Exogenous melatonin positively influences follicular dynamics, oocyte developmental competence and blastocyst output in a goat model
Abstract
Abstract: The role of melatonin in modulating mammalian reproduction is of particular interest; however, its effects on ovarian follicles and their oocytes still remain to be characterized. This study determined the influence of melatonin treatment on follicular growth patterns and on in vitro oocyte developmental competence. In a first experiment, the effects of melatonin supplementation on follicular dynamics were evaluated using daily transrectal ultrasonographies for 21 days, in 7 multiparous Sarda goats receiving a subcutaneous implant of 18 mg of melatonin and in 5 control untreated does. Melatonin caused more follicular waves (5.2 ± 0.2 versus 4 ± 0.3; P < 0.05) as the waves were shortened at around 2 days when compared with the non-melatonin treated control goats (P < 0.001). Oocyte developmental competence was evaluated in a second experiment by applying procedures for in vitro embryo production. There were no significant differences in the total number of oocytes obtained from 6 control (n = 192) and 7 melatonin-treated (n = 265) goats given follicle stimulating hormone to induce follicular development. Differences in oocyte developmental competence between the two groups became evident after in vitro fertilization and culture; melatonin increased the rate of cleaved oocytes in comparison with control animals (82.5 versus 63.4%; P < 0.001), advanced timing of embryo development and enhanced blastocyst output (31.5 versus 16.3%; P < 0.01). However, blastocyst quality, as evaluated by cryotolerance and gene expression analysis, was not found to be different between the groups. In conclusion, in vivo melatonin treatment is beneficial for increasing ovarian follicle turnover and improving oocyte developmental competence and kinetics of the blastocyst.
Introduction
Reproductive activity is governed, in most of the animal species and to some degree even in humans, by seasonal variations in the frequency of conception, although seasonality in humans is an open debate. Some researches indicate that humans have the capacity to reproduce continuously, unless inhibited by environmental influences such as nutrition, temperature or photoperiod [1]. Photoperiodism via the circadian melatonin rhythm registers changes in the annual cycle of day-length and this signal is used to synchronize seasonal reproductive fluctuations [2].
Melatonin also acts act directly on the ovary [3, 4]. Melatonin receptors have been found in the ovaries of rats, mice, and humans [5–7]. High levels of melatonin, influenced by seasonal variations [8], has been found in human follicular fluid in concentrations which are threefold higher than serum levels [9, 10]. Although it has been assumed that melatonin in ovarian follicles is derived from the general circulation, it may also be synthesized in the ovary as melatonin precursor and synthesizing enzyme activities have been detected both in rat [11] and human ovaries [12]. In vitro studies have demonstrated that melatonin stimulates progesterone production by ovarian granulosa cells in several mammalian species and acts synergistically with human chorionic gonadotrophin to increase the production of progesterone [8, 13]. Finally, melatonin has also been successfully tested for promoting in vitro embryo development in mice [14], buffaloes [15], heifers [16], and sows [17, 18]. Thus, melatonin treatment may be useful for infertility treatments [19].
Currently, many aspects of the in vivo actions of melatonin treatment on follicles and their oocytes still remain to be characterized. Therefore, this study was undertaken to examine the effects of melatonin on ovarian follicular growth patterns and on in vitro oocyte developmental competence in anestrous goats. The screening of follicular dynamics by real-time ultrasonography [20, 21] and the production of in vitro embryos [22, 23] are well established for this species and we used animals in their anestrous season so major changes in steroid and gonadotrophin levels could be avoided. Effects from exogenous melatonin on blastocyst quality, evaluated in terms of cryotolerance, and expression of a panel of genes related to developmental ability in pre-implantation embryos were also investigated.
Materials and methods
Animals and experimental design
All the animals used were adult multiparous Sarda goats housed outdoors with indoor access, and fed with a live-weight maintenance ration, at the experimental facilities of the Department of Animal Biology at the University of Sassari, Italy (latitude 40º43′N). These facilities meet the requirements of the European Union for Scientific Procedure Establishments. The experiments were performed during March and May, the seasonal anestrous period (late January–late August) described for this breed at this latitude.
Two consecutive trials were conducted to determine the effects of melatonin treatment during the seasonal anestrous season on follicular dynamics and oocyte developmental competence. In both experiments, does were randomly assigned to either a group receiving a subcutaneous melatonin implant (18 mg melatonin, Melovine®, CEVA VETEM S.p.A., Milano, Italy) at the base of the ear or a control group not receiving implants.
In the first experiment, the effects of melatonin supplementation on follicular dynamics was evaluated in 7 melatonin-treated and 5 control does. Follicular dynamics were evaluated by daily transrectal ultrasonographies performed from day 7 (day 0 = day of insertion of the melatonin implants) to day 27 (i.e. 21 days, the estimated duration of a cycle during seasonal breeding).
The second experiment arose from the results of the first trial, and was conducted to determine possible effects of in vivo melatonin supplementation on oocyte quality, as evaluated by its developmental competence. Follicular development was stimulated in 7 melatonin-treated and 6 control goats by the administration of 175 I.U. of FSH (Folltropin™, Bioniche Animal Health, Bio 98, Milano, Italy) administered, in 6 equal doses every 12 hr, from days 28–30 after insertion of the melatonin implants. At day 31, 12 hr after the last FSH injection, ovaries were collected by ovariectomy and processed for oocyte recovery and in vitro embryo production. Thereafter, developmental competence was assessed in terms of blastocysts output and cryotolerance after vitrification and thawing. Finally, a panel of gene transcripts whose expression has been proven to be correlated to embryo developmental competence [24] was determined, by real-time PCR, in in vitro produced blastocyst.
Ultrasonographic evaluation of follicle dynamics
Ovaries were examined by transrectal ultrasonography using a real-time B-mode scanner (Aloka SSD 500, Aloka Co., Tokyo, Japan) fitted to a 7.5 MHz linear-array probe. Scanning was performed as previously validated in our laboratory for goats [20, 25]. In brief, observations were conducted with the goat placed in dorsal recumbence on a metallic cradle as used for laparoscopy. After introducing a hydrosoluble contact gel into the rectum, the transducer was introduced perpendicularly to the abdomen wall. When the urinary bladder was surpassed and the uterine horns were located, the probe was rotated laterally 90º clockwise and 180º counter-clockwise to observe both ovaries and their structures. Each ovary was scanned several times from different angles to image all follicles ≥3 mm. The largest diameter of each of these follicles was measured and its position was recorded on a diagram of each ovary.
Oocyte in vitro maturation, fertilization and culture
Ovaries and, thereafter, oocytes and embryos derived from the two different groups were kept separated throughout the procedures. All reagents and media were from Chemical Co. (St. Louis, MO, USA) unless otherwise specified. Ovaries were placed in Dulbecco’s PBS at a temperature between 25°C and 35°C. After washing in fresh medium, ovaries were sliced using a micro-blade and the follicle content was released in medium TCM199 (with Earle’s salts and bicarbonate) supplemented with 25 mmol Hepes, penicillin and streptomycin and 0.1% (w/v) of polyvinyl alcohol (PVA). The cumulus–oocyte complexes (COCs) that presented 4–10 layers of granulosa cells and oocytes with a uniform cytoplasm, homogenous distribution of lipid droplets in the cytoplasm and with an outer diameter of about 90 μm (mean) were selected for the experimental procedure. The selected COCs, after three washes in the same fresh medium, were in vitro matured in TCM199 supplemented with 10% estrous goat serum, 10 μL/mL of FSH/LH and 100 μm of cysteamine. COCs were put, in groups of 30–35, in 600 μL of the maturation medium in a four-well Petri dish (Nunclon, Nalgene Nunc International, Denmark), layered with 300 μL mineral oil and cultured for 24 hr in 5% CO2 in air at 39°C.
After maturation, the COCs were partially stripped of the granulosa cells and fertilized in vitro at 39°C and 5% CO2, 5% O2 and 90% N2 atmosphere in four-well Petri dishes (Nunclon). Frozen-thawed spermatozoa from the same ejaculate of one buck were used across all experimental procedures. Semen has been previously cryopreserved in Tris-based extender (Tris 375 mm, citric acid 124 mm, glucose 41 mm) supplemented with egg yolk 20% (pH 7; osmolality 375 mOsm/kg) and glycerol (4%); it was cooled to 4°C, frozen in 0.25 mL pellets on dry ice and then plunged into liquid nitrogen. Thawing was carried out by plunging a sterilized glass falcon tube containing the pellet in a 39°C water bath for 20 s. Synthetic oviduct fluid (SOF) containing 3% bovine serum albumin (BSA-fraction V) supplemented with 25 mm Hepes (sperm-SOF) was used for sperm preparation. For IVF, SOF medium was supplemented with 10% estrous goat serum, 20 μg/mL heparin and 1 μg/mL hipotaurine (IVF-SOF). Percoll gradients were prepared as described by Rosenkrans et al. [26]. In brief, 100% Percoll solution was mixed with a 10× salt solution (NaCl 2.889 g; KCl 0.238 g; KH2PO4 0.116 g; CaCl2 0.112 g; Hepes 0.163 g; 50 mL of milli-Q water) to form 90% Percoll solution. A 45% Percoll solution was prepared from this by addition of an equal volume of sperm-SOF. The gradient was formed by pipetting 1 mL of 90% Percoll solution into a 15 mL conical tube and then overlaying it with 1 mL of 45% Percoll solution.
Frozen-thawed semen was placed onto the top of the 45% layer and then centrifuged at 800 g at room temperature for 15 min through the gradient. After removal of supernatant, the resulting pellet was transferred into in a sterilized glass conical tube below 1 mL of warmed IVF-SOF and incubated at 39°C in a humidified atmosphere at 5% CO2 in air for 15 min. Swim-up derived motile spermatozoa were diluted in IVF-SOF at a 1 × 106 spermatozoa/mL final concentration and incubated for 45 min at 39°C under 5% CO2, 5% O2 and 90% N2. For IVF, spermatozoa were co-incubated under mineral oil in four wells Petri dishes with a mean of 30 matured oocytes/well in the same atmosphere condition.
After 26 hr, presumptive zygotes were mechanically denuded of their cumulus cells and cultured in four-well Petri dishes containing SOF + essential and non-essential amino acids at oviduct concentration + 0.4% BSA under mineral oil in maximum humidified atmosphere with 5% CO2, 5% O2, 90% N2 to blastocyst stage. At 26 and 42 hr post-insemination the number of cleaved oocytes, showing two distinct blastomeres, was recorded. Thereafter, culture dishes were observed daily starting from the sixth day of culture and oocyte cleavage and evolution to blastocysts were recorded. As early cleavage is considered as a marker of embryo competence [27, 28], we analyzed the kinetic of the first embryonic division in the two experimental groups.
Blastocyst vitrification and warming
Vitrification and warming media were prepared using PBS supplemented with 20% (v/v) FCS as base media. Embryos were vitrified according to a simple method developed in our laboratory [29]. Briefly, blastocysts were put into 200 μL drops of 1.4 m glycerol for 5 min, then into 200 μL drops of 1.4 m glycerol and 3.6 m ethylene glycol for 5 min before being transferred into a 15 μL column of 3.4 m glycerol and 4.6 m ethylene glycol, and loaded into the center of 0.25 mL plastic insemination straws using a fine glass capillary pipette. In the straws, the embryos and vitrification media were separated from two columns of 0.5 m sucrose solution. After sealing, the straws were transferred directly into LN2 and kept in it.
For warming to a biological temperature, the straws were transferred from LN2 into a water bath at 35°C for 10 s. The content of each straw was expelled into a Petri dish and stirred gently to facilitate the mixture of the two solutions. The embryos were retrieved and transferred into 200 μL drops of 0.25 m sucrose solution supplemented with 20% FCS or 0.1% PVA for 3 min to allow for removal of intracellular cryoprotectants. Embryos were held 10 min in corresponding media of PBS containing 20% FCS or 0.1% PVA for rehydration and equilibration.
For determining in vitro viability of the embryos, these were cultured in TCM199 with 10% FCS in humidified atmosphere with 5% CO2 in air at 39°C. To assess differences in embryo quality between the two groups, we determined blastocyst cryotolerance after vitrification and warming, in terms of timing of re-expansion within 8 hr of in vitro post-warming culture. The embryos that re-expanded the blastocoelic cavity were considered to be viable [28], as this parameter represents a reliable indicator of in vitro produced blastocysts quality [24]. Thereafter, the embryos were examined at 12 hr intervals for 60 hr.
Gene expression analysis
The mRNA samples were prepared from pools of three vitrified/warmed re-expanded blastocyst. Re-expanded embryos were removed from culture, added to 2 mL of diethylpyrocarbonate (DEPC)-treated water, snap frozen in liquid nitrogen, and stored at −80°C until analysis. The poly(A) RNA was isolated with oligo(dT)25 attached magnetic beads (Dynal A.S. Oslo, Norway) following manufacturer’s instruction.
Ten μL of Lysis Buffer (100 mm Tris-HCl pH 7.5, 500 mm LiCl, 10 mm EDTA, 1% LiDS, 5 mm DTT) were immediately added to each sample. Samples were vortexed, briefly centrifugated, incubated at room temperature for 10 min and added with 10 μL of Dynabeads oligo (dT)25. Tubes were incubated for 5 min at 25°C, the tubes were shaken gently and put into a Dynal magnetic separator for 2 min. After removal of the supernatant, poly(A)+ RNAs were washed once with 40 μL of Washing Buffer A (10 mm TrisHCl pH 7.5, 0.15 m LiCl, 1 mm EDTA, 0.1% LiDS) and three times with Washing Buffer B (10 mm TrisHCl pH 7.5, 0.15 m LiCl, 1 mm EDTA). Poly(A)+ RNAs were then eluted from the magnetic beads by incubation in 11 μL of DEPC-treated water at 65°C for 2 min, and aliquots were immediately used for Reverse Transcription Polymerase Chain Reaction (RT-PCR).
Reverse-transcription reactions were performed in a final volume of 20 μL consisting of 50 mm Tris-HCl (pH 8.3), 75 mm KCl, 3 mm MgCl2, 5 mm DTT, 1 mm dNTPs, 2.5 μm of Random Hexamer primers (Invitrogen, Carlsbad, CA, USA), 20 U of RNase OUTTM (Invitrogen) and 100 U of SuperScriptTM III RT (Invitrogen). The reaction tubes were kept at 25°C for 10 min, then at 42°C for 1 hr and finally at 70°C for 15 min to inactivate the reaction. A tube without RNA and one with RNA, but without reverse transcriptase, were used as negative controls.
Quantitative real time-polymerase chain reaction
The quantification of β-actin, heat shock protein 90β (HSP90 β), cyclin b1 (Ccnd1), Na/K-ATPase (Atp1a1), Type I cadherin (E-Cad) and aquaporin 3 (Aqp3) transcripts in melatonin-treated (n = 4) and control (n = 5) embryos was carried out by Real-Time PCR in a BioRad iCycler™ (Bio-Rad, Hercules, CA, USA). PCRs were performed in 25 μL reaction volume containing 12.5 μL 2× Platinum SYBR Green qPCR Super Mix UDG (Invitrogen), 0.025 nm Fluorescein Reference Standard (Molecular Probes – Invitrogen Detection Technologies, Leiden, the Netherlands), 200 nm of each primer and cDNA equivalent to 0.1 embryo.
The PCR protocol involved two incubation steps (50°C for 5 min and 95°C for 2 min) followed by 40 cycles of amplification program [95°C for 15 s, gene specific annealing temperature (Table 1) for 30 s, 72°C for 30 s], a melting curve program (65°C–95°C, starting the fluorescence acquisition at 65°C and taking measurements every 10 s interval, until the temperature reached 95°C) and finally a cooling step to 4°C. Fluorescence data were acquired during the 72°C extension steps. The PCR products were then analyzed by generating a melting curve to check the specificity and identity of the amplification product. To minimize handling variation, samples and standard curves were run on the same plate, using a PCR master mix containing all reaction components apart from the sample.
| Gene | GenBank accession number | Sequence | Annealing temperature | Location | Size (bps) |
|---|---|---|---|---|---|
| HSP90β | AB072369 | 5′tggagatcaaccctgacca3′5′cttctcgcttgaggatccc3′ | 56°C | 1959–2101 | 143 |
| Ccnd1 | L26548 | 5′cagtgtatgacaggtaatgc3′5′cgtagtccagcatagttagt3′ | 56°C | 1089–1222 | 134 |
| β-actin | NM_001009784 | 5′ttcctgggtatggatcctg3′5′ggtgatctccttctgcatcc3′ | 60°C | 532–678 | 162 |
| Atp1a1 | X02813 | 5′gctgacttggtcatctgcaa3′5′cattccagggcagtaggaaa3′ | 58°C | 3052–3180 | 129 |
| E-Cad | NM_001002763 | 5′tgtgactgtgatgggatcgt3′5′acccttctcctccgaacaag3′ | 58°C | 2056–2207 | 155 |
| Aqp3 | AF123316 | 5′gggtgcccattgtctctcc3′5′acgaggagaatgtgaagttg3′ | 60°C | 500–618 | 119 |
The sizes of the PCR products were further confirmed by gel electrophoresis on a standard ethidium bromide stained 2% agarose gel and visualized by exposure to ultraviolet light. The PCR products were sequenced (Applied Biosystems, Model 3130 xl Genetic Analyzer, Foster City, CA 94404, USA) after purification and sequences were confirmed by Basic Local Alignment Search Tool (BLAST).
Statistical analyses
Ultrasonographic data were summarized to characterize patterns of ovarian follicular development during the period of study. First, all follicles present on the ovaries were classified by their largest diameter in four categories: total follicles (≥3 mm), small (3–3.4 mm), medium (3.5–4.4 mm) and large (≥4.5 mm). Thereafter, data on individual growth of follicles were used to characterize follicular waves on the basis of (a) wave onset (emergence): day in which follicles were firstly detected at 3 mm; (b) growth phase (length): defined as the time taken by a single follicle to grow from 3 mm to its maximum diameter; (c) regression phase: the time taken by a single follicle to regress from its maximum size until the day that it reach its smallest size; (d) wave end: day when the dominant follicle ending its regressing phase; (e) wave duration (total length): time taken by a single follicle to grow from 3 mm to its maximum diameter and to completely regress to its minimum size. Effects of day and wave of follicular development on individual characteristics of dominant follicle and on number and size of remaining follicles throughout the study were assessed by ANOVA.
The chi-square test, or Fisher exact test when appropriate, was used to determine differences on oocyte developmental competence and blastocysts re-expansion and hatching after vitrification/warming procedures.
The relative quantification of the target genes expression was performed with the 2-ddCt method [30] normalizing the target genes expression with the transcripts levels of the endogenous β-actin gene [31]. After testing for normality and equal variance using respectively the Kolmogorov–Smirnov and Levene tests, the transcript data were analyzed with ANOVA.
Statistical analysis was performed using the statistical software program Statgraphic Centurion XV (version 15.2.06 for Windows; StatPoint, Inc., Herndon, VA, USA) and a probability of P ≤ 0.05 was considered to be the minimum level of significance. All results are expressed as mean ± S.E.M.
Results
The ultrasonographic study indicated that both the melatonin-treated and control ovaries showed a well-defined wave-like pattern of follicle dynamics (Fig. 1); however, there were significant differences between groups in the characteristics of the follicular waves (Table 2). Melatonin treatment caused more waves during the period of study (5.2 ± 0.2 versus 4 ± 0.3; P < 0.05) because waves were shortened at around 2 days when compared with the controls (P < 0.001). These differences in total length of the waves were mainly caused by a more rapid growth phase of the dominant follicles (P < 0.005), as differences in regression phase length were not statistically significant. On the other hand, the maximum diameters of the dominant follicles of each wave were similar between groups.
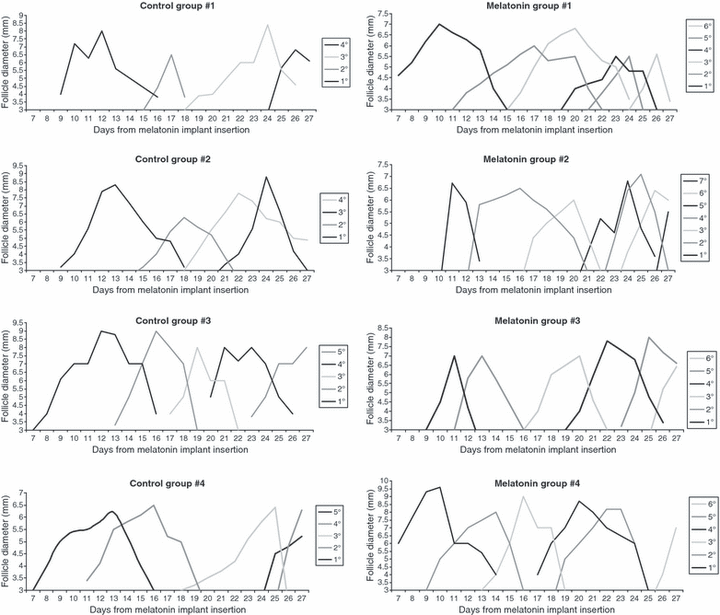
Wave-like pattern of follicle dynamics in Sarda goats treated with a subcutaneous implant of melatonin (melatonin treatment) or untreated (control) during seasonal anoestrous.
| CTR (mean ± S.E.) | MEL (mean ± S.E.) | |
|---|---|---|
| Number of follicular waves | 4 ± 0.3a | 5.2 ± 0.2b |
| Follicular wave length (days) | 8.7 ± 0.4c | 6.9 ± 0.3d |
| Maximum diameter reached by FD (mm) | 7.4 ± 0.2 | 7 ± 0.2 |
| DF growth rates (mm/day) | 0.9 ± 0.1 | 1.1 ± 0.1 |
| DF regression rates (mm/day) | 1.3 ± 0.2 | 1.4 ± 0.1 |
| DF growth phase (days) | 5.1 ± 0.3e | 4 ± 0.2f |
| DF regression phase (days) | 3.6 ± 0.3 | 2.9 ± 0.2 |
- In the same row, different superscripts indicate statistical difference (ANOVA a ≠ b: P < 0.05; c ≠ d: P = 0.001; e ≠ f: P = 0.005).
- DF, dominant follicles.
There were no significant differences in the total number of oocytes obtained from control (n = 192) and melatonin-treated (n = 265) animals (Table 3). Differences in oocyte developmental competence between the two groups became evident after in vitro fertilization and culture; melatonin increased the rate of cleaved oocytes in comparison with the controls (82.5 versus 63.4 %, respectively; P < 0.001) and advanced timing of cleavage. Oocytes collected from melatonin-treated does underwent cleavage within 26 hr of post-fertilization culture at a higher rate than in the controls (24.1 versus 11.5%, respectively, P < 0.05). Thereafter, such faster developmental rate was also observed in the blastocysts rate; after melatonin treatment a higher percentage of embryos had developed to blastocyst stage at day 7 post-fertilization (19.4 versus 8.6%; P < 0.05). Finally, total blastocysts output was significantly higher after melatonin treatment than in controls (31.5 versus 16.3; P < 0.01), showing a higher in vitro developmental competence in oocytes collected from melatonin-treated goats.
| Follicles (mean ± S.E.) | Oocytes | Embryos (%)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IVM | Fertilized | Cleavage | Blastocyst yield | ||||||||
| 26° hpf | 42° hpf | TOT | 6° dpf | 7° dpf | 8° dpf | TOT | |||||
| CTR | 1 | 30 | 17 | 16 | 1 | 9 | 10 | 1 | 0 | 0 | 1 |
| 2 | 25 | 15 | 13 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | |
| 3 | 58 | 29 | 25 | 4 | 14 | 18 | 1 | 4 | 1 | 6 | |
| 4 | 36 | 27 | 23 | 4 | 12 | 16 | 2 | 4 | 2 | 8 | |
| 5 | 23 | 9 | 9 | 1 | 5 | 6 | 0 | 1 | 0 | 1 | |
| 6 | 20 | 20 | 18 | 2 | 12 | 14 | 0 | 0 | 0 | 0 | |
| TOT | 192 (32 ± 6.4) | 117 | 104 | 12a (11.5) | 54 (51.9) | 66c (63.4) | 4 (3.8) | 9a (8.6) | 4 (3.8) | 17e (16.3) | |
| MEL | 1 | 38 | 17 | 15 | 3 | 10 | 13 | 1 | 3 | 2 | 6 |
| 2 | 39 | 29 | 19 | 5 | 9 | 14 | 0 | 3 | 0 | 3 | |
| 3 | 25 | 17 | 14 | 5 | 8 | 13 | 2 | 6 | 1 | 9 | |
| 4 | 73 | 42 | 39 | 10 | 20 | 30 | 1 | 13 | 2 | 16 | |
| 5 | 19 | 23 | 18 | 2 | 12 | 14 | 0 | 1 | 1 | 2 | |
| 6 | 34 | 27 | 26 | 7 | 17 | 24 | 2 | 3 | 1 | 6 | |
| 7 | 37 | 24 | 18 | 4 | 11 | 15 | 2 | 0 | 3 | 5 | |
| TOT | 265 (37 ± 6) | 179 | 149 | 36b (24.1) | 87 (58.3) | 123d (82.5) | 8 (5.3) | 29b (19.4) | 10 (6.7) | 47f (31.5) | |
- *Percentages are calculated on fertilized oocytes.
- Within the same column, different superscript indicate statistical difference (chi-square test: a ≠ b: P < 0.05; c ≠ d: P < 0.001; e ≠ f: P < 0.01).
- hpf, hours of culture post-fertilization; dpf, days of culture post-fertilization.
Blastocyst obtained from melatonin-treated and control animals did not differ in terms of re-expansion rates. For the melatonin group, of 47 vitrified/warmed blastocysts, 19 (40.4%) re-expanded within 8 hr of in vitro culture and 11 re-expanded in the following 8 hr (17%). On the other hand, for controls, of 17 vitrified/warmed blastocysts, 5 (29.4%) re-expanded within 8 hr of in vitro culture and 5 re-expanded in the following 8 hr (17%).
Gene expression was quantified for a panel of genes involved in important developmental processes such as blastocoel formation aquaporin 3, Aqp3: Na/K ATPase, Atp1a1 [32–34], cell cycle control Ccnd1: [35], chaperon-like activity (90-kDa heat shock protein β, Hsp90β: [36], and cytoskeletal constitution (E caderin, E-cad; actin, Act1). The transcript relative quantity was normalized on the basis of Act 1 mRNA levels. We detected transcripts of all the analyzed genes in both experimental groups. The normalized quantity of Aqp3, Atp1a1, Ccnd1, Hsp90β, and E-cad did not differ between the two groups of blastocysts (Fig. 2).
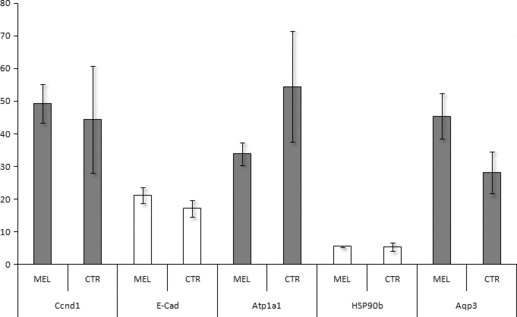
Relative mRNA expression of Ccnd1, E-Cad, Atp1a1, HSP90b and Aqp3 in vitrified/warmed and in vitro cultured re-expanded blastocysts. Embryos were in vitro produced by maturation, fertilization and culture of oocytes derived form Sarda goats treated with a subcutaneous implant of melatonin (MEL) or untreated (CTR) during seasonal anoestrous. Relative abundance values are expressed as Î6Î6CP and show the mean value  ± SEM of the three replicates for each group.
Discussion
Three main conclusions can be drawn from our study. First, we are reporting for the first time that melatonin treatment affects follicle features; it modifies the wave growth pattern by increasing the turnover of dominant follicles. Second, the oocytes inside these follicles have increased quality when compared with control untreated females and, third, developmental competence to the blastocyst stage is also improved.
The ultrasonographic assessment of follicle dynamics showed that melatonin treatment shortened follicular waves by reducing the period of emergence of the dominant follicle. In previous studies, melatonin has been proposed to regulate the pulsatile secretion of GnRH from the hypothalamus, thereby influencing LH secretion [1] and favoring follicle development. Despite intensive research during the past two decades, however, the precise targets for melatonin action on the control of reproduction remain to be identified. The effect of melatonin on GnRH secretion appears not to be related to a direct effect on GnRH neurons [37, 38]; melatonin might regulate GnRH secretion via two complementary mechanisms: a change in the steroid negative feedback on GnRH release and a steroid-independent modulation of GnRH secretion [39]. Recent data suggest that kisspeptin may be the missing link between melatonin and the hypothalamic-pituitary-gonadal axis, and that in seasonal breeders melatonin could act on the KiSS-1 cells to modulate reproductive activity [for review see: 40]. Considering that kisspeptins potently elicits LH/FSH secretion [41, 42], the activation of this endocrine pathway may have created a more suitable hormonal milieu for follicle growth, and hence oocyte maturation, in melatonin-treated does. In anestrus, LH secretion is diminished, as opposed to the breeding season when the pulsatile release of LH is at frequencies and amplitudes appropriate for controlling follicle dynamics [43]. Furthermore, the presence of an active growing follicle may indirectly support effects of melatonin on follicular function, and therefore on steroid secretion and function, as suggested in previous studies [39, 44]. At the same time, we cannot aside off evidences addressing direct effects of melatonin on the ovary [3, 4, 9, 10].
Improvement of follicle features by administration of melatonin was followed thereafter by improvement of the quality of their oocytes, confirming recent data [19]. In the current study, cleavage rates and total blastocysts output were significantly higher in melatonin-treated does than in control ones (31.5 versus 16.3; P < 0.01). Data concerning in vitro embryo production are limited for anestrous goats; a single study reports that the overall in vitro blastocyst development rates range from 20% to 24% in anestrous does treated with FSH [45]. Studies regarding in vivo embryo production have shown that melatonin treatment of embryo donor increases fertility, embryo quality and prolificacy after ovarian superovulation protocols [46–48]. Our results, in contrast to previous studies in sheep [47], also indicate that melatonin treatment, besides inducing higher developmental rates, advances timing of formation of zygotes and blastocysts in vitro. Early cleavage is considered as a marker of embryo competence for review see: [49]; fast developing blastocysts are those with the higher quality and cryotolerance [28]. On the other hand, the quality of the embryos reaching the blastocyst stage, as evaluated by cryotolerance and gene expression analysis [24], was not found to be different in melatonin and control group.
Melatonin effects on oocyte and blastocyst output may be related, like effects on follicle turnover, to both systemic and ovarian factors. Systemic effects, as detailed above, would be regulated by LH secretion [1, 40]. Oussaid et al. [50] showed that the temporary suppression of LH affects oocyte developmental competence in cyclic ewes; a more prolonged suppression of LH, such as during anestrus, may show even more detrimental effects on developmental competence of oocytes. In a previous study, we reported that the injection of a single dose of GnRH antagonist leads to weakness in oocyte maturation and limitations in their developmental competence; such effect being likely because of LH release deficiencies [51]. In a study performed in humans it was found that reduced preovulatory levels of LH, equivalent to the scenario taking place in anestrous does, are associated with impaired in vitro fertilization of the oocytes [52]. Melatonin treatment, through modifications in LH pulse frequency [1], may have contributed in creating a more suitable follicular environment as LH is the primary drive for ovarian steroid secretion and, thus, LH is essential for final oocyte maturation [53].
The enhanced oocyte quality obtained in melatonin-treated does may be also linked to a local, intrafollicular effect. Melatonin enters all tissues. High levels of melatonin have been found in human follicular fluid [9, 10] and such concentrations were found to be higher in mature follicles than in small atretic follicles [3]. The main role of melatonin within the follicle, as suggested by Tamura et al. [19], may be to act as a free radical scavenger which would reduce oocyte DNA damage. The antioxidant properties of melatonin have been extensively studied [54, 55] and the indole may have utility as a potential disease preventing agent [54, 56, 57]. The scavenger activity of melatonin or of its metabolites may account for its beneficial effect on in vitro embryo development, as found in bovine embryos under high oxygen environment [16]. Different authors suggest that the reduction of reactive oxygen species (ROS) could be an aspect of the mechanism by which melatonin exerts its beneficial effects during oocyte in vitro maturation [15, 18]. Detailed studies on the protective effects of melatonin against oxidative stress have found that such effects are particularly effective in the mitochondria, as it seemingly targets these organelles [58]. Accumulated oxidative damage to mitochondria may both decrease the number of functionally intact mitochondria and hence ATP levels and increase the production of ROS by the electron transport chain. This, in turn, may reduce the ratio of intracellular GSH/GSSG which contributes to perturbations of intracellular Ca2++ homeostasis [59]. All these variables may have effects on oocyte cytoskeletal fibers, balance of protein kinase/phosphatase activity, and synthesis, processing and degradation of proteins and RNA and DNA [60]. However, in this study, systemic and ovarian effects of melatonin may have acted synergistically. The enhanced LH pulse frequency, supported by faster growth phase of the dominant follicle, and the provided oxidative stress protection to the oocyte may have contributed to the higher oocyte quality. Moreover, we can also speculate that melatonin has a direct beneficial effect on follicle and oocyte maturation, considering the presence of melatonin receptors on granulosa cells [8, 13].
In conclusion, in vivo melatonin treatment prior to oocyte collection hastened dominant follicle growth phase, thus increasing ovarian follicle turnover. At the same time, although not affecting ovarian response to FSH, melatonin improved oocyte quality as evaluated by in vitro developmental kinetics and blastocyst output. Thus, this study may establish a basis for new protocols for in vitro embryo production; the key factor limiting the proportion of oocytes that develop to blastocysts within an in vitro embryo production system is the intrinsic quality of the oocyte itself. Melatonin treatments may provide a useful tool in improving the developmental competence of poor quality oocytes.
Author contributions
FB participated in the design of the study, performed the statistical analysis and drafted the manuscript. GGL participated in drafting the manuscript and supervised the gene expression analysis. SS and MM carried on the experiments on oocyte developmental competence and embryo cryopreservation. AS and ICS carried on the ovarian ultrasonographic scanning to evaluate follicular growth pattern. ICS also contributed to the performance of the statistical analysis. VS and DB performed the gene expression analysis. AGB conceptualized the idea, helped in drafting the manuscript and revising it critically for important intellectual content. SN conceived the study, participated in its design and coordination, and helped in drafting the manuscript. All co-authors provided inputs during final manuscript preparation. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by grants “Progetti di ricerca ed innovazione al di sotto della soglia de minimis” from P.O.R. Sardegna 2000/2006, Asse 3 – Risorse Umane, Misura 3.13 – Ricerca e sviluppo tecnologico delle Imprese e territorio and by PRIN from MIUR.




