The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells
Abstract
Abstract: Methamphetamine (METH) is a potent psychostimulant drug that may cause neuronal cell degeneration. The underlying mechanisms of METH-induced neuronal toxicity remains poorly understood. In this study, we investigated an important role of calpain-dependent cascades in methamphetamine-induced toxicity in human dopaminergic neuroblastoma SH-SY5Y cultured cell lines. In addition, the protective effect of melatonin against METH-induced calpain-dependent death pathway was also investigated. The results of this study show that METH significantly decreased cell viability and tyrosine hydroxylase phosphorylation in SH-SY5Y cultured cells. Melatonin reversed the toxic effect of METH by inducing cell viability. In addition, melatonin was able to restore the reduction in mitochondrial function and phosphorylation of tyrosine hydroxylase in SH-SY5Y treated cells. An induction of calpain expression and activity but a reduction of calpain inhibitor (calpastatin) protein levels were observed in SH-SY5Y cells treated with METH but these effects were diminished by melatonin. These results implicated calpain-dependent death pathways in the processes of METH-induced toxicity and also indicated that melatonin has the capacity to reverse this toxic effect in SH-SY5Y cultured cells.
Introduction
Methamphetamine (METH) is a derivative of amphetamine and a cationic lipophilic psychostimulant that enhances the release of dopamine (DA) from synaptic vesicles and nerve terminal stores in the central nervous system (CNS). Several pieces of evidence have demonstrated that an excessive release of DA and auto-oxidation of DA subsequently results in the formation of reactive oxygen species (ROS) and neuronal damage. METH can cause damage to the dopaminergic nerve terminals and neurons that are typical of neurodegenerative diseases such as Parkinson’s disease (PD). It has been reported that the mechanism of METH-induced neurotoxicity and neuronal cell degeneration involve multiple processes including the inhibition of the mitochondrial electron transport chain (ETC) [1], a decrease in anti-apoptotic Bcl-2-related proteins, an increase in pro-apototic proteins (BAX, BAD and BID) [2] and an activation of cysteine protease or caspase death pathways [3, 4].
In a number of research studies, it has been reported that other protease such as calpain, which is a calcium-dependent cysteine protease, is implicated in neurodegeneration such as in PD [5, 6], Alzheimer’s disease (AD) [7], Huntington’s disease [8] and postischemic brain damage [9]. MPP+-treated cerebellar granule neuron cultures exhibit a strong increase in calpain activity and the apoptotic cells [10]. The activation of calpain pathway and the degeneration of neuronal cells have been observed in the striatum of rat and rat hippocampal cultured cells treated with 3-nitropropionic acid (3-NP), an inhibitor of the mitochondrial complex II respiratory enzyme (succinate dehydrogenase) [11].
Melatonin (N-acetyl-5-methoxytryptamine) is a lipophilic molecule that is synthesized in the pineal gland. In addition to regulating several biological functions such as circadian rhythm regulation, immune enhancement, sleep induction, seasonal reproduction and light/dark signaling, the neuroprotective and neurorestorative functions of melatonin and its metabolites have been documented [4, 12–14]. Numerous studies provide strong evidence for the protective effects of melatonin on caspase-mediated death pathways. It has been demonstrated that melatonin reduces the toxicity induced by METH in SH-SY5Y cells by reducing the induction of Bax and caspase enzymes [4]. However, there have not been any studies, which have sought to elucidate the protective effects of melatonin on calpain-dependent death processes induced by METH in DA cells. Therefore, the aim of this study was to investigate the neuroprotective effect of melatonin on METH-induced induction in calpain-dependent death signaling cascades in human neuroblastoma SH-SY5Y cells.
Materials and methods
Reagents and chemicals
Minimum essential medium (MEM), Ham’s F-12 medium, fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco BRL (Gaithersburg, MD, USA). Melatonin, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and methamphetamine were obtained from Sigma–Aldrich (St. Louis, MO, USA). The rabbit polyclonal anti-phosphorylated TH at Ser40 (phospho-TH) and mouse monoclonal anti-spectrin and anti-actin were purchased from Chemicon International (Temecula, CA, USA.).The rabbit polyclonal anti-calpain (H-240) and anti-calpastatin (H-300) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The horseradish peroxidase-conjugated goat anti-rabbit IgG and anti-mouse IgG antibodies were purchased from Cell Signaling (Beverly, MA, USA). ECL Plus Western Blotting Reagent was purchased from Amersham Biosciences (Piscataway, NJ, USA). NunclonTM culture flasks and Corning culture plates were obtained from Corning Incorporated (Acton, MA, USA). Human dopaminergic neuroblastoma SH-SY5Y cell line was provided from American Type Culture Collection (Manassas, VA, USA). All other chemicals used in this study were of an analytical grade and obtained essentially either from Sigma–Aldrich or Lab-scan analytical science (Dublin, Ireland).
SH-SY5Y cell cultures
SH-SY5Y cells were grown in complete media containing 45% MEM, 45% Ham’s F-12, 10% inactivated FBS and 100 U/mL penicillin/streptomycin. Cells were maintained at 37ºC under a 5% CO2/95% humidified air incubator for the indicated time.
Cell viability assay
Cell viability was assessed by using the MTT assay, which is based on the conversion of the yellow color of MTT to dark blue formazan crystals by mitochondrial dehydrogenase [15]. SH-SY5Y cells were resuspended in 10% FBS-completed media and the cell suspensions were placed into a 96-well culture plates. The cells were incubated at 37ºC under a 5% CO2/95% humidified air incubator for 24 hr. The 10% FBS-completed media were replaced with 1% FBS-completed media then cells were treated with METH for 24 hr. In some experiments, melatonin was added to the medium for 1 hr prior to incubation with METH for another 24 hr. Control-untreated cells were incubated with a culture medium for 24 hr. MTT in 0.1 mm phosphate buffered saline (PBS) were added to each well and incubated at 37ºC for 4 hr. The solution was discarded and then the extraction buffer (0.04 N HCl in isopropanol) was added. The optimal densities were measured at 570 nm spectral wavelength using a microtiter plate reader.
Western immunoblotting
SH-SY5Y cells were grown in 10% FBS-completed media to subconfluent stage in six-well plates for 24 hr and then the culture medium was replaced with 1% FBS-completed media. Cells were then exposed to METH or melatonin for 24 hr. In some experiments, melatonin was added to the medium for 1 hr prior to incubation with METH for another 24 hr without changing the culture medium. The control-cultured cells were incubated with culture medium for 24 hr. After incubation, the cells were lysed by adding lysis buffer and scraped off the plate. The suspended cells were sonicated for 10 s and centrifuged for 15 min at 10,000 g. The supernatants were collected and run to SDS–PAGE. The protein bands were transferred to nitrocellulose membrane. After the transfer, the nitrocellulose membrane was washed with Tris-buffered saline (TBS) for 5 min. The membrane was incubated with blocking solution (5% nonfat dry milk in 0.1% Tween-TBS, TBST) and washed three times for 5 min each time with TBST. It was then incubated in primary antibodies at 4ºC overnight. After incubation, the membrane was washed three times for 5 min each time with TBST, incubated in HRP-conjugated secondary antibody for 1.30 hr and was then washed three times for 5 min each time with TBST. The blot membrane was developed signal with Chemiluminescence ECL Plus-Western Blotting detection reagents. The immunoblots were quantified by measuring the density of each band using densitometry analysis with Scion image program (National Institutes of Health, Bethesda, MD, USA).
Immunocytochemical analysis
SH-SY5Y cells were seeded on sterile glass coverslips at 37ºC for 24 hr then cells were exposed to METH for 24 hr. In some experiments, melatonin was added to the medium 1 hr prior to incubation with METH for 24 hr. The control cells were incubated with the culture medium for 24 hr. The cells were incubated with MitoTracker® (Invitrogen, San Diego, CA, USA) Red CMXRos for 30 min. After incubation the medium was removed, the cells were washed with ice-cold PBS. The cells were then fixed with 4% paraformaldehyde in PBS for 30 min at 4°C and washed with PBS three times for 5 min each time. The cells were then permeabilized with 1% Triton X-100 in PBS for 10 min at room temperature and rinsed with PBS three times. Cells were blocked nonspecific antibody binding sites by incubating for 10 min at room temperature with 10% Donkey serum in PBS containing 0.3% Triton X-100 and 1% Bovine serum albumin (BSA). Cells were incubated with primary antibody (1:1000 in PBS containing 0.3% Triton X-100 and 0.25% BSA) overnight at 4ºC followed by incubation in Fluorescein isothiocyanate (FITC)-conjugated with anti-rabbit IgG (1:200 in PBS containing 0.3% Triton X-100 and 0.25% BSA) for 2 hr at room temperature. Cells were washed three times with PBS. Stained slides were mounted using anti-fade reagent in glycerol buffer (Vector Laboratories, Burlingame, CA, USA) and visualized under Fluorescence microscopy (Olympus, Tokyo, Japan)
Statistical analysis
Data were expressed as mean ± S.E.M. Significance was assessed by a one-way ANOVA followed by Tukey–Kramer test using the scientific statistic software SPSS version 16 (SPSS Inc., Chicago, IL, USA). Probability (P) values of <0.05 were considered statistically significant.
Results
METH produced a dose- and time-dependent reduction in cell viability in SH-SY5Y cultured cells. METH at 1.0 mm for 6 and 24 hr significantly decreased cell viability to 84 ± 3.6% and 67 ± 6.2% of the untreated (0 mm METH) control values, respectively. METH at 2.0 mm for 6 and 24 hr significantly decreased cell viability to 64 ± 2.9% and 54 ± 1.2% of the control values, respectively (Fig. 1).
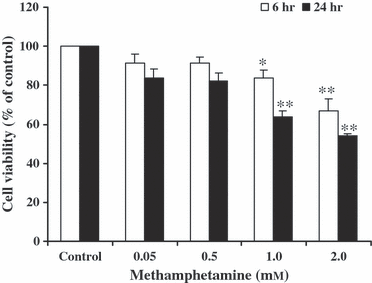
The effect of methamphetamine (METH)-induced reduction in cell viability. SH-SY5Y cells were treated with various concentrations of METH for 6 and 24 hr. The control-cultured cells were incubated with culture medium for 24 hr. Cell viability was measured using MTT assay. The results are expressed as mean ± S.E.M. of four independent experiments. ANOVA was performed for statistical analysis. *P < 0.05; **P < 0.001 compared with control.
METH-induced reduction in phosphorylation of TH was demonstrated in this study. Western immunoblotting was employed to determine the amount of phosphorylated TH in SH-SY5Y cultured cells. The results show that METH at 0.05, 0.5, 1.0 and 2.0 mm for 24 hr significantly decreased the amount of phospho-TH when compared with control-untreated cells. Although, there were no significant changes of TH, a trend to decrease was found in METH-treated cells (Fig. 2).
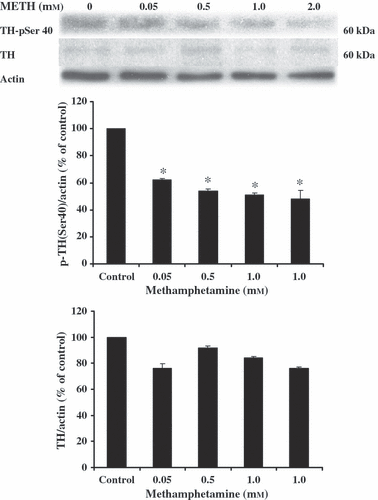
The effect of methamphetamine (METH)-induced reduction in phosphorylation of TH (p-TH). SH-SY5Y cells were treated with various concentrations of METH for 24 hr. The control-cultured cells were incubated with culture medium for 24 hr. The alteration of the amount of p-TH was determined using Western blot analysis. Protein bands were quantified by densitometry and the changes are represented in the graph. The amount of p-TH is expressed as a ratio of p-TH/actin protein bands. The results are expressed as mean ± S.E.M. of four independent experiments. ANOVA was performed for statistical analysis. *P < 0.05 compared with control.
The protective effect of melatonin on METH-reduced cell viability was ascertained in SH-SY5Y cultured cells. METH at 1.0 mm for 24 hr significantly reduced cell viability (70 ± 2.7% of the control) when compared with untreated control cells. Melatonin at 0.25 mm for 24 hr had no effect on cell viability (96 ± 2.0% of the control) when compared with control-untreated cells. Viability of SH-SY5Y cells, pretreated with 0.25 mm melatonin for 1 hr prior to incubation with 1.0 mm METH for another 24 hr was 90 ± 1.8% of the control values (Fig. 3). The results show that the pretreatment of melatonin significantly increased cell viability in METH-treated cells in comparison with METH-treated cells without melatonin.
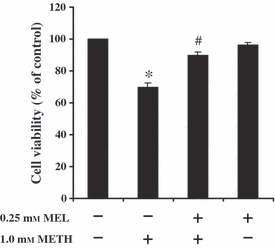
The effect of melatonin (MEL) on METH-induced reduction in cell viability. SH-SY5Y cells were treated with 1.0 mm METH for 24 hr. Some cells were pretreated with 0.25 mm MEL for 1 hr prior to incubation with 1.0 mm METH for another 24 hr without changing the culture medium. The control-cultured cells were incubated with culture medium for 24 hr. Cell viability was measured using MTT assay. The results are expressed as mean ± S.E.M. of four independent experiments. ANOVA was performed for statistical analysis. *P < 0.05 compared with control and #P < 0.05 compared with METH-treated cells.
Fluorescent dye and immunofluorescence staining were employed to confirm the induction in death processes of METH and protective or restorative effects of melatonin against this neurotoxicity. METH-induced disruption of the membrane integrity of mitochondria was demonstrated using fluorescent mitochondria-staining dye. MitoTracker®Red CMX-Ros is a red-fluorescent dye used for staining mitochondria in live cells and its accumulation is dependent on mitochondrial membrane potential, whereas the immunofluorescence staining of phosphorylation of TH (p-TH) was used to demonstrate the normal state of SH-SY5Y cells. The red and green colors indicate active mitochondrial sites and p-TH-positive immunostaining in SH-SY5Y cells, respectively (Fig. 4). Exposure to METH resulted in cell shrinkage and a decrease in mitochondrial site signals together with a reduction in p-TH immunofluorescence staining (Fig. 4F, arrows) when compared with control-untreated cells (Fig. 4C). Normal cell morphology and induction in mitochondrial sites and p-TH immunofluorescence staining in METH-treated cells were restored by melatonin (Fig. 4I).
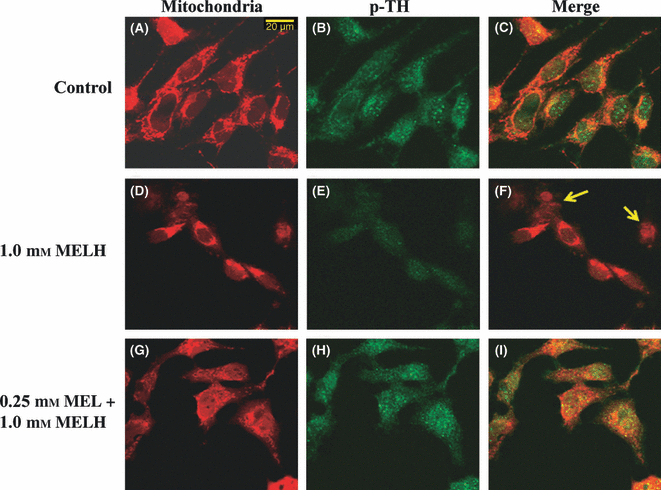
Fluorescence microscopic images of SH-SY5Y cells demonstrating the effect of melatonin (MEL) on METH-induced neurotoxicity in SH-SY5Y cultured cells. The control-cultured cells were incubated with culture medium for 24 hr (A–C). Some cells were treated with 1.0 mm METH for 24 hr (D–F). Some cells were pretreated with 0.25 mm MEL for 1 hr prior to incubation with 1 mm METH for another 24 hr (G–I). Cells were incubated with MitoTracker®Red before the cells were fixed. Cells were fixed with 4% paraformaldehyde and stained with rabbit polyclonal antibody against p-TH. The green color indicates p-TH immunostatining using fluorescein-5-isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG (B, E and H) and the red color indicates mitochondria sites using MitoTracker®Red (A, D and G). The double fluorescence (merge) images are shown in C, F and I. Scale bar = 20 μm.
The effect of melatonin on METH-induced induction in calpain levels and activity, and reduction in calpastatin levels were determined in SH-SY5Y cells using Western blot analysis. The calapin activity was determined in terms of the levels of spectrin break down products (SBDP). The 145-kDa SBDP (SBDP 145) is a specific product of spectrin cleaved by calpain. METH at 1.0 mm for 24 hr significantly increased the amount of calpain (376 ± 14.8% of the control) and SBDP 145 (290 ± 8.8% of the control) (Fig. 5) but decreased the amount of calpastatin (78 ± 2.5% of control) (Fig. 6) in SH-SY5Y cells, respectively. Pretreatment of SH-SY5Y cells with 0.25 mm melatonin for 1 hr prior to treatment with 1 mm METH for another 24 hr significantly decreased the amount of calpain (147 ± 9.1% of the control) and SBDP 145 (117 ± 2.5% of the control) (Fig. 5) but increased the amount of calpastatin (145 ± 3.6% of the control) (Fig. 6) in SH-SY5Y cells, respectively. Melatonin at 0.25 mm had no effect on the amount of calpain (130 ± 9.8% of the control) and SBDP 145 (72 ± 7.2% of the control) (Fig. 5) but significantly increased the amount of calpastatin (144 ± 4.1% of the control) (Fig. 6) in SH-SY5Y cells when compared with the control values, respectively.
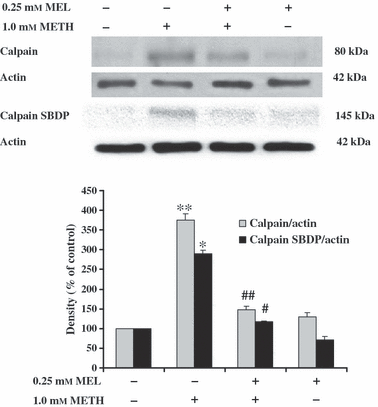
The effect of melatonin (MEL) on methamphetamine (METH)-induced induction in calpain expression and activity. SH-SY5Y cells were treated with 1.0 mm METH for 24 hr. Some cells were pretreated with 0.25 mm MEL for 1 hr prior to incubation with 1.0 mm METH for another 24 hr without changing the culture medium. The control-cultured cells were incubated with culture medium for 24 hr. The alteration of calpain levels and activity was determined using Western blot analysis of calpain and calpain spectrin break down products (SBDP), respectively. Protein bands were quantified by densitometry and the changes are represented in the graph. The calpain levels and activity is represented as a ratio of calpain/actin and calpain SBDP145/actin protein bands, respectively. The results are expressed as mean ± S.E.M. of four independent experiments. ANOVA was performed for statistical analysis. *,**P < 0.05 compared with control and #,##P < 0.05 compared with METH-treated cells.
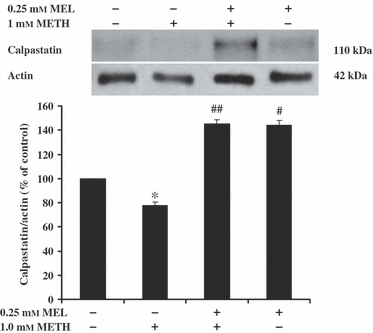
The effect of melatonin (MEL) on methamphetamine (METH)-induced reduction in calpastatin levels. SH-SY5Y cells were treated with 1.0 mm METH for 24 hr. Some cells were pretreated with 0.25 mm MEL for 1 hr prior to incubation with 1 mm METH for another 24 hr without changing the culture medium. The control-cultured cells were incubated with culture medium for 24 hr. The alteration in the amount of calpastatin was determined using Western blot analysis. Protein bands were quantified by densitometry and the changes are represented in the graph. The amount of calpastatin is expressed as a ratio of calpastatin/actin protein bands. The results are expressed as mean ± S.E.M. of four independent experiments. ANOVA was performed for statistical analysis. *,#P < 0.05 compared with control and ##P < 0.05 compared with METH-treated cells.
Discussion
This study demonstrates the capacity of melatonin to prevent the toxicity of METH in inducing calpain-dependent death pathway in neuroblastoma SH-SY5Y cells. The results of this study demonstrated that METH-induced dopaminergic SH-SY5Y cell death occurs with dose and time-dependent effects. The higher METH concentrations or longer the METH exposure time, the greater the reduction in cell viability was observed. METH-induced neurotoxicity has been demonstrated in animals [2] and humans [16]. In the human brain, the reward circuit responses to METH by the activation of mesolimbic system [17]. Several studies have shown that METH enters dopaminergic cell via the dopamine transporter (DAT) [4] and passive diffusion [18] and then induces DA release from synaptic vesicles into the cytoplasm [19]. Many studies suggest that high levels of DA auto-oxidize to generate ROS leading to oxidative stress, mitochondria dysfunction and an increase in free radical formation [20]. It has been demonstrated that METH decreases mitochondrial membrane potential and increases the levels of ROS and the occurrence of apoptosis in SH-SY5Y cultured cells [21]. In this study, METH-induced reduction in cell viability was also ascertained by the reduction in the phosphorylation of TH, the rate-limiting enzyme for DA synthesis. Several studies suggest that METH can cause a decrease in TH levels in different brain regions of rat [22] and mice [23, 24].
Recently, it has been reported that METH can induce an increase in intracellular calcium (Ca2+) concentrations both in in vivo and in vitro studies [16]. High levels of Ca2+ inside the cell activate several intracellular signaling cascades including calpain, which is a calcium-dependent cysteine protease. Calpain was localized in numerous cellular compartments such as mitochondria, cytosol and nucleus. It is a member of death signaling pathways that is regulated by intracellular Ca2+ concentration. Moreover, calpain activity is also regulated by an endogenous calpain inhibitor named calpastatin [25]. Calpain has been implicated in certain pathological conditions in the central nervous system (CNS) [26] including AD and PD [27]. Upregulation of calpain activity in microglia, astrocytes and neurons has been observed in spinal cord of MPTP-treated mice [28, 29]. The unilateral infusion of intrastriatal 6-OHDA increases calpain activation in caudate–putamen and substantia nigra, and dopaminergic denervation in caudate–putamen of rat brain [30]. In addition, it has been previously shown that activation of calpain in rat striatal synaptosome by increasing Ca2+ concentrations leads to an increase in the cleavage of DATs and this process is totally blocked by a calpain inhibitor [31].
This study demonstrated METH-induced reduction in levels of calpastatin but induction in calpain expression and activation in dopaminergic SH-SY5Y cells. It has been reported that an overproduction of reactive oxygen radical stimulates Ca2+ influx, which, in turn, increases calpain expression and Bax/Bcl-2 ratio and then promotes cytochrome c release and apoptosis in C6 cell [32, 33]. Nakagawa and Yuan [34] have demonstrated that calpain has the capacity to mediate caspase-12 activation and Bcl-xl inactivation in ischemic-induced apoptotic glial cell cultures and amyloid beta peptide-induced cell death in cortical neuron cultures.
Our previous study demonstrated induction in ROS formation in D-amphetamine-induced neurotoxicity in dopaminergic neuroblastoma SK-N-SH cultured cells [35]. We also found that METH can induce mitochondrial dysfunction, caspase activation and cell death in SH-SY5Y cells [4]. Although the exact mechanisms of METH-induced calpain activation and cell death in neuronal cells are unknown, a possible explanation might be an increase in ROS formation, which, in turn, results in mitochondrial dysfunction. This dysfunction leads to a leakage of mitochondrial Ca2+ into cytosol. High levels of intracellular Ca2+ may contribute to the activation of calpain and this activation eventually leads to a cleavage of cytoskeletal and myelin proteins and, also an activation of pro-apoptotic factors such as Bax and caspase-3 [36, 37].
Melatonin and its metabolites have protective effects on a variety of oxidative stress associated neuropathologies [4, 28–30, 32]. Melatonin is a lipophilic molecule, which easily crosses the blood–brain barrier, plasma membrane and enter several cellular compartments including mitochondria [38]. Mitochondria play some functional roles related to anti-oxidative properties and cell survival such as the stimulation of anti-oxidant enzymes for the direct scavenging of toxic reagents, and functional units of ATP synthesis that help to maintain cellular homeostasis, respectively [39]. Either the over production of intracellular ROS or the inhibition of mitochondrial protein complexes disturbs the normal function of mitochondria and induces mitochondrial Ca2+ dysregulation which leads to the activation of calpain and cell damage or death [40]. The restorative properties of melatonin on secretory functions, Ca2+ signals and mitochondrial potential have been demonstrated in aged exocrine cells [41]. Some of the action of melatonin prevents a ruthenium red-induced reduction in the activity of complexes I and IV mitochondrial in rats [42].
In this study, the protective effect of melatonin on METH-induced reduction in cell viability, calpastatin levels, mitochondrial function, phosphorylation of TH but induction in calpain expression and activity was determined in SH-SY5Y cells. The results of the this study clearly demonstrated that melatonin has a potential effect on protection and restoration of METH-induced clapian-dependent death processes in dopaminergic SH-SY5Y cells. Present findings are consistent with the previous studies, which demonstrated that treatment with melatonin decreases inflammation and calpain expression in spinal cord injury (SCI) of rats [43]. Treatment with melatonin also reduces the neurotoxic effects of MPP+ on induction in calpain activity, activation in cleavage of cdk5/p35 to cdk5/p25 and increases in the apoptotic cell population in cerebellar granule neuron cultures [10]. Furthermore, melatonin can reduce calcium overload from mitochondria and blocks mitochondrial permeability transition (MPT) pore-dependent cytochrome c release, and caspase 3 activation [44, 45]. In this study, we also observed the rescue effect of melatonin on reduction in calpastatin levels in METH-treated SH-SY5Y cells. This might be an indirect beneficial effect of melatonin regarding the prevention of cell death, as this death is brought about by calpain-dependent death processes. The results of this study are in accordance with previous studies which demonstrated that treatment with NGF and cAMP decreases calpain activity and increases calpastatin levels in PC12 cells [46]. The overexpression of calpastatin protects MPTP-treated mouse [47] and attenuates AIF in ischemic neuronal injury [48].
In conclusion, this study demonstrated the neurotoxicity of METH-induced calpain activation. This neurotoxicity leads to cell death. Melatonin protects METH-induced cell death by increasing calpastatin levels and decreasing calpain activity in SH-SY5Y cultured cells. This discovery points to the contribution of melatonin as a potential protective agent for calpain-dependent death processes. Finally, it is suggested that melatonin should be used as a calpain inhibitor in PD models before using it in therapies, which implicate human patients.
Acknowledgements
This work was supported by a research grant from the Thailand Research Fund through RMU 5180010 to B.C and the TRF-Senior Scholar Fellowship to P.G, and Mahidol University. We thank Dr Lynken Ghose (Editorial office of Graduate Studies, MU) for revising the language of the manuscript.




