptors. This kind of inhibition of SERT from the allosteric site has been recently described for high concentrations of 5-HT [34] which suggests that 5-HT and melatonin effects may be partly mediated by similar mechanisms. The results of the present study indicate that melatonin inhibits SERT activity; however, the mediation of melatonin metabolites in this effect can not be discarded in our epithelial model. The interrelationship between the serotoninergic system and melatonin has been previously described in the regulation of digestive processes [24], in the platelets [45, 46] and in the central nervous system, where melatonin was shown to affect serotoninergic neurotransmission [38].
In conclusion, the results of the present paper show the inhibitory effect of melatonin on SERT and the mechanism involved in this inhibition. This finding may contribute to a better understanding of the feedback system between melatonin and 5-HT in intestinal physiology and pathology, and it may also be useful in the treatment of disorders in which the serotoninergic system is affected.
Acknowledgements
This work was funded by a grant from the Spanish Ministry of Science and Innovation and the European Regional Development Fund (ERDF/FEDER) (BFI 2009-08149), as well as by a grant from the Aragon Regional Government (B61) and grants from the University of Zaragoza (UZ2007-BIO-01, UZ2008-BIO-18). The research group is a member of the Network for Cooperative Research on Membrane Transport Proteins (REIT), co-funded by the Spanish Ministry of Education and Science and the European Regional Development Fund (ERDF) (Grant BFU2007-30688-E/BFI).
Author contributions
N. Matheus, C. Mendoza and R. Iceta have contributed to the acquisition of the data and the analysis and interpretation of them. J. E. Mesonero and A. I. Alcalde have contributed to conception, design, analysis and interpretation of the data and the drafting of the article. They have also revised the article for intellectual content and they have contributed to the final approval of the version to be published.




