Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: melatonin receptor-mediated inhibition of signal transduction, linoleic acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts
Abstract
Abstract: Melatonin provides a circadian signal that regulates linoleic acid (LA)-dependent tumor growth. In rodent and human cancer xenografts of epithelial origin in vivo, melatonin suppresses the growth-stimulatory effects of linoleic acid (LA) by blocking its uptake and metabolism to the mitogenic agent, 13-hydroxyoctadecadienoic acid (13-HODE). This study tested the hypothesis that both acute and long-term inhibitory effects of melatonin are exerted on LA transport and metabolism, and growth activity in tissue-isolated human leiomyosarcoma (LMS), a rare, mesenchymally-derived smooth muscle tissue sarcoma, via melatonin receptor-mediated inhibition of signal transduction activity. Melatonin added to the drinking water of female nude rats bearing tissue-isolated LMS xenografts and fed a 5% corn oil (CO) diet caused the rapid regression of these tumors (0.17 ± 0.02 g/day) versus control xenografts that continued to grow at 0.22 ± 0.03 g/day over a 10-day period. LMS perfused in situ for 150 min with arterial donor blood augmented with physiological nocturnal levels of melatonin showed a dose-dependent suppression of tumor cAMP production, LA uptake, 13-HODE release, extracellular signal-regulated kinase (ERK 1/2), mitogen activated protein kinase (MEK), Akt activation, and [3H]thymidine incorporation into DNA and DNA content. The inhibitory effects of melatonin were reversible and preventable with either melatonin receptor antagonist S20928, pertussis toxin, forskolin, or 8-Br-cAMP. These results demonstrate that, as observed in epithelially-derived cancers, a nocturnal physiological melatonin concentration acutely suppress the proliferative activity of mesenchymal human LMS xenografts while long-term treatment of established tumors with a pharmacological dose of melatonin induced tumor regression via a melatonin receptor-mediated signal transduction mechanism involving the inhibition of tumor LA uptake and metabolism.
Introduction
In the United States alone, approximately 10,000 people will be diagnosed this year with soft tissue sarcoma, including malignant fibrous histiocytomas, liposarcomas and leiomyosarcomas [1]. Of this number, about 3500 people will be diagnosed with leiomyosarcoma (LMS) a rare and aggressive neoplasm derived from the smooth muscle cells of the uterus, stomach, blood vessel walls or skin. This sarcoma is a chemotherapy and radiation resistant cancer, particularly in advanced stages, with greater than 36% mortality rate within 5 yr of diagnosis.
Although most of the work on the anticancer role of melatonin has been performed on epithelially-derived cancers [2–5], a number of early studies in this field have addressed the potential role of melatonin in regulating the growth of mesenchymally-derived murine tumors including sarcomas and leukemia [6–8]. For example, it has been shown that pinealectomy (i.e. removal of the nocturnal melatonin signal) stimulates the growth of both transplantable and methylcholanthrene (MCA)-induced fibrosarcomas in both mice and rats as compared with their pineal-intact counterparts. The prevalence of tumor metastases to lymph nodes was also increased in pinealectomized animals. Although indirect, this was the first evidence to suggest that sarcomas are sensitive to the antiproliferative effects of the nocturnal, circadian melatonin signal. Conversely, pharmacological concentrations (50–100 μg) of melatonin injected into mice with MCA-induced fibrosarcomas have been shown to significantly inhibit the development and growth of these tumors [9–13]. Similarly, the growth of a transplantable form of leukemia (LSTRA cells) in mice was substantially suppressed by injections of a pharmacological dose of melatonin [8]. A more recent in vitro study demonstrated that melatonin was effective in inducing differentiation, bone marker protein expression and bone mineralization in rat osteoblast-like osteosarcoma 17/2.8 cells via melatonin receptors which are inhibitory G protein-coupled [14]. In contrast, other investigators failed to demonstrate any effects of melatonin on the proliferation of MG-63 human osteosarcoma cells in vitro [15].
Like melatonin, most of the work on the ability of LA to stimulate tumor growth has been performed in epithellially-derived rodent and human cancers. Of these few studies that exist in the literature, three of them appear to support a stimulatory role for LA in cancer cell proliferation/survival [16–18] while the other two support an inhibitory effect [19, 20]. With respect to mesenchymally-derived tumors, only one study addressed the role of growth signaling pathways by demonstrating that stimulation of a receptor tyrosine kinase (encoded by the c-Met proto-oncogene) by hepatocyte growth factor promotes the survival of the human leiomyosarcoma cell line (SK-LMS-1) [21].
Interestingly, the first clinical study of melatonin’s effects on any cancer type involved the treatment of a small number of individuals with either recurrent rhabdomyosarcoma, osteogenic sarcoma or synovial sarcoma. High doses of melatonin resulted in either a remarkable tumor regression or a total eradication of the disease [22]. In a more recent clinical study of a small group of patients with soft tissue sarcoma, oral melatonin therapy during the evening coupled with supportive care had a slight positive effect on disease stabilization as compared with supportive care alone [23, 24]. In spite of these encouraging earlier basic science and clinical studies, melatonin’s antineoplastic effect on sarcoma growth and its mechanisms of action have not been pursued further. In fact, melatonin’s effects on LMS development or growth have never been investigated.
Here we used a unique animal model, developed in our laboratory [25] that allows us to grow and perfuse tissue-isolated human vulvar-derived LMS (SK-LMS-1) xenografts in nude rats. We tested the hypothesis that a physiological nocturnal circulating concentration of melatonin, that normally provides a regular circadian signal regulating LA-dependent tumor growth, would inhibit LA metabolism and the proliferation of human leiomyosarcoma (LMS) in vivo. We further investigated whether a melatonin receptor-mediated signal transduction mechanism was involved.
Materials and methods
Animals, housing conditions, and diet
Female, homozygous, inbred, athymic, nude mice (BALBc/nu+/nu+) were purchased from Taconic Farms (Germantown, NY, USA). Female, inbred, athymic nude rats (Hsd:RH-Foxn1rnu) were purchased from Harlan (Indianapolis, IN, USA). Adult male Buffalo rats (BUF/CrCrl), which provided donor blood for perfusions, were purchased from Charles River Laboratories (Wilmington, MA, USA). All specific-pathogen-free strains were maintained in environmentally controlled rooms (23°C; 45–50% humidity) in micro-isolator units (Thoren Caging Systems, Hazelton, PA, USA) and were subjected to diurnal lighting, 12L:12D (lights on 06:00 hr; 123 lux; 300 μW/cm2). There was no light contamination during the dark phase. To ensure that all animals remained uninfected with both bacterial and viral agents, semiannually and during the course of this study, serum samples from sentinel rats were tested by ELISA (Comprehensive Health Monitoring Program, Charles River Laboratories, Kingston, NY, USA). Animals were given free access to food (Harlan Teklad 1800 Diet, Washington, DC, USA) and water. Quadruplicate determinations of this diet contained 4.1 g total FA/100 g of diet composed of 0.04% myristic (C14:0), 12.50% palmitic (C16:0), 0.24% palmitoleic (C16:1), 3.12% stearic (C18:0), 21.70% oleic (C18:1n9), 56.13% linoleic (C18:2n6), 5.80%α-linolenic, and 0.23% arachidonic (C20:4n6) acids. Minor amounts of other FA comprised 0.24%. Conjugated linoleic acids (CLAs) and trans FAs were not found. Over 90% of the total fatty acids (TFA) was in the form of triglycerides; more than 5% was in the form of free fatty acids. Animals were maintained in an AAALAC-accredited facility in accordance with The Guide for the Care and Use of Laboratory Animals [26]. All procedures for animal use were approved by the Institutional Animal Care and Use Committee.
Dietary regimens, plasma melatonin levels, and SK-LMS-1 xenograft implantation
In an initial experiment, 20 female nude rats were maintained on the standard lab diet for an initial acclimation period of 7 days. After this time, the animals were provided free access to a 5% corn oil (CO) semi-purified diet, as described in detail previously [16]. Following a 4-wk period, all animals were subjected to two cardiac blood samplings (1 mL), one at 12:00 hr and another 3 days later at 24:00 hr under dim red light for extraction and analysis of diurnal melatonin levels [27–29]. Following a 1-wk recovery period steroid-independent human vulvar-derived SK-LMS-1 xenografts were implanted in the nude rats and grew as tissue-isolated tumors in a manner previously described [25, 27–33], with a single arterial and venous connection to the host. Originally, human vulvar-derived SK-LMS-1 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in our laboratory. Using a 1 cc syringe with a 22-gauge needle (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), 1 × 107 human SK-LMS-1 cells in 0.1 cc of incubation media were inoculated s.c. on the flank immediately caudal to the axilla of the nude mouse. The SK-LMS-1 tumors grew as a solid mass. When tumors reached approximately 1–2 g, the mice were euthanized via CO2 narcosis and the tumors were excised and placed in ice-cold saline for subsequent tissue-isolated tumor transplantation in the nude rats. The LMS tumor xenografts were verified histopathologically to be a grade II human uterine leiomyosarcoma cell line. Latency-to-onset of tumor growth was noted and estimated tumor weights were measured and recorded, as described previously [27]. When tumor weights were estimated to be 3.5–4.5 g, the rats were randomized into two groups (n = 10/group) and were fed either 5% CO diet (controls) or 5% CO diet +50 μg/day melatonin-HOH (treatment). Arterial and venous blood samples were collected across tumors [27, 33], when estimated tumor weights reached 4–6 g (controls) or 1.5–2.5 g (treatment), prior to complete regression. Following collection of the blood samples tumors were freeze-clamped in situ between aluminum blocks chilled in liquid nitrogen and stored at −80°C until analyzed for cAMP, FA’s, and [3H]thymidine incorporation into tumor DNA.
Arterial and venous difference measurements during perfusions in situ
In separate experiments tissue-isolated SK-LMS-1 tumor xenografts were used for in situ perfusions (n = 3/group; total, 54) to examine the growth signaling pathway in SK-LMS-1 tumors in vivo. When tumors reached 4–6 g estimated weight animals were prepared for arteriovenous difference measurements made across the perfused xenografts. Animal preparation, including anesthesia administration, heparin-treatment, surgical preparation of the tumor, and blood sample collection was described previously [25, 27–32]. Perfusions were carried out for a period of either 60 or 150 min with blood from fed, donor rats, as previously described [28, 29, 32, 34–37]. Blood flow from the tumor vein was collected passively in ice-chilled tubes at 30-min intervals. Arterial samples were collected from a side-port of the arterial catheterization line leading to the tumor. In a first set of perfusions (n = 15), donor blood was supplemented with different concentrations of melatonin (0–1 nm; Sigma Scientific, St. Louis, MO, USA) to measure the kinetic effects on tumor growth and metabolism. The purity of the melatonin was listed at greater than 99%. Inhibitions of LA uptake (as a percent of supply to the tumor), 13-HODE formation and [3H]thymidine incorporation into tumor DNA were measured in tumors perfused for 60 min.
In a second set of perfusions of SK-LMS-1 tumors (n = 18), to determine the direct association of the melatonin receptor-mediated system with the growth signal transduction pathways (i.e. MEK>ERK 1/2 and PI3K>Akt>mTOR), sequential additions of melatonin (1 nm) at 36 min following initiation of perfusion with fed donor blood and the following agents at 86 min on TFA and LA uptake and 13-HODE release were examined: (a) 1 nm S20928, nonselective melatonin receptor 1 (MT1)/melatonin receptor 2 (MT2) antagonist (Servier, Courbevoie Cedex, France); (b) 1 μm forskolin (Sigma Scientific); (c) 0.5 μg/mL plasma pertussis toxin (PTX; Sigma Scientific), or (d) 10 μm 8-Bromo-cyclic-AMP (Calbiochem, La Jolla, CA, USA) for a total perfusion time of 150 min. Agents introduced into the donor blood reservoir required 8.1 min to reach the tumor. Changes in TFA and LA uptake, and tumor 13-HODE formation were measured over the time course of each perfusion.
In a third set of perfusions (n = 21), SK-LMS-1 tumor xenografts were perfused for 60 min with donor blood either deplete of the growth inhibitory agent melatonin (controls), supplemented with melatonin (1 nm), or supplemented with melatonin (1 nm) plus 1 nm S20928, or 0.5 μg/mL plasma PTX, or forskolin, or 10 μm 8-Br-cAMP; perfusion of tumors with donor blood containing melatonin (1 nm) + 13-HODE (0.032 μm; Cayman Chemical, Ann Arbor, MI, USA) was also carried out in this group. In all perfusions, 20 min prior to the end of the experiment 20 μL of a saline solution containing 2 μCi [3H]thymidine/g estimated tumor weight was injected into the arterial catheter side-port for measurement of incorporation into tumor DNA. Each tumor was freeze-clamped between 2 liquid-nitrogen chilled aluminum blocks and stored at −80°C for determination of cAMP content, FA content, 13-HODE release, MEK, ERK1/2, Akt levels and [3H]thymidine incorporation, as previously described [27–32, 34–37]. All [3H]thymidine incorporated into tumor DNA was measured by liquid scintillation using internal standardization. Values are reported here as dpms/μg DNA. Tumor DNA was measured in 20% homogenates (w/w) flourometrically using Hoechst dye 33258 as described in Bulletin #119, Hoefer Scientific Instruments (San Francisco, CA, USA).
Fatty acid extraction and analysis
Arterial and human SK-LMS-1 tumor venous plasma total fatty acids were extracted from 0.1 mL arterial and venous samples following the addition of heptadecanoic acid (C17:0), methylated and analyzed via gas chromatography, as described previously [27–32, 34–37]. A–V difference measurements were calculated as rates of FA uptake or release and were expressed as μg/min/g tumor tissue. Values for total fatty acids (TFA) represent the sum of the seven major fatty acids (myristic, palmitic, palmitoleic, stearic, oleic, linoleic and arachidonic acids) in the blood plasma as free fatty acids, cholesterol esters, triglycerides, phospholipids, as well as other plasma lipids and is expressed here as μg or mg per liter plasma. The SK-LMS-1 xenografts have a large capacity for removal of TFA and LA from the whole blood perfusate. The uptake of TFA and LA is dependent upon supply in the arterial blood to the tumor. Physiological levels of TFA in different batches of donor arterial blood collected from fed rats differed by as much as 10%. Analyses showed that this variation altered the rate of TFA and LA uptake tumor somewhat, but not the rate of uptake as a percent of supply to the tumor, which remained consistent at about 18–22%. Rates of TFA and LA uptake are presented here for statistical comparisons as both absolute values (μg/min/g wet weight tumor) and as percent of supply (defined as the TFA or LA uptake/arterial supply × 100). Zero values indicate no amount of TFA or LA uptake could be detected within the sensitivity range of the instrument (10−8 m and above). Tumor tissue TFA and LA levels in control and treatment groups were extracted from 0.1 mL of 20% homogenates as previously described [27–32, 34–37].
HPLC analysis of 13-HODE concentration
Plasma samples (0.2 mL) collected in vivo were extracted for 13-HODE with a known quantity of internal standard, (±)5-hydroxy, 6, 8, 11, 14-eicosatetraenoic acid (5-HETE, racemic; Cayman Chemicals, Ann Arbor, MI, USA) and analyzed by HPLC [23–28, 30–33]. All SK-LMS-1 xenograft 13-HODE production values are expressed as ng/min/g tumor. Zero values indicate no amount of 13-HODE could be detected within the sensitivity range of the instrument (10−18 m and above).
Determination of intratumor cAMP content
A portion of the freeze-clamped tumor was pulverized under liquid nitrogen and cAMP was analyzed in duplicate 10-mg aliquots using Biotrak Enzyme Immunoassay System (RPN 225; Amersham-Pharmacia, Piscataway, NJ, USA), as described previously [30, 31].
Western blot measurement of tumor phosphorylated ERK 1/2, MEK and Akt
Cytosol and membrane fractions were isolated from the frozen, pulverized tumors as described by Allgeier et al. [38]. Homogenizing buffer (2 mL) contained 20 μL Halt Protease Inhibitor Cocktails 1 and 2 (Sigma Chemical). Protein was determined by Folin-phenol reagent [39]. Electrophoresis, polyvinylidine difluoride membrane transfer, and immunodetection of total and phosphorylated ERK 1/2 were performed as previously described [36, 37]. Primary antibodies for the detection of phosphorylated and total MEK were #9121 and #9122 (Cell Signaling, Beverly, MA, USA) respectively; primary antibodies for the detection of phosphorylated and total Akt (Ser 473) were #9271 and #9272 (Cell Signaling) respectively. All antibodies were diluted 1:1000. The bands were visualized and quantified using Storm PhosphoImage and Image Quant software (Amersham Biosciences).
Statistical analysis
All data are presented as the mean ± 1 standard deviation (S.D.) and were compared and using one-way analysis of variance followed by Student-Neuman-Keul’s post hoc test. Differences among the group mean were considered statistically different when the P was <0.05.
Results
Prior to implant, mid-light phase (12:00 hr) and mid-dark phase (24:00 hr) plasma melatonin levels in the nude female rats (n = 20) maintained on the 5% CO control diet measured 6.1 ± 0.4 and 119.0 ± 17.2 pg/mL respectively. Following tumor xenograft implantation, latency-to-onset of tumor appearance, which measured the time of implant to first palpable mass (approximately pea size, 10 mm3), and tumor growth rates in all animals were 22 days and 0.22 ± 0.03 g/day (n = 20), respectively, as shown in Fig. 1. After animal randomization into control (5% CO diet) and treatment (5% CO +50 μg/day melatonin) groups at day 33 following tumor implantation, control tumors continued to grow as before, but melatonin-treated tumors regressed at a rate of 0.17 ± 0.02 g/day. Dietary and water intake, and final body weight at time of tumor harvest, which remained remarkably consistent and did not differ between the control and treatment groups during the course of this experiment were, respectively, 19.1 ± 0.5 g/day and 22.1 ± 1.9 mL HOH/day, and 209.7 ± 31.8 g. Tumor cAMP levels, TFA and LA uptake, 13-HODE release, [3H]thymidine incorporation into tumor DNA and DNA content are shown in Table 1. Tumor cAMP levels were depressed by 68% in the melatonin-treated group as compared with the control group. Tumor TFA and LA uptake, as a percent of arterial LA supply to the tumors, measured, respectively, 19.9 ± 3.0 and 20.5 ± 1.5% in controls, but was completely abrogated in treatment group tumors. Leiomyosarcoma [3H]thymidine incorporation and DNA content, a measure of tumor proliferation, was significantly decreased (P < 0.001) by 80% and 30%, respectively, in melatonin-treated, as compared with control xenografts. Figure 2 depicts tumor ERK 1/2, MEK, and Akt activation in the 5% CO controls and 5% CO + 50 μg/day melatonin treatment groups. The presence of melatonin (50 μg/day) in the drinking water significantly decreased the amount of phosphorylated forms of ERK 1/2, MEK and Akt in vivo.
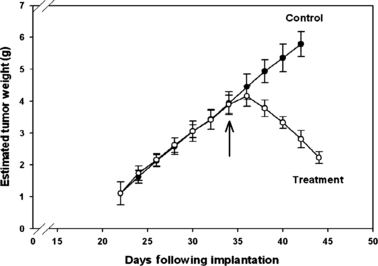
Effects of dietary intake of control 5% corn oil (CO) (•) and treatment 5% CO-50 μg/day melatonin (MLT)-HOH diets (○) [switched from control to treatment diet on day 33 following implantation] on estimated tumor growth rates in female nude rats bearing tissue-isolated SK-LMS-1 tumor xenografts. Each point represents the mean ± S.D. (n = 10) estimated tumor weights. Tumor growth rates of 5% CO group were significantly different from the 5% CO + 50 μg/day melatonin diets group (P < 0.001).
| Treatmenta (10/group) | cAMP (nmol/g tissue) | Total fatty acid uptake (μg/min/g) | LA uptake (μg/min/g) | 13-HODE (ng/min/g) | [3H]thymidine incorporation (dpm/μg DNA) | DNA content (mg/g) | |
|---|---|---|---|---|---|---|---|
| Arterial supply | Venous output | ||||||
| Control | 1.21 ± 0.31 | 3.12 ± 0.56 | 0.86 ± 0.10 | 0 | 5.80 ± 0.82 | 32.8 ± 1.6 | 2.3 ± 0.1 |
| Melatonin | 0.39 ± 0.12b | 0b | 0b | 0 | 0b | 6.5 ± 0.5b | 1.6 ± 0.1b |
- aThere were 10 animals (tumors) per group; mean tumor weight ± 1 S.D. = 5.8 + 0.4 g. bP < 0.001 versus control.
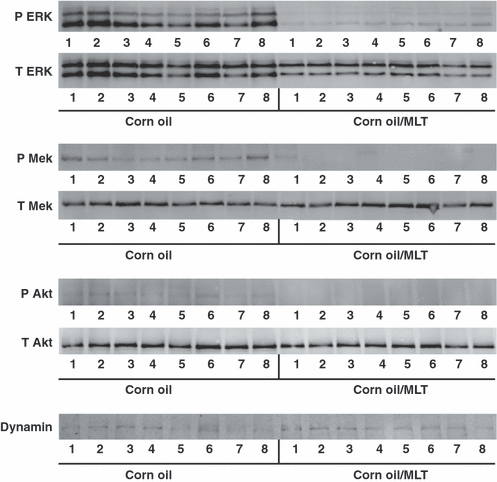
Western blot analysis for the expression of total (lower panels) and phosphorylated (upper panels) forms of ERK 1/2, MEK, Akt and Dynamin II housekeeping protein in SK-LMS-1 leiomyosarcoma xenografts harvested from animals fed either control (5% corn oil) (right side, lanes 1–8) or treatment (5% CO + 50 μg/day melatonin diets) (left side, lanes 1–8). Animals began receiving 5% CO diets for 2 wk prior to tumor implantation and continued until day 33 following implantation when the tumors were approximately 4 g estimated tumor weight; treatment group was switched from control to treatment diet on day 33 following implantation. Each lane depicts either ERK 1/2, MEK, AKT or Dynamin II housekeeping protein bands from one tumor (n = 8).
Arterial and venous differences for total plasma lipid fatty acids, LA, and tumor 13-HODE formation in vivo were measured across tissue-isolated SK-LMS-1 xenografts perfused in situ with increasing concentrations of melatonin in nocturnal circulating range. The rates of LA uptake and 13-HODE release (Fig. 3, upper panel) were ultimately reduced to zero, as the plasma concentration of melatonin increased. Control rates for LA and TFA uptake, respectively, were 0.75 ± 0.13 and 2.94 ± 0.81 μg/min/g tumor (n = 15), which represented 19.5 ± 2.5% and 16.5 ± 2.9% uptake of arterial supply to the tumor respectively. The TFA uptake showed a similar response to increasing concentrations of melatonin (data not shown) as that of LA uptake. The calculated value for Ki in the arterial blood plasma, based on the decrease in LA uptake, was approximately 1 × 10−12 m melatonin. Tumor [3H]thymidine incorporation (Fig. 3, lower panel) and DNA content decreased significantly (P < 0.001) from 27.0 ± 2.0 dpm/μg DNA (2.3 ± 0.1 mg/g tumor), respectively to a minimum of 6.6 ± 0.9 dpm/μg DNA (1.6 ± 0.1 μg/g DNA), respectively, at 10−11 m melatonin. Regression analysis revealed that LA uptake (r = −0.9930), 13-HODE production (r = −0.9550), and [3H]thymidine incorporation (r = −0.9040) were inversely correlated with whole blood melatonin concentrations (P < 0.05). Correlation between LA-uptake by SK-LMS-1 xenografts and the rates 13-HODE release into the tumor venous blood in vivo and during perfusion in situ was significant (P < 0.001): rate of 13-HODE release = 7.853 (rate of tumor LA uptake) + (0.948), r = 0.92, n = 45. Tumors converted 0.68 ± 0.06% of the available arterial supply of LA to the mitogen 13-HODE.
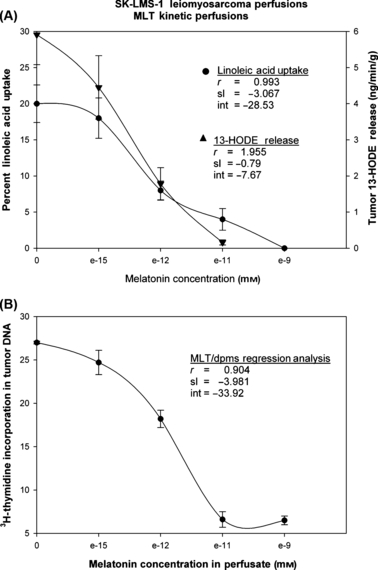
The kinetic effects of increasing concentrations in arterial blood plasma of melatonin on LA uptake (•) (as a percent of supply) and 13-HODE release () (upper panel) in tumor venous blood, and [3H]thymidine incorporation (•) (lower panel) in tissue-isolated SK-LMS-1 leiomyosarcoma tumor xenografts perfused in situ. Each point represents the mean value ± 1 S.D. (n = 3). Mean tumor weight was 5.64 ± 0.59 g.
During the perfusion of SK-LMS-1 xenografts in situ control steady-state rates of TFA and LA uptake and 13-HODE release to the tumor blood were completely inhibited for 60 min following the addition of 1 nm melatonin to the arterial blood at 36 min into the perfusion (Fig. 4). Subsequent addition of either melatonin-receptor antagonist S20928 (Fig. 4A), forskolin (Fig. 4B), PTX (Fig. 4C) or 8-Br-cAMP (Fig. 4D) at 86 min perfusion time resulted in a complete and sustained reversal, restoring the rates of TFA and LA uptake and 13-HODE formation to control values. The inhibition of TFA and LA uptake and 13-HODE release caused by melatonin treatment and its reversal by S20928, forskolin, PTX, and 8-Br-cAMP occurred rapidly. At an arterial flow rate to the tumor of about 0.127 mL/min these agents reach the tumor from the reservoir in about 8 min. Hence, inhibitions of TFA and LA uptake and 13-HODE release by melatonin must occurs beginning at about 45–50 min; reversal of the inhibitory effect by the aforementioned agents must begin to occur at about 95–100 min into the perfusion.
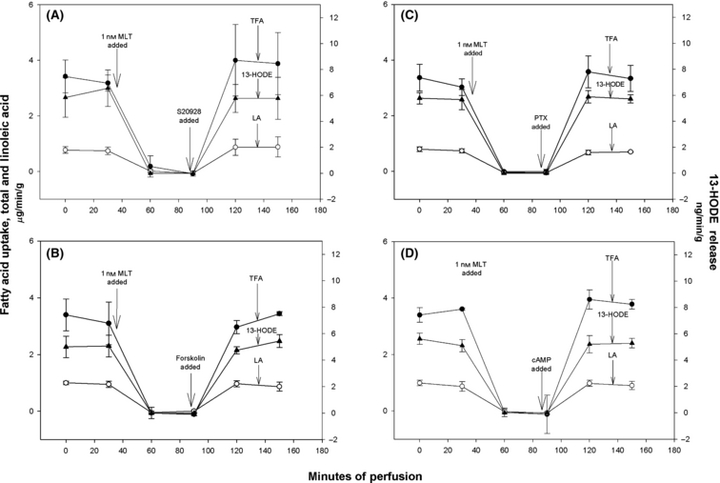
Kinetic changes in total FA and LA uptakes, and 13-HODE release in SK-LMS-1 human leiomyosarcoma perfused in situ after consecutive supplementation to the arterial blood perfusate of melatonin (1 nm) followed by either MT1/MT2 receptor antagonist S20928 (A), or forskolin (B), or PTX (C), or 8-Bromo-cyclic-AMP (D). Melatonin was added 6 min after the 30-min sample collections; S20928, or forskolin, or PTX, or 8-Bromo-cyclic-AMP was added 4-min prior to the 90-min sample collections. Each value depicted here represents the mean value ± 1 S.D. for three tumors. Mean tumor weight was 5.73 ± 0.59 g.
The effects of melatonin (1 nm), either alone in the whole blood perfusate or perfused together with S20928, PTX, forskolin, 8-Br-cAMP or 13-HODE for 150 min, on tumor cAMP content, TFA and LA uptake, 13-HODE release, incorporation of [3H]thymidine into DNA, and DNA content in human SK-LMS-1 xenografts were evaluated among the different treatment groups (Table 2). The inhibition of TFA and LA uptake by melatonin and the reversal by S20928, forskolin, PTX, and 8-Br-cAMP occurred rapidly. The supply rates of TFA and LA to tumors in situ reveals clear differences in the rates of TFA and LA uptake and associated downstream events by human LMS cancer xenografts perfused in situ with melatonin. Donor whole blood perfusates supplemented with 1 nm melatonin attenuated tumor cAMP levels by more than 40%, completely inhibited TFA and LA uptake and 13-HODE production, and resulted in over a 75% reduction in tumor [3H]thymidine incorporation into DNA and a 30% reduction in tumor DNA content. Addition of melatonin receptor antagonist S20928, forskolin, PTX, or 8-Br-cAMP to the melatonin-replete donor blood perfusate prevented the melatonin growth inhibitory effects. Under normal physiologic conditions the amount of 13-HODE release accounted for about 0.7% of the LA uptake by the LMS tumor. Interestingly, addition of exogenous 13-HODE to the melatonin-replete arterial blood increased tumor 13-HODE uptake, boosted tumor cAMP levels by nearly 40% and nearly doubled the rate of [3H]thymidine incorporation and tumor DNA content, but had no effect on melatonin-induced inhibitions of TFA or LA uptakes. Protein content of control and treated tumors were not significantly different (P > 0.001), and were 183.0 ± 20.0 and 183.0 ± 16.1 mg/g respectively.
| Treatmenta (3/group) | cAMP (nmol/g tissue) | Total fatty acid uptake (μg/min/g) | LA uptake (μg/min/g) | 13-HODE (ng/min/g) | [3H]thymidine incorporation (dpm/μg DNA) | DNA content (mg/g) | |
|---|---|---|---|---|---|---|---|
| Arterial supply | Venous output | ||||||
| Control | 1.07 ± 0.01 | 3.20 ± 1.56 | 0.81 ± 0.15 | 0 | 5.91 ± 0.83 | 27.0 ± 1.6 | 2.3 ± 0.1 |
| Melatonin | 0.62 ± 0.05b | 0b | 0b | 0 | 0b | 6.5 ± 0.5c | 1.6 ± 0.1c |
| Melatonin + S20928 | 0.89 ± 0.21 | 3.94 ± 1.20 | 0.86 ± 0.31 | 0 | 5.78 ± 1.35 | 30.1 ± 2.4 | 2.3 ± 0.1 |
| Melatonin + PTX | 1.06 ± 0.02 | 3.29 ± 0.26 | 0.80 ± 0.07 | 0 | 5.77 ± 0.57 | 27.0 ± 1.6 | 2.3 ± 0.1 |
| Melatonin + Forskolin | 0.94 ± 0.28 | 3.15 ± 0.47 | 0.92 ± 0.12 | 0 | 5.10 ± 0.35 | 28.8 ± 5.4 | 2.3 ± 0.1 |
| Melatonin + 8-Br-cAMP | 1.43 ± 0.16 | 3.86 ± 0.24 | 0.94 ± 0.13 | 0 | 5.71 ± 0.49 | 28.4 ± 2.0 | 2.3 ± 0.2 |
| Melatonin + 13-HODEd | 1.42 ± 0.16 | 0b | 0b | 149.7 ± 0.04 | 132.9 ± 12.5c | 49.2 ± 2.4c | 3.9 ± 0.1c |
- aThere were three animals (tumors) per group; (+S.D.) tumor weight = 5.7 + 0.6 g. bP < 0.05 versus control and all other melatonin-treated groups. cP < 0.05 versus control group. dTumors removed 11.3% of the 13-HODE.
Mean tumor weight at time of perfusions in situ was 5.64 ± 0.59 g (n = 54). Although spontaneous changes in flow rate sometimes occur [25, 26], these perfusions showed little or no alterations during the course of this study. Alterations in flow rate can affect the rates of uptake and release of nutrients [26, 27, 40, 41]. Perfusion of tumors with blood from donor rats yielded a venous flow rate of 0.122 ± 0.002 mL/min; arterial flow to the tumors was 0.127 ± 0.002 mL/min (n = 198 measurements). The hematocrit for arterial and venous samples, respectively, was 44.7 ± 2.2% and 46.7 ± 2.2% (n = 74); these values did not differ significantly between perfusions using fed donor blood, and was comparable to epithelial tumors.
Figure 5 depicts Western blots of phosphorylated (upper panel) and total (lower panel) forms of ERK 1/2, MEK, Akt and the housekeeping protein Dynamin II in human LMS tumors. The presence of melatonin (1 nm) in the arterial blood significantly decreased the amount of phosphorylated forms of ERK 1/2, MEK and Akt in situ. The addition of melatonin-receptor antagonist, PTX, forskolin, 8-Br-cAMP or 13-HODE resulted in activation of ERK 1/2, MEK and Akt that were similar to control levels, and correlated directly with 13-HODE release rates and [3H]thymidine incorporation (Table 2).
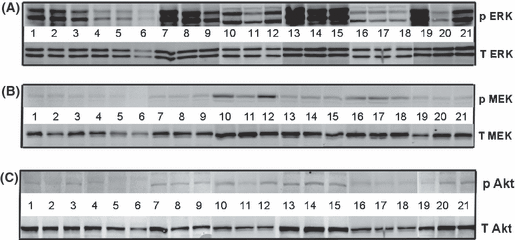
Western blot analysis for the expression of total (lower panels) and phosphorylated (upper panels) forms of ERK 1/2 (A), MEK (B) and Akt (C) in SK-LMS-1 leiomyosarcoma xenografts perfused in situ. Each lane depicts either ERK 1/2, MEK or Akt bands from one tumor perfused for 150 min. Perfusions were: lanes 1–3, no melatonin (MLT); lanes 4–6, 1 nm MLT; lanes 7–9, 1 nm S20928; lanes 10–12, 1 nm forskolin; lanes 13–15, 0.5 mg/mL PTX; lanes 16–18 1 mm 8-Br-cAMP; lanes 19–21, MLT + 13-HODE (2.1 μm).
Discussion
This investigation tested the postulate that both acute and long-term inhibitory effects of melatonin are exerted on LA transport and metabolism, and growth activity in tissue-isolated SK-LMS-1 human leiomyosarcoma xenografts via melatonin receptor-mediated inhibition of signal transduction activity. This is the first study to show that the long-term treatment of LMS-bearing nude female rats with a pharmacological dose of melatonin administered in the drinking water induced a brief period of growth stabilization followed by a rapid regression of a human cancer of any type in vivo. The fact that tumor cAMP levels, LA uptake, 13-HODE production, MEK, ERK 1/2 and Akt activation, DNA content and [3H]thymidine incorporation into DNA were substantially reduced in these regressed LMS xenografts suggests that melatonin-induced down-regulation of these tumor growth stimulatory signaling pathways was responsible for tumor stabilization and regression.
The acute perfusion experiments provided the first mechanistic evidence in vivo that melatonin, at a nocturnal physiological concentration in the arterial blood, was indeed responsible for suppressing a signal transduction pathway for cell proliferation in human LMS cancer xenografts. The initial steps in the signaling pathway were the binding of melatonin to a cell surface melatonin-receptor coupled inhibitory Gi-protein, inhibition of adenylate cyclase activity, and subsequently a reduction in intratumoral cAMP. Consequently the tumor transport of plasma TFA and LA decreased; LA was rate-limiting for the formation of the EGF-induced mitogen, 13-HODE. The phosphorylation of ERK 1/2, as well as MEK and Akt, was diminished and [3H]thymidine incorporation into tumor DNA decreased dramatically. Addition of PTX, which prevents the dissociation of the inhibitory Gi-protein heterodimer and blocks melatonin’s suppression of adenylate cyclase, reversed the melatonin inhibitory response. Furthermore, addition of either forskolin, an activator of adenylate cyclase, or 8-Br-cAMP, an activator of protein kinase A (PKA), also removed each of the inhibitions, providing compelling evidence that cAMP is involved early on, probably in LA transport. Exogenous 13-HODE, entering the pathway downstream of cAMP restored the activation state of ERK 1/2, MEK, and Akt and increased [3H]thymidine incorporation into tumor DNA, but not TFA or LA uptake.
In the Western diet, there exists an overabundance of LA, the increased consumption of which promotes tumorigenesis and growth in human cancer both in vitro [42] and in vivo [27–29, 37, 41]. Indeed, ample supplies of dietary LA are required for maximal growth of solid tumors in vivo [29, 41]. Studies from our laboratory using tissue-isolated rodent [27–29, 35, 36, 40, 41] and human [30–32, 36, 37] tumors showed that augmented arterial blood concentrations of LA, as a result of increased dietary intake or mobilization of host fat stores, are taken up by the tumors, resulting in a stimulation of growth and metabolism in vivo. As dietary concentrations and animal intake of LA increase, blood plasma levels rise in turn causing a stimulation of tumor growth rates due to higher levels of cAMP, ostensively inducing increased entry of LA into the tumor cells. Historically, one school of thought has argued for the simple diffusion of TFA into cells [43]. However, most recently a number of investigators have shown a role for facilitated transport [44] and activation of G-protein coupled systems by either short-chain [45] or long-chain [46–51] fatty acids associated with either the stimulation or inhibition of tumorigenesis in vitro. Some of the LA taken up by the tumor tissue is converted to 13-HODE, via 15-lipoxygenase-1 activity, which is the mitogenic signal for LA-dependent growth of these tumors. In the case of human vulvar LMS tumor xenografts in vivo, we demonstrated that S20928 blocks the ability of melatonin to inhibit tumor activity, indicating that these tumors express functional MT1 and/or MT2 melatonin receptors. Future studies will examine whether the selective MT2 antagonist, 4-phenyl-acetamidotetraline, is effective as well.
Previous studies [52, 53] demonstrated that 13-HODE enhances EGF-responsive mitogenesis through EGF receptor phosphorylation and tyrosine phosphorylation of important downstream signal transduction proteins such as ERK 1/2 and Akt. Most certainly there exist a host of receptors [45, 49, 50, 54] and signaling pathways involved in cancer progression, invasion and metastasis [see 55 for review] and ‘cross-talk’ between associated receptors is not uncommon, resulting in the interaction of various signaling pathways. While an examination of the many other known receptors and signaling pathways is beyond the scope of the principal signaling pathways involved here, preliminary evidence provided here suggests that possibly the serine–threonine kinase Akt pathway is also involved (Fig. 5). This kinase is directly linked to the phosphorylation and regulation of a number of targets including other kinases, like ERK 1/2 and MEK, as well as transcription factors and regulatory molecules associated with tumor cell proliferation and apoptosis [55]. Studies now underway in our laboratory are examining several human tumors in our laboratory animal model system and other associated receptor/pathways including Ras, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), phosphoinositide 3-kinase (PI(3)K), and mammalian target of rapamycin (mTOR).
Melatonin plays a critical role in a number of physiological and pathophysiological processes. These include circadian rhythm regulation [56, 57], seasonal reproduction [58], immune function [59] and carcinogenesis [60, 61]. In experimental models of cancer, in some cases melatonin inhibits tumorigenesis in a circadian time-dependent manner [12, 60–64]. A few studies have suggested that melatonin may exert a modulatory role on normal lipid metabolism [58, 63, 65–68]. An early observation in relation to tumor growth suggested that melatonin’s oncostatic effect was accentuated in diet-restricted animals, as compared with animals provided free access food [63]. Subsequently, we demonstrated that suppression of melatonin production increased rates of tumor growth, LA uptake, and 13-HODE formation in rodent hepatoma 7288CTC [29, 35, 36], human breast cancer [30, 32, 36] and in human head/neck cancer [37, 66].
These investigations provide, for the first time, compelling evidence for the hypothesis that nocturnal physiologic levels of melatonin markedly, and dose-dependently, inhibited tumor proliferative activity in vivo in this rare, aggressive and difficult-to-treat, mesenchymally-derived human sarcoma. Similar to what we have previously reported for epithelially-derived cancers, the present results indicate that melatonin suppressed LA-dependent proliferative activity in human LMS via an inhibitory G-protein-coupled, melatonin receptor-mediated signal transduction pathway. Furthermore, it is apparent that the long-term treatment with a pharmacological dose of melatonin administered in the drinking water caused the stabilization followed by the rapid regression of this tumor via the suppression of these signal transduction mechanisms. Additional studies are warranted to further examine long-term, potentially beneficial effects of melatonin on the control of LA-uptake in human cancer that could lead to new approaches for therapeutic intervention and/or cancer prevention in LMS in particular, and other cancers in general.
Acknowledgments
This work was supported by the National Leiomyosarcoma Foundation. The authors thank the Institute de Recherches Internationales Servier (Courbevoie Cedex, France) for the generous gift of the melatonin receptor antagonist S20928, and Mr. Darin T. Lynch for his assistance with the laboratory animals and the initial preparation of the tumor samples.




