Cloning and characterization of a Chlamydomonas reinhardtii cDNA arylalkylamine N-acetyltransferase and its use in the genetic engineering of melatonin content in the Micro-Tom tomato
Abstract
Abstract: Melatonin is found in a wide variety of plant species. Several investigators have studied the physiological roles of melatonin in plants. However, its role is not well understood because of the limited information on its biosynthetic pathway. To clarify melatonin biosynthesis in plants, we isolated a cDNA-coded arylalkylamine N-acetyltransferase (AANAT), a possible limiting enzyme for melatonin biosynthesis, from Chlamydomonas reinhardtii (designated as CrAANAT). The predicted amino acid sequence of CrAANAT shares 39.0% homology to AANAT from Ostreococcus tauri and lacks cAMP-dependent protein kinase phosphorylation sites in the N- and C-terminal regions that are conserved in vertebrates. The enzyme activity of CrAANAT was confirmed by in vitro assay using Escherichia coli. Transgenic plants constitutively expressing the CrAANAT were produced using Micro-Tom, a model cultivar of tomato (Solanum lycopersicum L.). The transgenic Micro-Tom exhibited higher melatonin content compared with wild type, suggesting that melatonin was synthesized from serotonin via N-acetylserotonin in plants. Moreover, the melatonin-rich transgenic Micro-Tom can be used to elucidate the role of melatonin in plant development.
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is found in a considerable variety of plant species, and it seems to have a role in developmental and physiological processes [1–6]. Several investigators have studied the physiological role of melatonin in plants [7]. These studies suggest that melatonin is involved in many functions, including regulating mitosis [8, 9]; delaying flower induction [10]; stimulating hypocotyl, coleoptile, and root growth [11–13]; regenerating adventitious- and lateral roots [14]; protecting against cold-induced apoptosis [15], chlorophyll degradation [16], and toxic copper ions [17]. However, its biosynthetic pathway is not fully known, which has been a rate limiting factor in understanding its function in plants.
In vertebrates, melatonin is biosynthesized from tryptophan [18]. Tryptophan is first converted to 5-hydroxytryptophan by tryptophan 5-hydroxylase. Aromatic amino acid decarboxylase then catalyzes the conversion of 5-hydroxytryptophan to serotonin, which is converted to N-acetylserotonin by arylalkylamine N-acetyltransferase (AANAT) [19]. The last step in this pathway is catalyzed by hydroxyindole-O-methyltransferase (HIOMT), which leads to the formation of melatonin. The nocturnal increase in circulating melatonin in vertebrates is regulated by 10- to 100-fold increases in pineal AANAT activity, which is low during the day [20]. Therefore, AANAT may be the rate-limiting enzyme for melatonin biosynthesis. Although, the biosynthetic pathway of melatonin in plants is not fully understood, tryptophan, 5-hydroxytryptophan, tryptamine, and serotonin are found in plant cells [21, 22], and tryptophan is converted to tryptamine by tryptophan decarboxylase, which is converted to serotonin by tryptamine 5-hydroxylase [23, 24]. Melatonin is biosynthesized from tryptophan in plants [25]. This indicates that melatonin might be biosynthesized in a pathway similar to that in vertebrates.
Arylalkylamine N-acetyltransferase homologs have been detected in the genomes of vertebrates, yeast, insects, and Gram-positive bacteria but not in other published genomes including plants and nematodes [26]. According to a search of genomic databases by Coon and Klein [27], a homologous AANAT sequence was not detected in the published genomes of higher plants. However, a possible homolog was detected in the unicellular green alga Chlamydomonas reinhardtii.
Melatonin was first detected in tomato (Solanum lycopersicum L.) in 1995 [2], it was also reported that melatonin accumulates in tomato fruits as the mature [28] and melatonin is highly accumulated in seeds [29]. Moreover, we found that melatonin is biosynthesized from N-acetylserotonin in Micro-Tom (M. Okazaki and H. Ezura, unpublished). These results show that Micro-Tom could serve as a model plant in the study of the function of melatonin.
In this paper, we describe the cloning and characterization of the Chlamydomonas AANAT gene, CrAANAT, the first reported AANAT gene in plants. Transgenic Micro-Tom plants expressing CrAANAT had higher melatonin content, demonstrating the involvement of the CrAANAT gene in melatonin biosynthesis and the possibility of genetic engineering of melatonin content in plants.
Materials and methods
Database searches
We searched for the homologous candidate AANAT gene in Chlamydomonas using Chlamy Center Blast (http://www.chlamy.org/cgi-bin/webblast.pl) and compared it with the serotonin N-acetyltransferase of Ostreococcus tauri (GenBank accession no. CAL56689) using tBLASTn.
Culture conditions for Chlamydomonas reinhardtii
Chlamydomonas cells were grown to OD750 = 0.9 in Tris-acetate-phosphate (TAP) medium (modified TAP medium: 15.4 mm Tris HCl, 7.0 mm NH4Cl, 830 mm MgSO4, 337 mm CaCl2, 490 mm K2HPO4, 400 mm KH2PO4, 17.4 mm acetic acid, and Hutner trace elements, pH 7.0) [30] for 36 hr at a light intensity of 45 μmol/m2/s (20°C, 100 rpm). After pre-culture, the medium was centrifuged (2670 g, at 20°C), and the pellets were washed twice with Sager–Granick medium (modified SG medium: 1.22 mm MgSO4·7H2O, 0.57 mm K2HPO4, 0.73 mm KH2PO4, 0.36 mm CaCl2, 37 μm FeCl3·6H2O, 16.2 μm H3BO4, 3.5 μm ZnSO4·H2O, 2.02 μm MnCl·4H2O, 0.24 μm CuSO4·5H2O, 0.84 μm CoCl2·6H2O, 0.83 μm Na2MoO4·H2O, pH 7.0) [31]. The cells were resuspended in new SG medium and cultured for 1 hr at a light intensity of 45 μmol/ m2/s (25°C, 100 rpm). The medium was centrifuged at 4200 × g and dissolved in 100 μL of DW. The solution was frozen with liquid nitrogen and stored at −80°C until use.
Cloning of CrAANAT
RNA was extracted from the Chlamydomonas cell pellets using the RNeasy Midi kit (Qiagen, Valencia, CA, USA) following the manufacture’s instructions. After extraction, DNase was added and the solution was left for 10 min. RNA was immediately used for cDNA synthesis with a first-strand cDNA synthesis kit (TaKaRa, Shiga, Japan) following the manufacture’s instructions. Fragment of the CrAANAT sequence (EST clone 963025G12.y2, 645 bp) were amplified with internal specific primers for the cDNA (forward primer: 5′-CTCTAGGCTAGCTGCGCCCTGACCC-3′, reverse primer: 5′-GGATGCACAGTAGTGCGCCGTCGGCATC-3′). The PCR cycles were as follows: 94°C for 3 min, 30 cycles at 94°C for 30 s and 72°C for 30 s, and 72°C for 2 hr. The PCR products were subcloned into the pCR®8/GW/TOPO® vector (Invitrogen, Carlsbad, CA, USA) using the pCR®8/GW/TOPO® TA cloning kit following the manufacture’s protocol. The pCR®8 vectors, containing partial fragments of CrAANAT were sequenced using a PRISM 3130 DNA sequencer (Avant Applied Biosystems, Foster City, CA, USA). 3′-RACE (rapid amplification of cDNA ends) for CrAANAT was performed using the SMARTTM RACE cDNA Amplification Kit (Clontech, Palo Alto, CA, USA), with gene-specific primers and the supplied adaptor primer as below: first PCR for cDNA (forward primer: 5′-CTCTAGGCTAGCTGCGCCCTGACC C-3′, reverse primer [Universal primer (UPM)]: 5′-CTAATACGACTCACTATAGGGC-3′); second PCR, from the first PCR product (forward primer: 5′-CCGGTGCAGCCAGAGATGCTGGACCGA-3′, reverse primer [Nested universal primer (NUP)], 5′-AAGCAGTGGTATCAACGCAGAGT-3′); third PCR from the second PCR product (forward primer: 5′-GTGGTGGTGGACGCCGCGCTGCGC-3′, reverse primer: 5′-CACCACTGCCCTAGGCTTCCGCCACCT-3′). All of the PCR cycles were performed as follows: 95°C for 5 min; 30 cycles at 94°C for 30 s, 50°C for 30 s, and 72°C for 3 min; and 72°C for 2 hr. The complete open reading frame (ORF) of CrAANAT was amplified using the following primers: forward primer: 5′-ATGGCCGAGGAATCTCTAGACGCCAGTG-3′, reverse primer: 5′-TACACCCCAGCCCCCATGCCACCAC-3′. The PCR cycles were performed as follows: 95°C for 5 min; 30 cycles at 94°C for 30 s, 50°C for 30 s, and 72°C for 3 min; and 72°C for 2 hr. The full-length cDNA sequence of CrAANAT was registered in DDBJ under accession number AB474787.
Sequence characterization of CrAANAT and phylogenetic analysis
Multiple sequence alignments of AANATs (Ovis, Homo, Gallus, Xenopus, Medaka, O. tauri, Ostreococcus lucimarinus, Trichoplax, Coprinopsis, Laccaria, Saccharomyces, Schizosaccharomyces, Pseudomonas, Bordetella, and Reinekea) were constructed using EBI ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html). IdentityX (http://home.hiroshima-u.ac.jp/kei/IdentityX/index.html) was used in rearrangements of the resulting alignment file in EBI ClustalW2. Phylogenetic trees were constructed using FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Measurement of activity of CrAANAT proteins
The pCR®8 vector containing CrAANAT was subcloned into the Gateway® pDESTTM15 vector (Invitrogen) using the Gateway® LR Clonase® Enzyme Mix (Invitrogen) to produce a fusion protein with N-terminal glutathione-S-transferase (GST)-tags. After transformation of the pDESTTM15 vectors containing CrAANAT to Escherichia coli strain BL21, a single colony was pre-cultured in TB medium (containing 2% glucose and carbenicillin; MO BIO Laboratories, Inc., Solana Beach, CA, USA). When the medium OD600 reached 0.6, isopropyl-β-D-1-thiogalactopyranoside was added to 1 mm. After incubation for 3 hr, we performed a protein extraction. The medium was cooled for 5 min and centrifuged (5000 g, at 4°C) for 10 min. After washing with TE (50 mm Tris-HCl [pH 8.0], 2 mm EDTA) and centrifugation, the pellet was extracted using the BugBuster Protein Extraction Reagent (Novagen, Madison, WI, USA). A total of 600 μL of regent was added and gently mixed on a rotary shaker for 10 min. The mixture was centrifuged (12,000 g, at 4°C) for 10 min and the supernatant was stored as a soluble fraction. This fraction was purified with GSTrapTM columns (GE Healthcare, NY, USA). The following experiments were conducted in a cold room, except where mentioned. GSTrap columns were equilibrated with a quintuple volume of binding buffer (PBS, 140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, and 1.8 mm KH2PO4, adjusted to pH 7.4 and filtered) to column volume. After loading, the sample columns were washed with a quintuple volume of binding buffer, and a flow-through of elution buffer (50 mm Tris-HCl, pH 8.0, with 10 mm reduced glutathione) was collected. The purity of the GST-CrAANAT protein was confirmed by SDS–PAGE.
To measure the activity of GST-CrAANAT, we used Ellman’s reagent, 5,5′-dithobis(2-butribenzoic acid) (DTNB) [32]. DTNB can be used in the assay of acetyltransferases to examine the thiol content in CoA [33]. The reaction mixture consisted of 60 μL purified protein, 0.28 mm acetyl CoA, 2.0 mm serotonin, and up to 100 μL buffer (50 mm PBS, 500 mm NaCl, 2 mm EDTA, and 5 mg BSA, pH 6.8). The mixture was reacted at 37°C for 0, 5, and 10 min. After the reaction, 200 μL stop solution (3.2 m Guanidine–HCl, 10 mm PBS, pH 6.8) was added to stop the reaction, and 30 μL DTNB reagent (2 mm DTNB, 88 mm PBS, 10 mm EDTA) was added to the solution and left for 5 min at room temperature. The absorbances at 415 nm were measured, along with those of CoA, which is used for standard curve, and the CoA content was calculated.
Generation of transgenic Micro-Tom plants
The pCR®8 vectors containing CrAANAT were subcloned into pGWR1 vector under the control of the cauliflower mosaic virus (CMV) 35 S promoter using the Gateway® LR Clonase® Enzyme Mix (Invitrogen), which can overexpress the genes in a whole plant. The CrAANAT vector was transformed to Agrobacterium tumefaciens strain GV2260 by electroporation. Transgenic Micro-Tom plants were generated using Agrobacterium according to the procedure of Sun et al. [34]. The transgenic plants were grown on rock wool (Toyobo, Osaka, Japan) in a growth chamber with a day/night photoperiod of 16/8 hr at 60.5 μmol photons/m2/s at 25°C and were supplied with a mixed nutrient solution of Otsuka. House No. 1: Otsuka House No. 2 (6:4; Otsuka Chemical, Osaka, Japan; N, P, K, Ca, and Mg =18.6, 5.1, 8.6, 8.2, and 3.0 mequiv./L in the applied concentration).
Genomic DNA was isolated from the young leaves of putative transgenic plants (T0) and their first generation (T1) using a modified Murray and Thompson’s CTAB method [35]. The presence of the CrAANAT gene was confirmed by genomic PCR amplification of rooting plantlets with the following primers: forward primer: 5′-ACAGAACTCGCCGTAAAGACTGG-3′, reverse primer: 5′-GCTTGGTCAGCAGCCGTATC-3′ for the 500 bp CMV 35 S promoter fragment and 439 bp CrAANAT fragment, in extracted genomic DNAs. The PCR cycles were performed as follows: 95°C for 2 min; 30 cycles at 94°C for 5 min, 95°C for 30 s, 65°C for 1 min, and 72°C for 1 min; and 72°C for 5 min. To screen diploid plants, we confirmed the ploidy level of rooted shoots by using a ploidy analyzer (Partec, Germany). T0 and T1 plants were used in the experiments.
Transgene expression in transgenic Micro-Tom plants
We used transgenic tomato lines (T0 and T1 plants) as confirmed by genomic PCR. Total RNA was extracted from the leaves of transgenic and non-transgenic Micro-Tom using the RNeasy Plant Mini Kit (Qiagen) following the manufacture’s protocol. DNase treatment was performed using the RNase-Free DNase Set (Qiagen), and total RNA was measured on a spectrophotometer (Beckman Instruments, San Ramon, CA, USA) and quality was checked by RNA electrophoresis. cDNA was synthesized from the RNA using the SuperScript®III First Strand Synthesis System (Invitrogen) following the manufacturer’s instructions. The expression levels of the cDNA were analyzed by Real Time RT-PCR using the Thermal Cycler Dice® Real-Time System (TaKaRa, Japan). Gene-specific primers for PCR amplification were designed using Primer3 (http://frodo.wi.mit.edu/) for the 140 bp CrAANAT fragment (forward primer: 5′-CGCACTACTGTGCATCCACTC-3′, reverse primer: 5′-GCTTGGTCAGCAGCCGTATC-3′) and for the 93 bp actin fragment (forward primer: 5′-TATTGTGTTGGACTCTGGTGATGG-3′, reverse primer: 5′-GCCAAGTCAAGACGGAGAATG-3′). Reaction conditions were as follows: CrAANAT was amplified at 95°C for 10 s with 42 cycles at 95°C for 5 s and 65°C for 35 s. We confirmed that the amplification was primer specific.
Measurement of melatonin content in transgenic Micro-Tom plants
Melatonin, serotonin, CoA, and acetyl CoA were purchased from Sigma (Sigma Chemicals, MO, USA), and BSA was purchased from Wako (Wako Pure Chemicals, Tokyo, Japan). The melatonin content in the leaves of transgenic and non-transgenic Micro-Tom plants was measured by enzyme-linked immunosorbent assay (ELISA) according to the procedure of Okazaki and Ezura [29].
Statistical analysis
Statistical analyses for the melatonin-level data were performed using Student’s t-test, with differences considered significant if P < 0.05.
Results
Isolation and characterization of CrAANAT
Homologous sequences of AANAT were not found in higher plant databases. However, an EST clone (963025G12.y2) of 645 bp that lacks the 3′-sequence of CrAANAT was found in the Chlamydomonas EST library (Chlamy Center) when compared with the serotonin N-acetyltransferase of O. tauri. The 3′-sequence of the EST fragment, 963025G12.y2, was obtained by 3′-RACE-PCR. We found that the full-length sequence of CrAANAT has a 5′-untranslated region (UTR; 118 bp), a coding sequence (CDS; 576 bp), and a 3′-UTR (975 bp). After reading the 3′-sequence, we amplified a complete ORF of the CrAANAT and subcloned it into the pCR®8 vector. The amino acid sequence of CrAANAT contains motifs A, B, C, and D, which are characteristic of AANAT (Fig. 1). The parts of the CrAANAT sequence that are similar to vertebrate AANATs are the catalytic core, specifically motifs A, B, and D/c-2. A histidine residue in motif A and a tyrosine residue in motif B also exist in CrAANAT. However, CrAANAT lacks cAMP-dependent protein kinase (PKA) phosphorylation sites in the N- and C-terminal regions that are conserved in vertebrate AANAT. We found that CrAANAT has a sequence homology of 39.0% compared with O. tauri AANAT, based on the amino acid sequence. Unicellular green algae lack regulatory sequences in the N- and C-terminal regions. Phylogenetic analysis of the whole amino acid sequence of CrAANAT revealed that the algal AANAT sequences are similar to those in proteobacteria, ascomycota, basidomycota, and placozoa in that they all lack regulatory sequences in the N- and C-terminal regions (Fig. 2).
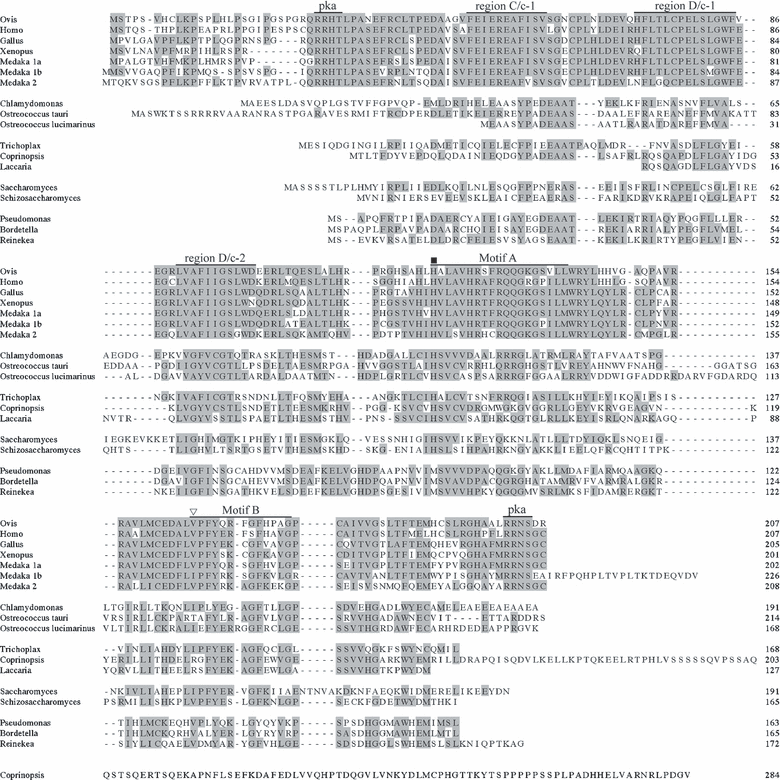
Multiple sequence alignment of AANAT based on amino acid sequences. The included sequences are as follows (numbers are GenBank accession numbers unless otherwise noted): Ovis (NP_001009461), Homo (NP_001079), Gallus (NP_990489), Xenopus (AAP57668), Medaka (AANAT1a; NP_001098302, AANAT1b; NP_001098330, AANAT2; NP_001098316), Ostreococcus tauri (CAL56689), Ostreococcus lucimarinus (XP_001422427), Trichoplax (XP_002113076), Coprinopsis (XP_001834952), Laccaria (XP_001889540), Saccharomyces (Q12447), Schizosaccharomyces (NP_593345), Pseudomonas (YP_001669494), Bordetella (NP_887628), and Reinekea (ZP_01114978), (- - -) inserted space: () His106 and () Tyr153 in the catalytic site. Regions C/c-1, D/c-1, D/c-2, and motifs A and B are conserved in AANAT.
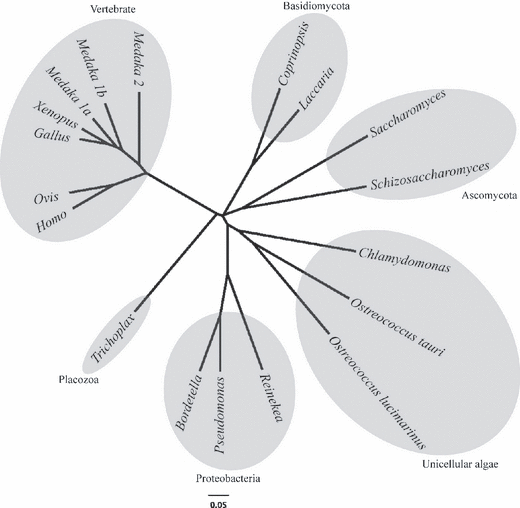
Phylogenetic tree of AANAT based on amino acid sequences. The sequences included are as in Fig. 1.
Enzyme activity of the CrAANAT fusion protein
CrAANAT proteins were expressed as GST fusion proteins in E. coli strain BL21. The resulting protein was purified from crude extracts using GSTrap columns, as indicated by Coomassie Blue staining of the SDS–PAGE gel (Fig. 3), which revealed a single major band of protein. The apparent molecular mass of the band was more than 40 kDa (Fig. 3A), which was consistent with the predicted mass of 48.7 kDa based on the amino acid sequence. The activity of the GST-CrAANAT protein was estimated by measuring CoA production after reaction with acetyl CoA and serotonin. CoA production was seen at the 20 min, 40 min, and 60 min time points (Fig. 3B). The CrAANAT protein was functional in vitro, although its substrate specificity is unknown.
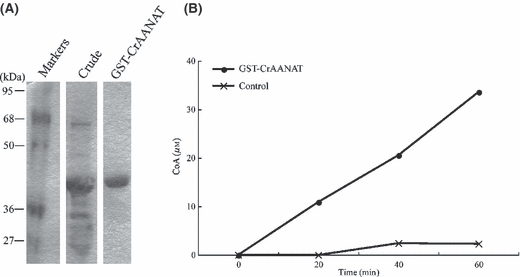
Induction of the CrAANAT protein and evaluation of enzyme activity. (A) A GST-CrAANAT recombinant fusion protein was produced using Escherichia coli strain BL21. Estimated protein size is 48.7 kDa. Crude: bacterial lysate containing GST-CrAANAT protein, GST-CrAANAT: elution (bounded fraction) from GSTrap columns. (B) GST-CrAANAT protein, or the buffer (control), was used to measure AANAT activity.
Generation of transgenic Micro-Tom plants
cDNA of was transformed into the Micro-Tom tomato using the pGWR1 vector (Fig. 4A). Candidate transgenic plants were screened by rooting on MS medium containing kanamycin. After confirming the ploidy level using a ploidy analyzer, we obtained 15 T0 lines of diploid, putative transgenic plants. The presence and expression of CrAANAT in the putative transgenic lines were confirmed by genomic PCR (Fig. 4B) and quantitative Real Time RT-PCR, respectively (Fig. 4C). Variation in the level of mRNA expression was observed in the transgenic lines. These transgenic plants were allowed to self-pollinate and T1 seeds were obtained. The T1 seeds were used in the experiments.
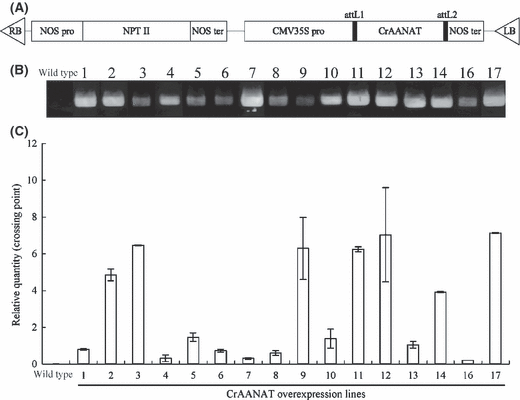
Production of transgenic Micro-Tom plants overexpressing CrAANAT. (A) Vector construction of pGWR1: CrAANAT. NOS pro: nopaline synthase promoter; NPT II: neomycin phosphotransferase II, which provides kanamycin resistance; NOS ter; nopaline synthase terminator, attL1, attL2: gateway cloning site; CMV35S pro: cauliflower mosaic virus promoter. (B) Detection of transgene by genomic PCR amplification in the leaves of T0 transgenic plants. (C) Detection of transgene expression by quantitative Real Time RT-PCR in the leaves of T0 transgenic plants. The values are the means of two parallel measurements ± S.D.
Analysis of the T1 plants of the transgenic Micro-Tom
T1 seeds from the CrAANAT 2 line were sown and 9 T1 plants obtained. Genomic PCR amplification of CrAANAT in these plants showed that 2 had the CrAANAT transgene, whereas 7 plants were azygous for the transgene (Fig. 5A). Additionally, 9 T1 plants of the CrAANAT 11 line were obtained. All of the T1 plants had the CrAANAT transgene as determined by genomic PCR, indicating the integration of multiple transgenes into the genome.
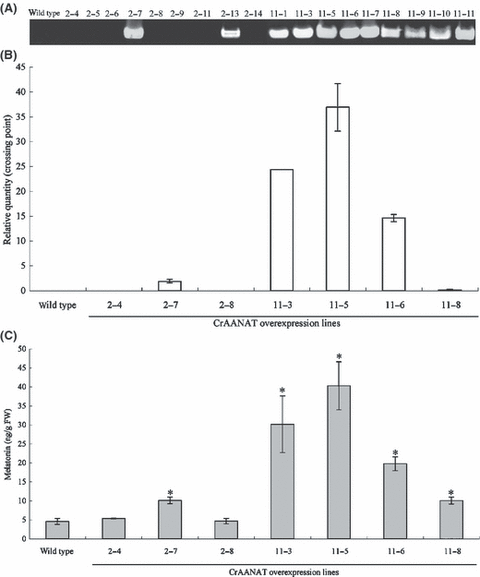
The presence of the CrAANAT gene (A), its mRNA expression levels (B), and melatonin content (C) in the leaves of Micro-Tom and transgenic T1 plants expressing CrAANAT. (A) Detection of transgene by genomic PCR amplification in the leaves of T1 transgenic plants. (B) Detection of transgene expression by quantitative Real Time RT-PCR in the leaves of T1 transgenic plants. The values are the means of two parallel measurements ± S.E.M. (C) Melatonin content in the leaves of transgenic T1 plants. The values are the means of three parallel measurements ± S.E.M. The values shown are *P < 0.05, in comparison with wild type, based on Student’s t-test.
Transgene expression in transgenic T1 Micro-Tom plants
Three T1 plants, #2-4, #2-7, and #2-8 from the CrAANAT 2 line, were evaluated for mRNA expression. According to genomic PCR, #2-4 and #2-8 were azygous and #2-7 was transgenic (Fig. 5A). Plants #2-4 and #2-8 did not show CrAANAT mRNA expression, as in the wild type, whereas plant #2-7 showed CrAANAT mRNA expression (Fig. 5B). Four T1 plants, #11-3, #11-5, #11-6, and #11-8 from the CrAANAT 11 line, were also analyzed for mRNA expression. According to genomic PCR, all plants from CAANAT 11 had the transgene (Fig. 5A). CrAANAT mRNA expression was detected in all plants from the CAANAT 11 line, although the expression levels varied, with the highest being in plant #11-5 (Fig. 5B). These results demonstrate that the CrAANAT transgene is inherited and expressed in the T1 generation.
Melatonin content in the leaves of T1 transgenic Micro-Tom
Melatonin content was significantly higher in the leaves of transgenic lines #2-7, #11-3, #11-5, #11-6, and #11-8 (10.1 ng/g FW, 30.2 ng/g FW, 40.3 ng/g FW, 20.0 ng/g FW, and 10.1 ng/g FW, respectively) than in wild type (4.6 ng/g FW) and azygous plants #2-4 (5.3 ng/g FW) and #2-8 (4.7 ng/g FW; Fig. 5C). The melatonin content was nearly correlated with the mRNA expression level (Fig. 5B), indicating that CrAANAT expression is involved in melatonin accumulation in transgenic Micro-Tom.
Discussion
In this study, the CrAANAT gene encoding AANAT in C. reinhardtii was identified in a search of public domain databases, isolated, characterized using the E. coli expression system, and transferred to tomato. To study the role of endogenous melatonin in plant development more closely, researchers need information about it biosynthetic pathway. Previously, it was shown that melatonin was biosynthesized from tryptophan in plants [25] and N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) exist in water hyacinth [36], which is a major metabolite of melatonin in mammals. However, information concerning about enzymes responsible for melatonin biosynthesis and metabolism is currently not available in plants.
Melatonin is present in various algae [37]. The enzymatic activities of AANAT and HIOMT were detected in Gonyaulax [38], suggesting that melatonin in plants is synthesized from serotonin via N-acetylserotonin, as in vertebrates. Previous to this study, two AANAT homologs were identified in phototrophic organisms by Coon et al. [27]. In 2007, one of the AANAT homologs, serotonin N-acetyltransferase (CAL56689), was first isolated in O. tauri [39]; however, the function of this gene has not been elucidated. CrAANAT isolated from unicellular green algae was characterized and its enzyme activity was shown in vitro. Its characteristics show what role CrAANAT plays in Chlamydomonas.
Chlamydomonas exhibits circadian photoperiodic responses, e.g. zygospore maturation [40] and the regulation of ferredoxin-nitrite reductase activity [41]. The expression levels of CrAANAT were 100 times higher in Chlamydomonas cultured in media without phosphorus than in media without nitrogen (data not shown). It is interesting whether Chlamydomonas responds to environmental cues via AANAT activity and melatonin accumulation, as in Gonyaulax, which responds to decreased temperature and daylight length [42].
CrAANAT contains the four conserved sequence motifs (A–D) of AANAT and the conserved residues found in vertebrates. In vertebrates, motif A has an α/β conformation characteristic of the phylogenetically conserved cofactor binding site for acetyl CoA [43]. Motif B forms an α/β structure for the serotonin-binding pocket. Two histidine residues in motif A, His120 and His122, of AANAT may promote catalysis by facilitating the removal of protons generated at the active site [44], and mutations of either of these histidines raise the Km significantly and change the pH dependence [45]. The tyrosine residue in motif B may also serve as a catalytic acid facilitating CoASH departure. Thus, the residues in similar regions of CrAANAT may play such roles. Phosphorylation of PKA sites in the N- and C-terminal regions promotes binding to 14-3-3 proteins, which reduces the Km for the arylalkylamine substrates and also protects the enzyme from proteasomal proteolysis [46]. The stability of the CrAANAT protein may be extremely different from that of vertebrates, because CrAANAT lacks the PKA sites. In vitro AANAT enzyme activity showed that the CrAANAT protein has AANAT activity. However, it is still not known whether these residues in CrAANAT play such roles. Further analyses, such as site-directed mutagenesis in the regions and its kinetics of CrAANAT protein will be needed to understand this function.
Using the enzyme encoding melatonin biosynthesis, we generated transgenic Micro-Tom plants expressing CrAANAT. mRNA expression levels of CrAANAT varied from 0.2–6.9 times the expression level of actin, although these genes were both under the control of the CMV 35 S promoter. The instability of transgene expression is often attributable to the transgene copy number, the position of transgene integration, and/or the degree of homology to endogenous genes [47]. Transgenic plants derived from Agrobacterium-mediated transformation often carry multiple copies of the integrated transgene [48]. Actually, all T1 plants of the CrAANAT 11 line had the transgene, indicating that the T0 line has multiple copies and that the T1 plants vary in copy number of transgenes. The differences in copy number and integration position of the transgenes resulted in the generation of various expression levels between lines. As a result of the multiple copies of the ectopic gene, the integrated gene can become unstable because of enhanced gene silencing [49]. Detailed analysis of the transgenic plants obtained in this study will provide clues for resolving this problem.
We found that the melatonin content increased in these transgenic Micro-Tom plants, which overexpress CrAANAT. This result strongly suggests that the biosynthetic pathway of melatonin in Micro-Tom is similar to that in vertebrates. Because AANAT homolog was not detected in published genomes of plants, another enzyme, such as GCN5-related N-acetyltransferase (GNAT) superfamily [50], may catalyze the reaction in plants, which is capable of N-acetylating serotonin. In hamster skin, arylamine N-acetyltransferase as having two isozymic forms of which one (NAT-2) could acetylate serotonin to N-acetylserotonin [51]. GNAT protein is also distributed in plants, e.g. glucosamine-6-phosphate acetyltransferase [52], hookless [53], and nuclear shuttle protein [54]. Nonetheless, it demonstrates that it is possible to regulate endogenous melatonin content in Micro-Tom. These transgenic lines will be a powerful tool in the elucidation of the function and role of melatonin in plant development.
In contrast to serotonin content, melatonin content appears to be relatively low. Tomato fruits contain 0.18–12 μg/g FW of serotonin [21, 22]. Possible explanations for the low relative melatonin content are as follows: (i) The enzyme activity of CrAANAT is too low to acetylate huge amounts of serotonin, which presumably relates to the lack of PKA phosphorylation sites in the N- and C-terminal regions of the CrAANAT sequence. 14-3-3 protein cannot bind to CrAANAT, and unbound CrAANAT is degraded by proteasomal proteolysis, as in Saccharomyces cerevisiae [55], which also does not contain PKA phosphorylation sites. (ii) The enzyme activity of HIOMT is low, although N-acetylserotonin accumulates. (iii) Melatonin is metabolized to other metabolites by the enzymes, such as indoleamine 2,3-dioxygenase (IDO) [56], cytochrome P450 monooxygenases [57], and arylalkyl acylamidase [58] as well as non-enzymatic degradation by oxidants [59]. These pathway may exist in plants, according to the report that melatonin metabolite, AFMK, was detected in water hyacinth [36]. The expression levels of CrAANAT directly affected melatonin content, therefore, it seems to relate to the enzyme activity of CrAANAT. To clarify the explanations, it will be necessary to investigate the enzymatic activities of AANAT, HIOMT, and IDO or the accumulation of the precursors and metabolites of melatonin.
Despite increased melatonin concentration in the CrAANAT 11-5 line, significant phenotypical changes were not observed. In a previous report, a germ plasm line of St. John’s wort (Hypericum perforatum L.) with high levels of melatonin was produced in vitro using mutagenized tissues [60]. However, the mutant was morphologically similar to the wild type, as is transgenic Micro-Tom. The reported functions of melatonin are as a growth regulator, antioxidant, and signal molecule for circadian rhythms. It is difficult to observe these functions, especially antioxidant capacity and circadian maintenance, through morphology only. The melatonin-rich, transgenic Micro-Tom can be used in detailed studies of fruits, leaves, and seedlings to elucidate the role of melatonin and metabolism in plants.
Acknowledgment
We are grateful to Dr Takashi Matozaki of Gunma University for providing anti-melatonin serum (HAC-AA92-03RBP86). Micro-Tom seeds (TOMJPF00001) were provided by the Gene Research Center of the University of Tsukuba through the National Bio-Resource Project (NBRP) of the MEXT, Japan.




