Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy
Abstract
Abstract: Melatonin participates in circadian, seasonal and reproductive physiology. Melatonin also acts as a potent endogenous antioxidant by scavenging free radicals and upregulating antioxidant pathways. The placenta expresses melatonin receptors and melatonin protects against oxidative damage induced in rat placenta by ischemia-reperfusion. One of the most common complications in pregnancy is a reduction in fetal nutrient delivery, which is known to promote oxidative stress. However, whether melatonin protects placental function and fetal development in undernourished pregnancy is unknown. Here, we investigated the effects of maternal treatment with melatonin on placental efficiency, fetal growth, birth weight and protein expression of placental oxidative stress markers in undernourished pregnancy. On day 15 of pregnancy, rats were divided into control and undernourished pregnancy (35% reduction in food intake), with and without melatonin treatment (5 μg/mL drinking water). On day 20 of gestation, fetal biometry was carried out, the placenta was weighed and subsequently analyzed by Western blot for xanthine oxidase, heat shock protein (HSP) 27 and 70, catalase, manganese superoxide dismutase (Mn-SOD) and glutathione peroxidase 1 (GPx-1). A separate cohort was allowed to deliver to assess effects on birth weight. Maternal undernutrition led to a fall in placental efficiency, disproportionate intrauterine growth retardation and a reduction in birth weight. Maternal treatment with melatonin in undernourished pregnancy improved placental efficiency and restored birth weight, and it increased the expression of placental Mn-SOD and catalase. The data show that in pregnancy complicated by undernutrition, melatonin may improve placental efficiency and birth weight by upregulating placental antioxidant enzymes.
Introduction
The neurohormone melatonin participates in circadian and seasonal rhythmicity, and it is also important in reproduction. For instance, maternal plasma melatonin levels are elevated during pregnancy, reaching a maximum at term and then returning to basal levels soon after delivery. Concentrations of melatonin in maternal plasma are also higher in twin than in single pregnancies [1–4]. Melatonin may contribute to the maintenance of pregnancy by stimulating progesterone production, while inhibiting the synthesis of prostaglandins and uterine contractility [5]. The expression of melatonin receptors in the human and rat placenta and the ability of melatonin to stimulate human chorionic gonadotropin and to downregulate rat placental lactogen II mRNA levels, further suggest that melatonin plays a functional role in feto-placental development [6–8].
Melatonin is also a powerful antioxidant [9–11] and the potency of its antioxidant actions relates directly to its combined ability to scavenge free radicals [12], to enhance the expression and/or activity of antioxidant enzymes [13–17], and to reduce the leakage of electrons from the mitochondrial electron transport chain [18]. In addition, metabolites of melatonin, products of its reaction with free radicals, are themselves also powerful antioxidants [19–21].
In complicated pregnancy, such as placental insufficiency or preeclampsia, the feto-placental unit may become compromised with alterations in placental efficiency, fetal growth and birth weight [22]. Intrauterine growth retardation is one of the greatest killers in obstetric medicine, being a major cause of both pre- and postnatal mortality and morbidity [23, 24]. Thus, the prevention of intrauterine growth retardation remains a major concern in perinatal practice today. Maternal undernutrition during pregnancy promotes asymmetric growth retardation [25] and it is associated with increased oxidative stress [26–30]. It has been reported that melatonin increases glutathione peroxidase activity in human chorion [31], and that it also protects against oxidative damage induced in rat placenta by ischemia-reperfusion injury [32]. However, it remains unknown whether treatment with antioxidants protects against the detrimental effects on placental and fetal development of pregnancy complicated by undernutrition.
This study tested the hypothesis that melatonin has a protective effect in pregnancy complicated by undernutrition and that this protection is associated with an increase in placental antioxidant capacity. The hypothesis was tested by investigating in rat undernourished pregnancy the effect of maternal treatment with melatonin on placental efficiency, fetal growth, birth weight and on the placental expression of pro- and anti-oxidant proteins and indices of oxidative stress.
Materials and methods
Animals
All experiments were carried out under the UK Animals (Scientific Procedures) Act 1986. Virgin, female Wistar rats (3 months old), fed ad libitum on maintenance diet (Charles River, UK) were delivered to Central Biomedical Services (CBS) at the University of Cambridge. At CBS, rats were mated after a minimum of 10 days acclimatization. The presence of a copulatory plug in the rat cage was considered to be day 0 of pregnancy. The pregnant rats were housed singly in individual ventilated cages (IVC units, 21% O2, 70–80 air changes per hour) in rooms with controlled humidity (60%), controlled temperature (21°C) and a 12:12 hr light–dark cycle (lights on at 07:00 hr). From the beginning of pregnancy females were fed a VRF1 diet (Special Diet Services, Essex, UK; the diet composition is shown in Table 1). From this point on, maternal weight, food and water intake were quantified and documented daily.
| Dietary component | Result |
|---|---|
| Moisture (%) | 9.9 |
| Crude fat (%) | 6.1 |
| Crude protein (%) | 19.6 |
| Crude fiber (%) | 4.6 |
| Ash (%) | 6.1 |
| NFE (by difference) (%) | 53.7 |
| Starch (%) | 33.6 |
| Total Sugars (Luff Schoorl) (%) | 4.3 |
| Calcium (mg/kg) | 8860 |
| Phosphorus (mg/kg) | 5210 |
| Sodium (mg/kg) | 2610 |
| Potassium (mg/kg) | 8610 |
| Copper (mg/kg) | 22.4 |
| Manganese (mg/kg) | 125 |
| Vitamin A (iu/kg) | 23,300 |
| Vitamin E (mg/kg) | 99 |
- NFE, nitrogen-free extract.
Experimental procedures
On day 15 of pregnancy, rats were randomly divided into 4 groups (n = 7 per group): control and undernourished pregnancy (ca. 35% reduction in food intake), with and without melatonin treatment (5 μg/mL maternal drinking water freshly prepared every day). The dose of melatonin was adopted from other studies showing potent actions on the expression of antioxidant enzymes [15] and it was also less than the maximal dose recommended for overcoming jet lag in humans [33]. On the morning of day 20 of gestation (term is approximately 21 days) dams were weighed. Anesthesia was induced with isoflurane and then maintained by a mixture of ketamine (40 mg/kg) and xylazine (5 mg/kg) injected intraperitoneally. Once anesthetized, a maternal blood sample (1 mL) was taken by cardiac puncture, the pregnant uterus was exposed via a mid-line incision and the anesthetized pups were killed via spinal transection. Fetal blood was collected from the neck incision by capillarity into haematocrit tubes and pooled per litter. All fetuses and their associated placentae were weighed and the placentae were immediately frozen in liquid nitrogen for subsequent protein isolation. In all pups, the ano-genital distance was measured with digital calipers for determination of sex and only tissues associated with two male pups from anyone litter were used to control for sex and within-litter variation. In a subgroup of the fetuses, the brain was dissected and weighed. A separate cohort of pregnancies was allowed to deliver to determine the effects on birth weight of maternal undernutrition with and without melatonin treatment.
Melatonin assay
Maternal and pooled fetal blood samples were collected in chilled heparinized/EDTA tubes and centrifuged. The plasma was separated and plasma aliquots were snap frozen in liquid N2 and maintained frozen at −80°C until analysis. Melatonin concentration in both maternal and fetal plasma was measured using the 2-[125I]iodomelatonin Rat Radioimmunoassay Kit (MP Biomedicals, Eschwege, Germany; code 07L-130102), following the manufacturer’s instructions. All samples were analyzed at the same time. The sensitivity of the assay was 1.5 pg/mL and the inter-assay and intra-assay coefficients of variation were <12%. The melatonin antiserum showed <1% cross-reactivity with N-acetylserotonin, 5-methoxytryptophol and 5-methoxytryptamine and less than 0.01% with other related amines.
Western blot analysis
Tissue homogenization to obtain protein lysates and subsequent sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were performed as previously described for human placental tissue [34]. The specific primary antibodies were: rabbit polyclonal to catalase (1:2500; Abcam, Cambridge, UK, code Ab1877), manganese superoxide dismutase (Mn-SOD) (1:1000; Upstate Biotech, Lake Placid, NY, USA, code 06-984), xanthine oxidase (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA, code sc-20991), heat shock protein (HSP) 27 (1:1000; Cell Signaling, Danvers, MA, USA, code 2442) and HSP70 (1:20,000; Stressgen, York, UK, code Spa812C); sheep polyclonal to glutathione peroxidase 1 (GPx-1) (1:20,000; Novus Biologicals, Littleton, CO, USA, code S-072-100); and mouse monoclonal to β-actin (1:50,000; Sigma, St Louis, USA, code A5441). The horseradish peroxidase-conjugated secondary antibodies were from GE Healthcare UK Ltd (anti-rabbit and -mouse) and Novus Biologicals (anti-sheep). Membranes were re-probed with antibody recognizing β-actin to control for protein loading and to normalize relative levels of protein expression. The optical density of the immunoreactive bands was analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/index.html); and the ratio protein to β-actin was calculated for each sample.
Statistical analysis
The mean ratio of each protein to β-actin was first transformed to arcsin [35]. Data are expressed as mean ± S.E.M. The effect of melatonin treatment on any variable measured was assessed by one-way ANOVA on absolute or ranked data (followed by the Tukey or Dunn’s post hoc test, respectively), or the Student’s t-test for unpaired data or the Mann–Whitney rank sum test; depending on the distribution of the data. Statistical analyses were performed using GraphPad Prism software (version 3.02; GraphPad Software Inc, San Diego, CA, USA). In all cases, significance was accepted when P < 0.05.
Results
Maternal and fetal plasma concentration of melatonin are shown in Table 2. In general, concentrations of plasma melatonin were highly variable in maternal and fetal samples within groups. In control pregnancies, concentrations of melatonin were higher in maternal than in fetal plasma (13.6 ± 4.8 versus 6.1 ± 1.4 pg/mL, P < 0.05). Undernourished pregnancy tended to elevate circulating plasma levels of melatonin in both maternal and fetal compartments; however, these comparisons did not establish any significant differences. Maternal treatment with melatonin significantly increased circulating plasma melatonin concentrations by at least sixfold in both control and undernourished pregnancy (Table 2).
| Plasma sample | Pregnancy conditions and treatments | |||
|---|---|---|---|---|
| C | CM | U | UM | |
| Maternal (pg/mL) | 13.6 ± 4.8 | 171.2 ± 81.5* | 44.6 ± 28.1 | 275.7 ± 98.8* |
| Fetal (pg/mL) | 6.1 ± 1.4 | 135.3 ± 51.2* | 13.2 ± 3.7 | 164.5 ± 53.5* |
- Values are mean ± S.E.M. for melatonin concentration (pg/mL) from maternal plasma samples (n = 7 per group) or fetal pooled plasma samples (n = 6 per group). *Different from the corresponding untreated group (P < 0.05; two-tailed unpaired t-test or Mann–Whitney rank sum test as appropriate). C, control; CM, control plus melatonin; U, undernourished; UM, undernourished plus melatonin.
In control pregnancies, maternal food intake between 0 and 20 days of gestation did not vary significantly and averaged 22.7 ± 0.9 g per day. Between 15 and 20 days of gestation, undernourished pregnancies had a ca. 35% reduction in maternal food intake relative to control pregnancies (mean ± S.E.M. for maternal food intake expressed as area under the curve, AUC, for days 15–20 of gestation: C = 109.5 ± 5.75 versus U = 71.1 ± 0.97 g, P < 0.05). Maternal treatment with melatonin did not affect maternal food intake in either control (AUC days 0–20 of gestation: C = 433.4 ± 16.9 versus CM = 458.7 ± 19.4 g) or undernourished pregnancies (AUC days 15–20 of gestation: U = 71.1 ± 0.97 versus UM = 73.1 ± 2.8 g).
At day 20 of gestation, undernourished relative to control pregnancies had significantly lower values for fetal body weight (U = 3.05 ± 0.04, n = 80 versus C = 3.73 ± 0.05 g, n = 76; P < 0.05) and for the fetal body:placental weight ratio (U = 6.68 ± 0.12, n = 80 versus C = 7.94 ± 0.14, n = 76; P < 0.05), with a significant increase in the values for the fetal brain:fetal body weight ratio (U = 0.053 ± 0.001, n = 14 versus C = 0.046 ± 0.001, n = 14; P < 0.05, Fig. 1). Maternal treatment with melatonin in undernourished pregnancy did not affect the reduction in fetal body weight (UM = 3.18 ± 0.08 g, n = 81 versus U = 3.05 ± 0.04, n = 80) or the increase in the fetal brain:fetal body weight ratio (UM = 0.054 ± 0.001, n = 14 versus U = 0.053 ± 0.001, n = 14). However, maternal treatment with melatonin in undernourished pregnancy did reduce placental weight (UM = 0.41 ± 0.01, n = 81 versus U = 0.46 ± 0.01 g, n = 80; P < 0.05), which restored the fetal body:placental weight ratio to control values (U = 6.68 ± 0.11, n = 80 versus UM = 7.97 ± 0.24, n = 81; P < 0.05, Fig. 1). Maternal treatment with melatonin in control pregnancies also led to significantly lighter placentae (CM = 0.43 ± 0.01, n = 95 versus C = 0.48 ± 0.01 g, n = 76; P < 0.05) but it had no effect on other variables (Fig. 1).
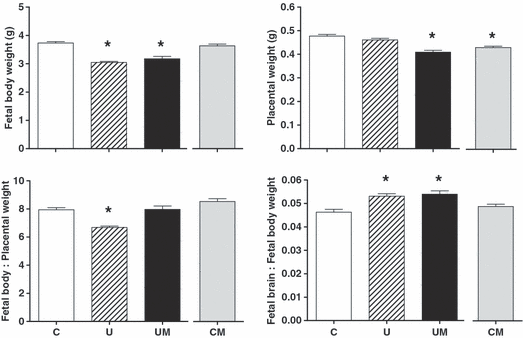
Effects of maternal undernutrition with or without maternal melatonin treatment on fetal body weight, placental weight, placental efficiency (fetal body weight:placental weight ratio) and fetal brain weight : fetal body weight ratio. Bars represent mean ± S.E.M. at day 20 of gestation (n = 7 dams per group). C, control (n = 76); U, undernourished (n = 80); UM, undernourished plus melatonin (n = 81); CM, control plus melatonin (n = 95). Significant (P < 0.05) differences are: *versus other treatments (one-way ANOVA with the Dunn’s or Tukey’s post hoc test, where appropriate). Statistical differences persisted whether comparisons were made by litter or by individual pup number.
When a separate cohort of animals was allowed to deliver to determine any effects on birth weight, undernourished relative to control pregnancies still had significantly lower values for birth weight (U = 5.81 ± 0.04, n = 80 versus C = 6.52 ± 0.06 g, n = 80; P < 0.05). However, maternal treatment with melatonin in undernourished pregnancy restored birth weight to control values (UM=6.24 ± 0.04, n = 89 versus U = 5.81 ± 0.04, n = 80; P < 0.05). In contrast, melatonin treatment in control pregnancy had no effect on birth weight (CM = 6.30 ± 0.06, n = 79 versus C = 6.52 ± 0.06 g, n = 80; Fig. 2).
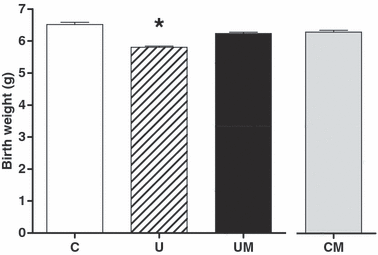
Effects of maternal undernutrition with or without maternal melatonin treatment on birth weight. Bars represent the mean ± S.E.M. at delivery (n = 7 dams per group). C, control (n = 80); U, undernourished (n = 80); UM, undernourished plus melatonin (n = 89); CM, control plus melatonin (n = 79). Significant (P < 0.05) differences are: *versus other treatments (one-way ANOVA with the Dunn’s post hoc test). Statistical differences persisted whether comparisons were made by litter or by individual pup number.
Maternal treatment with melatonin increased the expression of the antioxidant enzymes Mn-SOD and catalase, but not GPx-1, in placentae from undernourished pregnancy (P < 0.001; n = 7 per group). In contrast, there was no significant effect of melatonin on the expression of any antioxidant enzyme measured in control pregnancy (Fig. 3). No significant differences between groups were found in the placental expression of xanthine oxidase or HSP27 and HSP70 (Table 3). However, comparison by two-tailed unpaired t-test of the placental expression of xanthine oxidase in control pregnancy with and without melatonin treatment revealed a significant reduction in the expression of this protein following treatment (Table 3).
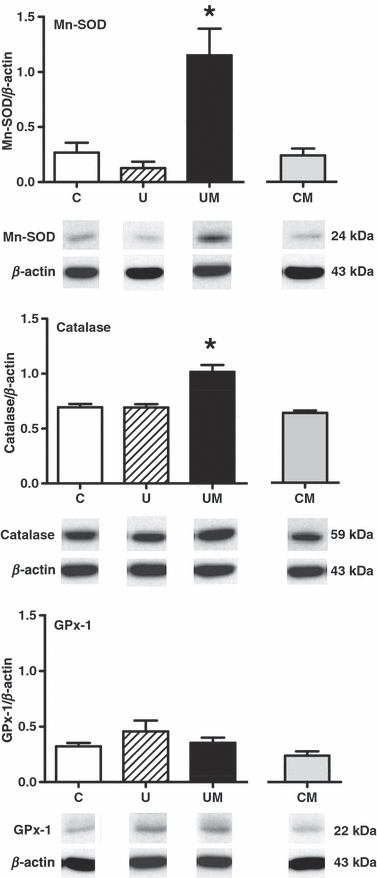
Effects of maternal undernutrition with or without maternal melatonin treatment on the relative expression of Mn-SOD, catalase and GPx-1 in the rat placenta. Bars represent mean ± S.E.M. (n = 7 per group). Total protein samples (25 μg per lane) were subjected to immunoblotting using commercial polyclonal antibodies. Respective representative immunodetected bands of 24 kDa (Mn-SOD), 59 kDa (catalase), 22 kDa (GPx-1) and 43 kDa (β-actin) are also shown. C, control; U, undernourished; UM, undernourished plus melatonin; CM, control plus melatonin. Significant (P < 0.001) differences are: *versus other treatments (one-way ANOVA with the Tukey’s post hoc test).
| Placental protein | Pregnancy conditions and treatments | |||
|---|---|---|---|---|
| C | CM | U | UM | |
| Xanthine oxidase | 1.03 ± 0.12 | 0.58 ± 0.12* | 0.89 ± 0.08 | 0.84 ± 0.07 |
| HSP27 | 0.74 ± 0.07 | 0.63 ± 0.06 | 0.59 ± 0.07 | 0.93 ± 0.17 |
| HSP70 | 0.74 ± 0.03 | 0.88 ± 0.05 | 0.61 ± 0.03 | 0.81 ± 0.10 |
- Values are mean ± S.E.M. for the ratios protein/β-actin obtained by digital band densitometry (n = 7). No significant differences were found between groups by one-way ANOVA and Tukey’s Multiple Comparison Test. However, comparison of C versus CM by two-tailed unpaired t-test revealed a significant effect of melatonin on xanthine oxidase expression (*versus C, P = 0.0014). C, control; CM, control plus melatonin; U, undernourished; UM, undernourished plus melatonin.
Discussion
During pregnancy, nutrient delivery to the unborn child is determined by nutrient content and blood flow in the placental and umbilical circulations. Consequently, reductions in nutrient delivery to the unborn child may occur because of reductions in content, blood flow or both. Diminished nutrient content during pregnancy may occur because of maternal malnutrition in developing countries [36]. In developing and developed societies, complications during pregnancy, such as placental insufficiency and preeclampsia, will also promote a fall in nutrient delivery to the fetus because of reductions in placental and umbilical blood flow [37]. Although the prevalence of maternal malnutrition in developing countries clearly varies, fetal undernutrition resulting in growth restriction in complicated pregnancy is still surprisingly common despite advances in obstetric practice, and it occurs in ca. 3% of pregnancies even in developed societies [24]. Therefore, intrauterine growth retardation resulting from reductions in nutrient delivery to the fetus during pregnancy is an important burden on the population’s health and wealth not only because the robust association between the condition and perinatal morbidity and mortality [23], but also because of the growing body of evidence linking slow fetal growth with the developmental programming of cardiovascular and metabolic diseases [25].
Converging evidence from different lines of research suggests the possibility that in complicated pregnancy maternal treatment with antioxidants may improve placental function and thereby diminish intrauterine growth retardation and its burden. For instance, it is known that the mammalian placenta expresses all major antioxidant systems, including Mn-SOD, Cu/Zn-SOD, GPx and catalase [38]. It has also been reported in rats that maternal undernutrition can overwhelm the endogenous placental antioxidant capacity and promote oxidative stress in the offspring [26, 27, 29, 30]. Maternal undernutrition may further aggravate oxidant stress in the placental and fetal tissues by reduced intake of thiols, transferrin and vitamins C and E, all of which play an important role in the defense against oxidant damage [28]. In support of this line of thinking, data in this study show that maternal treatment with melatonin in undernourished pregnancy improved placental efficiency and restored birth weight to control levels.
Our data show that basal plasma melatonin concentrations were higher in the maternal than the fetal compartment, confirming other studies reporting in different species that melatonin crosses the placenta, and that fetal circulating melatonin is of maternal origin [39–42]. The concentrations measured in maternal plasma following melatonin treatment were similar to those found in other studies using pregnant rats [39, 43]. They were also within the range or lower than those measured in human plasma following treatment with a known dose to avoid jet lag [33, 44]. To our knowledge, there are no reports indicating fetal plasma melatonin concentrations in rats. Against this background, it is worth mentioning that melatonin treatment does not adversely influence the development of rat pups when pregnant dams are treated with even exceptionally high doses of melatonin (up to 200 mg/kg/day) from gestational day 6–19 [45]. In fact, these and other authors have failed to find adverse effects of melatonin on prenatal growth, viability or morphology of the conceptus.
The molecular basis underlying the mechanism of protection of melatonin in undernourished pregnancy is unknown but it may be because of alterations in placental and umbilical perfusion. For instance, only comparatively recently, it has become appreciated that blood flow in most circulations can be regulated by the balance between free radical production and the bioavailability of nitric oxide (NO) [46]. The increased reactive oxygen species, in particular O2•−, will react with NO to form peroxynitrite (ONOO−). In addition to causing oxidative cellular damage directly, enhanced O2•− production will also promote reductions in blood flow as it will increase the sequestration of NO, reducing its bioavailability [47]. Reductions in blood flow in any circulation may therefore occur because of an increase in O2•− production and/or a fall in antioxidant capacity. Conversely, an increase in antioxidant capacity and/or a fall in free radical production will favor vasodilatation. This tenet is particularly relevant during pregnancy as NO is indispensable for the maintenance of blood flow in the placental and umbilical circulations and, thereby, appropriate nutrient and oxygen delivery to the fetus [48]. Further data in this study show that undernutrition during pregnancy did not have an effect on the protein expression of xanthine oxidase or in established indices of oxidative stress in the placenta [49], but that maternal treatment with melatonin in undernourished pregnancy significantly increased the protein expression of the antioxidant enzymes Mn-SOD and catalase. Similar effects of melatonin increasing the expression of antioxidant enzymes in tissues other than placenta have been previously reported. For instance, a single and relatively low dose of melatonin increased the levels of mRNA encoding Mn- and Cu/Zn-SOD in the Harderian gland of Syrian hamsters [13]. Several investigators have also reported an increase in antioxidant enzymes in the fetal and adult rat brain following treatment with melatonin [39, 50]. The mechanism of protection of melatonin in undernourished pregnancy may therefore be related to an increase in the endogenous placental antioxidant capacity, shifting the vascular oxidant ratio toward dilatation and, thereby, improving placental efficiency and maintaining perfusion and nutrient and oxygen delivery to the fetus. However, it is important to recognize that glucose and amino acid transport is not flow limited unless there are extreme reductions in placental blood flow [51]. Alternative explanations for the protective effect of melatonin on birth weight in undernourished pregnancy independent of an effect on flow may include its effects on the relative proportions of transport surface within each gram of placenta, or in the density of nutrient transporters within that surface area or in changes in concentration gradients. It is known that the human and rat placenta express receptors for melatonin [6–8], but whether melatonin has any receptor-mediated or indirect effects on placental transporters or in the concentration gradient of glucose either in normal or complicated pregnancy remains unknown.
The mechanism underlying the effect of melatonin on placental weight in normal or compromised pregnancy also remains unknown. However, it may be related to the extensively reported anti-proliferative actions of melatonin. For instance, down-regulation of cell proliferation by melatonin administration has been described not only in cancer, but also in normal human epithelial and endothelial cell lines [14, 52–55]. Anti-proliferative effects of melatonin on the fetus may be counter-balanced by the antioxidant effects of melatonin, which increase NO bioavailability, placental perfusion and thereby nutrient and oxygen delivery to the fetus.
An additional point in this study is that maternal treatment with melatonin reduced the placental expression of xanthine oxidase in control pregnancy. This is of interest because the data indicate tonic activation of this pathway in healthy pregnancy and that melatonin may be capable of limiting free radical production secondary to antagonizing its tonic activation.
In this study, it is of interest that maternal undernutrition led to a significant fall in the fetal body weight and an increase in the fetal brain:fetal body weight ratio at day 20 of gestation, and that maternal treatment with melatonin did not affect these changes at day 20 of gestation, but it restored the weight of the pups at birth. An increase in the fetal brain:fetal body weight ratio suggests a greater effect of undernutrition on the fetal body than brain weight and, therefore, relative brain sparing. Disproportionate intrauterine growth retardation is more commonly associated with sustained hypoxia during pregnancy as a result of the redistribution of the fetal cardiac output away from peripheral vascular beds toward essential circulations, such as those perfusing the brain [56]. The data in this work extend the few observations in human pregnancy and experimental studies in the literature indicating that fetal brain sparing may also occur as a result of reductions in nutrient as well as oxygen delivery to the fetus [57–59]. The fact that melatonin did not affect fetal brain sparing in undernourished pregnancy is not only reassuring, but the data also highlight that fetuses in undernourished pregnancies treated with melatonin were still compromised by day 20 of gestation. In these pregnancies the beneficial effects of melatonin on fetal growth became progressively apparent over the last 2 days of pregnancy, a time during which pronounced fetal growth occurs in rats [60] and pup weight almost doubled in the control group described in this study (3.73 ± 0.05 versus 6.52 ± 0.06 g). Biometry was not performed on the cohort of newborn rats to prevent maternal rejection.
The lack of effect of melatonin on placental GPx-1 in contrast to Mn-SOD and catalase expression in undernourished pregnancy in this study might be because of the cellular localization. While Mn-SOD and catalase are both localized to organelles (mitochondria and peroxisome, respectively), GPx-1 is mostly cytosolic [61]. However, at the transcriptional level, there is evidence indicating that melatonin induces the expression of γ-glutamylcysteine synthetase, the rate-limiting enzyme of GSH synthesis, mediated by AP-1 binding [14]. In this context, the present data do not preclude the possibility that melatonin may protect against oxidative damage by increasing the bioavailability of GSH [17], independent of an effect on GPx-1 protein expression.
In conclusion, the data in this study show that maternal undernutrition in late pregnancy in the rat led to a fall in placental efficiency, disproportionate intrauterine growth retardation and a reduction in birth weight. Maternal treatment with melatonin in undernourished pregnancy improved placental efficiency and restored birth weight, and it increased the expression of placental Mn-SOD and catalase. In pregnancy complicated by undernutrition, melatonin may improve placental efficiency and birth weight by upregulating placental antioxidant enzymes.
Acknowledgment
This research was supported by The British Heart Foundation and The BBSRC, UK.




