Expression of the melatonin receptor (MT) 1 in benign and malignant human bone tumors
Abstract
Abstract: The beneficial effects of melatonin on bone homeostasis have been shown in various diseases. As this indoleamine causes dose-dependent modulation of bone-forming osteoblast and bone-resorbing osteoclast activities by receptor-independent and -dependent pathways, we investigated the expression of G-protein-coupled melatonin receptors (MTs) in malignant and non-malignant human bone lesions. By TaqMan polymerase chain reaction (PCR), we analyzed 30 specimens from osteosarcoma and 11 from benign bone tumors for MT1-mRNA expression. Furthermore, we determined mRNA expression levels of the osteoclast activity-stimulating receptor activator of nuclear factor-κ B ligand (RANKL) and its counterpart osteoprotegerin (OPG). Although mean MT1-mRNA levels were similar (P = 0.596) in malignant (4.39 ± 4.98-fold) and benign samples (4.64 ± 6.81-fold), the highest MT1-mRNA levels (up to 27-fold) were observed in individual osteosarcomas, particularly, in two specimens of patients with local recurrence of the tumor. Moreover, mean RANKL- and OPG-mRNA levels were similar in malignant and benign specimens (RANKL: 7.38 ± 9.61-fold versus 3.57 ± 3.11-fold, P = 0.207; OPG: 23.45 ± 32.76 versus 8.07 ± 7.23-fold, P = 0.133). Again, highest RANKL- and OPG-mRNA levels (up to 41- and 160-fold, respectively) were observed in individual osteosarcomas. Expression of MT1-mRNA was confirmed in two human osteosarcoma cell lines (HOS, MG63). High expression levels of MT1-mRNA together with low OPG-mRNA were found in both osteosarcoma cell lines, while in normal human osteoblasts and bone marrow stromal cells, high OPG-mRNA levels were associated with low MT1-mRNA levels. These data on the abundant expression of MT1-mRNA in human bone tumors and osteosarcoma cells lines suggest an important role for MT1 in bone pathology.
Introduction
Bone tissue is constantly remodeled through the synthesis of bone matrix by osteoblasts and the resorption of bone by osteoclasts. The latter is controlled by the receptor activator of nuclear factor-κ B (RANK), its ligand RANKL, and the decoy receptor for RANKL, osteoprotegerin (OPG), which is produced by osteoblasts. OPG negatively regulates RANKL binding to RANK and thereby inhibits bone turnover by osteoclasts [1]. Several factors influence the regulation of bone growth and bone remodeling, including hormones, local tissue factors, neurotransmitters, and neuropeptides [2]. Various studies also show that melatonin participates in the regulation of bone homeostasis by influencing osteoblast and osteoclast functions either in a stimulatory or inhibitory way [3]. For these effects, not only the melatonin released from the pineal gland, but also the concentration of the indolamine produced locally in peripheral tissues as well as the bone marrow might be important [4]. Many effects of melatonin are mediated by its binding and activation of high-affinity receptors, namely the melatonin receptor MT1 (type 1A) and MT2 (type 1B). Both receptors share a close pharmacological profile and are activated by the indoleamine at nanomolar concentrations. They are capable of regulating a number of second messenger systems through the heterotrimeric guanine nucleotide-binding proteins (G proteins) [5]. For example, in pre-osteoblast and rat osteoblast-like osteosarcoma cells, melatonin directly promotes osteoblast maturation, leading to induction of osteoblast marker expression, e.g. bone sialoprotein, alkaline phosphatase, osteopontin, and osteocalcin [6]. Other effects seem to be independent of these receptors, because high (micromolar) concentrations of melatonin are required for example to stimulate proliferation of osteoblastic cell lines and augment collagen synthesis [7]. Contrary to the data on the induction of differentiation by melatonin, a blocking effect of the indoleamine on both, osteoblast and osteoclast differentiation was described in another system, e.g. a sandwich culture of goldfish scale [8]. For this effect, an interaction between the G-protein-coupled melatonin receptors and the estrogen receptor might be responsible [9, 10]. In a recent study, melatonin was found to inhibit triglyceride accumulation as an indicator of adipogenic differentiation in the rat osteosarcoma cell line ROS17/2.8, thereby promoting the osteoblastic pathway in cells with an adipocytic and osteoblastic potential [11]. These findings point to an important role of melatonin in bone cell growth and differentiation, which might require the presence of melatonin receptors in these cells [12, 13]. In fact, expression of MT1 was shown in osteoblasts isolated from healthy human bone specimens recently [14]. Melatonin receptor expression could also be important for the oncostatic effect of the indoleamine, which has been shown in vitro and in vivo in the case of many malignancies [15]. Moreover, in osteosarcoma, it has been hypothesized that the use of melatonin as an adjunct to chemotherapy might improve the quality of life and clinical outcome [16], which is particularly important as many of these patients are of young age [17]. In osteosarcoma, malignant cells arise from osteoblasts, but the pathogenesis and the etiology of this tumor are still unknown and its prognosis is poor. Even if osteosarcoma is localized and treated by surgery and chemotherapy, 30% of patients experience local recurrence and subsequent systemic disease, with a 5-yr survival rate of <55% [15]. Furthermore, benign bone tumors such as benign aneurysmal bone cysts are quite common in young adults. These cysts are expandable osteolytic lesions with a thin wall and blood-filled cystic cavities and are often formed by hemodynamic changes caused by pre-existing non-malignant and malignant tumors [18]. For example, chondromas, which are benign tumors with cartilaginous differentiation, or even osteosarcomas, are frequently found to form a base for this lesion [19]. Although many effects of melatonin on malignant and non-malignant bone cells have been described, to our knowledge no studies investigated whether melatonin receptors are present in these bone tumor cells. Therefore, we evaluated melatonin receptor expression in malignant and non-malignant human bone tumors as well as in human osteoblasts and osteoblastic bone tumor cell lines and we compared the expression of MT1 with that of key factors in bone metabolism, i.e. RANKL and OPG.
Material and methods
Bone tumor samples
Frozen samples were taken from patients (n = 35) undergoing routine surgery for primary bone malignancies and benign bone lesions at the Department of Orthopedic Surgery, Vienna General Hospital. Informed consent was obtained from all patients and the permission for the study was obtained from the Ethics Committee of the Institution.
All surgical samples were routinely subjected to pathological examination. Tumorous parts of specimens not required for further routine diagnosis were used for this investigation. In total, samples from 41 primary bone tumors were investigated. Of the 30 specimens in the osteosarcoma group (24 patients), 12 specimens were of patients, who had osteosarcoma on two different locations (two specimens per patient). Eleven benign specimens were derived from patients with bone cysts (n = 8) and chondroma (n = 3). The characteristics of the tumors of the 24 patients with osteosarcoma are given in Table 1.
| Location | Total (n = 24) | Right (n = 16) | Left (n = 7) |
|---|---|---|---|
| (A) Tumor locationa | |||
| Femur | 14 | 9 | 5 |
| Humerus | 2 | 2 | 0 |
| Tibia | 3 | 2 | 1 |
| Fibula | 2 | 2 | 0 |
| Illium | 1 | 1 | 0 |
| Pelvis | 1 | 0 | 1 |
| Sacrum | 1 | ||
| (B) Histological grading of samples (n = 30) | |||
|---|---|---|---|
| G1 | G2 | G3 | G4 |
| 2 | 3 | 23 | 2 |
| (C) Stageb (n = 24) | |||
|---|---|---|---|
| Stage IB | Stage IIB | Stage III–IV | n.d. |
| 2 | 15 | 6 | 1 |
| (D) Preoperative chemotherapy regimen (22/24 patients)c | |||
|---|---|---|---|
| COSS 96 | EURAMOS | Other | None |
| 18 | 2 | 2 | 2 |
| (E) Regression grade (RG) of tumors in patients receiving preoperative chemotherapy (n = 22)c | |||
|---|---|---|---|
| RG1–3 | RG4–6 | n.d. | |
| 5 | 14 | 3 | |
- aTwelve specimens were from patients (n = 6) with multiple osteosarcoma lesions in femur and tibia.
- bTumor staging was made according to the TNM classification, as described by Enneking [39]. Stage IB, includes tumors classified as T2 (tumor size > 8cm) with moderate differentiation (grade ≤ 2), while stage IIB comprises highly undifferentiated T2 tumors (>8cm, grade ≥ 3). Stage IB and IIB: tumor free lymph nodes and absence of peripheral and skip metastases. Stage III, IV: advanced tumors.
- cTwenty-two patients received neoadjuvant chemotherapy according to the cooperative osteosarcoma study protocol (COSS 96), the Euramos protocol or according to another protocol. The COSS 96 regimen included doxorubicin, methotrexate, cisplatin and ifosfamide [40], methotrexate, doxorubicin and cisplatin was given in the EURAMOS regimen [41]. Preoperative chemotherapy was followed by surgery to remove the primary tumor.
- n.d.: not determined.
Cell culture
hOB cells
The human osteoblast outgrowth (hOB) cells from the explant culture were isolated from human cancellous bone obtained during orthopedic surgery (hip replacement), as described previously [20]. Cells were cultured in RPMI-1640 medium with Glutamax (GibcoBRL, Paisley, UK) supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin G, 100 μg/mL streptomycin and 0.5 μg/mL amphotericin-B.
Human BMSC
Bone marrow stromal cells (BMSC) were harvested from an aspirate of human bone marrow derived from the superior iliac crest of the pelvis [21] and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), (Gibco, BRL) supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin and 0.5 μg/mL amphotericin B. Cells from passage 2 were used.
Tumor cell lines
The human osteosarcoma cell lines, HOS and MG63, were originally derived from the American Tissue Culture Collection (ATCC, Manassas, VA, USA) and the Ewing's sarcoma cell line TC71 from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). Cells were routinely cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin under standard tissue culture conditions. For RNA isolation, sub-confluent cells were harvested and total RNA was extracted using the TRIzol reagent (Invitrogen Life Technologies, Paisley, UK), according to the manufacturer's recommendation.
Quantitative (TaqMan) PCR
RNA from 41 frozen tissue specimens was analyzed for MT1-, OPG- and RANKL-mRNA expression levels. Two micrograms of total RNA was transcribed to cDNA using random hexamer primers (Fermentas, Vilnius, Lithuania) and the Superscript II RT system (Invitrogen, Carlsbad, CA, USA). Polymerase chain reaction (PCR) was carried out with prefabricated TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) for investigation of RANKL and OPG. The endogenous control was 18S rRNA. We accomplished multiplex real-time PCR in an amplification mixture volume of 20 μL. The amplification mixture contained 10 μL of 2X TaqMan Universal PCR Master Mix (Applied Biosystems, no. 4304437), 1 μL of the appropriate 20X Assays-on-DemandTM Gene Expression Assay Mix, 1 μL of 18S rRNA endogenous control assay (Applied Biosystems), 3 μL of nuclease-free water and 10 ng of template cDNA diluted in 5 μL of nuclease-free water. For amplification of MT1 cDNA, we prepared multiplex reactions in a final volume of 25 μL containing 12.5 μL of 2X TaqMan Universal PCR Master Mix (Applied Biosystems, no. 4304437), 0.225 μL of forward primer, 0.225 μL of reverse primer, 1 μL of MT1 TaqMan probe (Applied Biosystems), 1.25 μL of 18S rRNA endogenous control assay (Applied Biosystems), 4.8 μL of nuclease-free water and 10 ng of template cDNA, diluted in 5 μL of nuclease-free water. Universal thermal cycling conditions comprised 2 min at 50°C, 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Fluorescence generation caused by TaqMan probe cleavage by the 5′→3′ exonuclease activity of the DNA polymerase was measured with the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). All samples were amplified in triplicates. To cover the range of expected threshold cycle Ct values that included our amount of target mRNA, a standard curve of six serial dilutions from 50 ng to 500 pg was analyzed for MT1, OPG, RANKL and the endogenous control in multiplex reactions. A cDNA standard was established by pooling bone tissue cDNA as well as cDNA from the osteosarcoma cell lines, HOS and MG63. For analysis, sequence detection software (SDS 1.9.1., Applied Biosystems) results were imported into Microsoft Excel. To calculate the comparable cDNA amount in the experimental samples, the Ct values of the standard dilutions were plotted against the logarithmic input (in nanograms) of cDNA. Relative gene expression data are given as the n-fold change in transcription of the target genes normalized to the endogenous control. The obtained results were calculated in relation to the expression level of a cDNA pool containing bone tissue cDNA and cDNA from the osteosarcoma cell lines HOS and MG63 specified as calibrator (Cal).
Primers and probe sequences
MT1 forward primer 5′-CGG TGT ATC GGA ACA AGA AGC T-3′, reverse primer 5′-AGG TCT GCC ACC GCT AAG C-3′; TaqMan probe: 5′-6-FAM-TCA CCA CAA AGA TGT TTC CTG CGT TCC T-TAMRA-3′ were used; TaqMan Gene Expression Assays (Applied Biosystems) containing intron-spanning primers for OPG Assay ID: Hs00171068_m1; RANKL Assay ID: Hs00243519_m1; TaqMan Endogenous Control Eukaryotic 18S rRNA Part no. 4310893E.
Statistical analysis
Results were analyzed using a commercially available software package (Analyse-it® version 1.73; Analyse-it Software, Ltd, Leeds, UK). Non-normally distributed continuous variables were shown as median, first (Q1) and third (Q3) quartile together with minimum and maximum values (range); normal distributed mean values were compared with a two-sided Student's t-test for unpaired variables, skewed mean values were compared with the non-parametric Wilcoxon rank-sum test or Mann–Whitney U-test, where appropriate. Discrete variables were expressed as numbers (percentages), if appropriate, and compared applying chi-squared test (with Yates’ correction) or Fisher's exact test, respectively. To assess the statistical strength and direction of the relationship between two continuous variables, Spearman's rank correlation was used [22].
Results
Our investigations revealed that MT1-, but not MT2-mRNA is expressed in the two human osteosarcoma tumor cells lines HOS and MG63 at similar levels (Fig. 1, left). Therefore, expression of MT2-mRNA was not further studied in the other samples. Compared with the MT1-mRNA levels of the calibrator consisting of pooled cDNA from osteosarcoma cell lines and osteosarcoma tissue (Fig. 1; Cal), MT1-mRNA expression levels were slightly higher (twofold increase) in the HOS or similar in the MG63 cell line, while in the cell line TC71, originating from a neuroectodermal Ewing's sarcoma, a 5.5-fold increase was observed. In hOB and BMSC, MT1-mRNA levels were near the detection limit. Remarkably, the MT1-mRNA expression pattern was opposite to that of the osteogenic factor OPG (Fig. 1, right), presenting highest OPG mRNA expression levels in hOB (109-fold) and BMSC (33-fold). In HOS and MG63 cells, OPG-mRNA expression levels were moderately higher compared with the calibrator (4- and 8-fold enrichment, respectively), while OPG-mRNA levels were near the detection limit in TC71 cells.
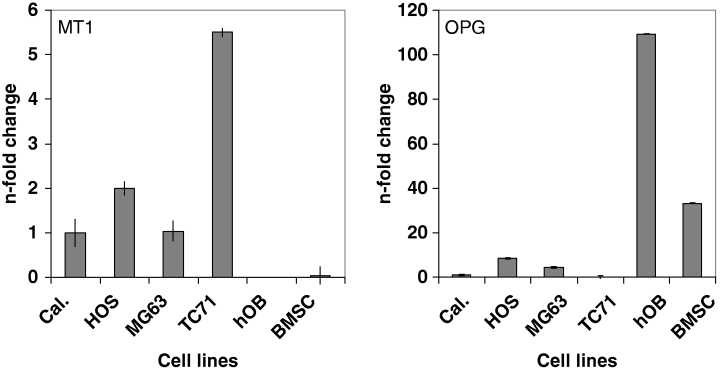
Expression of MT1-(left) and OPG-(right) mRNA in human osteosarcoma cell lines HOS, MG63, human osteoblasts (hOB) and bone marrow stromal cells (BMSC). Relative expression levels of MT1- and OPG-mRNA were determined by TaqMan multiplex PCR. mRNA values represent the average of triplicates (n-fold change) standardized to the expression level of a cDNA pool (Cal), for which a cDNA from human bone tissue and osteosarcoma cell lines (HOS, MG63) was created. The TC-71 Ewing cell line with a high expression rate of MT1-mRNA was used as an additional control.
To evaluate MT1-mRNA expression in human primary bone tumors, we investigated 41 specimens, which were derived from a collective of 35 patients, 18 females and 17 males. The median age of the patients was 17 yr (range 6–69 yr; Q1: 15, Q3: 25 yr). Thirty specimens were from osteosarcoma and 11 from non-malignant primary bone tumors. There was no difference in the distribution of benign and malignant tumor samples derived from females (n = 5 and 15, respectively) and males (n = 6 and 15, respectively) (chi-squared test with Yates’ correction, P = 0.92). From the group of benign tumors, eight specimens were from bone cysts (five aneurysmal and three juvenile bone cysts) and three specimens from chondroma, a benign tumor, which often underlies these cysts. In the group of osteosarcoma patients (30 specimens), six patients had multiple tumors. Two samples were taken from each of these patients. MT1-mRNA expression was detectable in all specimens from osteosarcoma and bone cysts and in the chondroma specimens (Fig. 2). However, MT1-mRNA expression levels varied over a broad range. For example, MT1-mRNA levels near the detection limit were observed in the osteosarcoma specimens 9, 13, 18, 19 derived from patients with G3 tumors in the fibula, tibia and femurs (see Table 2). This was also observed in the chondroma specimen 40, taken from the femur of a 19-yr-old patient. On the other hand, in the osteosarcoma group, the highest expression levels were seen in individual samples (specimen 4, 8, 12, 17 and 20). Remarkably, in this group, local tumor recurrences were detected within a period of 6 months in two patients (Table 2A). However, taking the group of malignant and benign samples as a whole, no difference was observed between mean MT1-mRNA levels (n-fold enrichment compared with Cal) in malignant (4.39 ± 4.98-fold) and benign (4.64 ± 6.81-fold) samples (P = 0.596, Mann–Whitney, two-tailed test). In the latter, MT1-mRNA enrichment was 3.80 ± 2.38-fold in the bone cyst group and 5.97 ± 9.95-fold in the chondroma group. In the group of osteosarcomas, MT1-mRNA levels were not uniformly associated with preoperative chemotherapy sensitivity. The clinical data for patients with respect to chemotherapy, who had the highest and lowest MT1 mRNA levels in the tumor specimens, are summarized in Table 2. For example, very low MT1-mRNA expression levels were seen in the osteosarcoma specimens 18 and 19 from a 23- and a 16-yr-old male patient, respectively (Table 2B). Patient 18 suffered from a tumor in the right femur (G3, stage IIB), and patient 19 from one in the right proximal tibia (G3, stage III). Patient 18 responded poorly to preoperative chemotherapy with a regression grade (RG) of 5, while patient 19 was a good responder (RG1). Interestingly, high enrichment in MT1-mRNA (27-fold compared with the Cal) was seen in the osteosarcoma specimen 8, derived from a 37-yr-old female patient (G3, stage IIB) in the right femur. This patient responded poorly to preoperative chemotherapy (COSS96, RG5) and local recurrence of the tumor was observed. Local recurrence of the tumor was also seen in patient 17 with high MT1 mRNA expression (15-fold), suffering from a tumor in the femur (COSS96, RG5). Furthermore, 18-fold MT1-mRNA levels were observed in specimen 20, a tumor taken from an osteosarcoma in the pelvis of a non-responder (RG6) to COSS96 chemotherapy. Similarly, an 18-fold enrichment was seen in the osteosarcoma specimen 4 from a G3 osteosarcoma taken from the femur of an 18-yr-old patient responding better to preoperative chemotherapy (RG3).
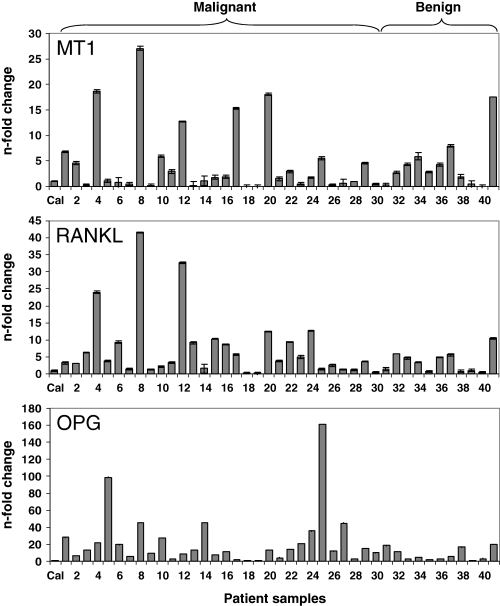
Expression of MT1-, RANKL-, and OPG-mRNA in human malignant and benign bone tumors. Total RNA was isolated from osteosarcoma (n = 30, specimens 1–30) and benign (n = 11) human bone tumors (n = 41). In the latter, 8 samples were from bone cysts (specimens 31–38) and 3 from chondroma (specimens 39–41). Relative expression levels of MT1-, RANKL- and OPG-mRNA were determined by TaqMan multiplex PCR and are expressed as n-fold change relative to the calibrator (Cal).
| Patient no. | Age | Gender | G | Location | Stage | Chemotherapy | RG | MT1 | RANKL | OPG |
|---|---|---|---|---|---|---|---|---|---|---|
| (A) High MT1-mRNA levels | ||||||||||
| 4 | 18 | m | 3 | Femur | IIB | COSS 96 | 3 | 18.6 | 23.9 | 9.3 |
| 8* | 37 | f | 3 | Femur | IIB | COSS 96 | 5 | 27.0 | 41.6 | 45.6 |
| 12 | 25 | m | 3 | Femur | IIB | COSS 96 | 1 | 12.8 | 32.6 | 8.4 |
| 17* | 14 | m | 3 | Femur | IIB | COSS 96 | 5 | 15.4 | 5.7 | 1.9 |
| 20 | 20 | m | 2 | Pelvis | IIB | COSS 96 | 6 | 18.0 | 12.5 | 13.2 |
| (B) Low MT1-mRNA levels | ||||||||||
| 9 | 17 | f | 3 | Fibula | IIB | EURAMOS | n.d. | 0.20 | 1.4 | 22.1 |
| 13 | 15 | f | 3 | Femur | IIB | COSS 96 | 5 | 0.16 | 9.1 | 8.4 |
| 18 | 23 | m | 3 | Femur | IIB | COSS 96 | 5 | 0.03 | 0.1 | 1.9 |
| 19 | 16 | m | 3 | Tibia | III | COSS 96 | 1 | 0.06 | 0.2 | 13.2 |
| (C) High OPG-mRNA levels | ||||||||||
| 5 | 25 | m | 3 | Sacrum | IIB | COSS 96 | 4 | 1.1 | 3.8 | 98.9 |
| 25 | 22 | m | 3 | Femur | IV | COSS 96 | 6 | 5.5 | 1.4 | 160.9 |
- Patient numbers correspond to sample numbers (Fig. 2). MT1, RANKL and OPG values are given as n-fold enrichment (see Fig. 2) Mean MT1-, RANKL- and OPG-mRNA levels in the osteosarcoma group were 3.8-, 7.4- and 23.5-fold higher than in the calculator specimen, respectively. Local recurrences were detected within a period of 6 months following wide surgical resection of the tumor.
- n.d.: not determined.
To compare the expression of MT1-mRNA with that of osteogenic factors, we assessed OPG- and RANKL-mRNA expression in the tumor samples (Fig. 2). As observed for MT1-mRNA expression, mean RANKL-mRNA expression in the malignant group (7.38 ± 9.61-fold) did not differ from that in the benign group (3.57 ± 3.11 n-fold) (P = 0.207). RANKL-mRNA levels did not correlate significantly with that of MT1-mRNA. However, as shown in Fig. 2 and Table 2A, in some individual osteosarcoma specimens, namely specimen 4, 8, 12, 20, high expression levels of MT1-mRNA were associated with high RANKL-mRNA levels (24-, 42-, 33-, and 12-fold, respectively). Remarkably, in specimen 8, presenting the highest MT1-mRNA level, RANKL-mRNA expression was also the highest. As observed for MT1- and RANKL-mRNA, mean OPG-mRNA level in malignant (23.45 ± 32.76-fold) versus benign (8.07 ± 7.23-fold) samples was not significantly different (P = 0.133), and overall OPG-mRNA levels showed no significant correlation with that of MT1-mRNA. This was also true for mean RANKL/OPG-mRNA expression levels (0.64 ± 0.85) in malignant samples, which did not differ (P = 0.812) from that in benign samples (0.71 ± 0.59). Additionally, no association of MT1- and OPG-mRNA levels was seen in individual samples (Table 2A–C). Highest OPG-mRNA expression (161-fold) with moderate MT1- and low RANKL-mRNA levels (5.5- and 1.4-fold, respectively) was seen in the osteosarcoma specimen 25 taken from the right femur of a 22-yr-old male with an advanced tumor (stage IV). This patient did not respond (RG6) to preoperative chemotherapy (COSS96). This was also observed in the osteosarcoma specimen 5 showing an increase in OPG-mRNA expression of 99-fold. This specimen was derived from a 25-yr-old male with a G3 tumor in the sacrum who responded poorly (RG4) to preoperative chemotherapy (COSS96) (Table 2C).
For the total collective of the specimens, we also calculated whether MT1-mRNA levels might correlate with the patients’ age and gender. No correlation was observed between age and MT1-mRNA (rs = 0.21; P = 0.197), RANKL-mRNA (rs = −0.05; P = 0.753), OPG-mRNA (rs = 0.26; P = 0.098), or RANKL/OPG-mRNA (rs = −0.16; P = 0.307) expression levels (Spearman's rank correlation). Likewise, no gender preference for MT1-, RANKL-, or OPG-mRNA expression levels was seen (P = 0.658, 0.297 and 0.549, respectively).
Discussion
We found that MT1-mRNA is abundantly expressed in benign and malignant human bone tumor specimens. The presence of this receptor in osteosarcoma, bone cyst and chondroma specimens as well as in hOB, BMSC and osteosarcoma cells (HOS and MG63) agrees with previous findings that showed that melatonin acts directly on bone cells [12]. Therefore, in addition to its well-known antioxidative effects [23–25], which were thought to prevent osteoclast activity [26], other effects of melatonin on bone cells might be mediated by G-protein receptor signaling [3]. Indeed, induction of osteoblast formation and differentiation have been attributed to a receptor-mediated effect [27]. The receptor isoform MT1, which we detected in osteosarcoma cell lines and other bone tumor specimens, is widely present in a number of cells from normal and malignant human tissues [28]. It has been detected in neuronal and non-neuronal tumors of the prostate and the breast [29, 30]. In the latter, higher MT1 levels were associated with a more malignant phenotype [31]. In our study, despite the finding that the relative expression levels of MT1-mRNA in malignant and non-malignant bone tumors did not differ significantly, the highest MT1 levels were detected in individual osteosarcoma specimens, i.e. in two specimens from patients with a local recurrence of the tumor. Whether this may be a prognostic parameter remains to be established. Furthermore, the high expression in malignant and non-malignant tumors as well as in both osteoblast-derived osteosarcoma cell lines when compared with that in normal hOB and BMSC cells raises the hypothesis that high MT1 expression is associated with a high degree of cell proliferation. High expression levels of melatonin receptors have been found in embryonal tissues exhibiting a high degree of cell proliferation [32, 33]. The sensitivity of some tumor cells to the growth inhibitory effects of melatonin are dependent on the expression of the MT1 receptor [9, 34]. In cancer patients, who often have low melatonin levels, high amounts of MT1 might counteract tumor progression by effectively conferring growth-inhibitory effects on the indoleamine. Melatonin enhances differentiation of human adult mesenchymal stem cell to osteoblasts via activation of the MEK/ERK pathway [27]. Furthermore, recent studies have shown that melatonin induces the expression of transcription factors such as HOXA4, SOX17, which are involved in the regulation of development and differentiation in human mononuclear blood cells [35]. Furthermore, for these mechanisms, melatonin receptors might be important. In humans, melatonin excretion levels do not show gender differences [36], but nighttime levels and the circadian amplitude of the melatonin rhythm decline with age, whereas daytime melatonin levels remain uninfluenced [37]. Although in osteoblasts, higher MT1 levels are rather associated with younger age [14], we did not find a significant relationship between age and MT1 expression levels in the bone lesions investigated. This suggests that expression of MT1 in bone tumors might be regulated independently of circulating melatonin, and perhaps by local concentrations of the indoleamine. This would mean that melatonin synthesis in bone marrow might be of greater importance for local effects on proliferating bone tumor cells [4].
To account for osteoblast-specific factors, we also measured OPG- and RANKL-mRNA expression in the tumor specimens. The expression of OPG and RANKL is developmentally regulated. Whereas OPG increases during osteoblast differentiation, RANKL expression is inversely related to the degree of differentiation. Comparing the two human osteoblastic culture systems that we used, OPG expression was more prominent in the well-differentiated cultured hOB than in the MG63 and HOS cell lines. Moreover, low levels of the osteogenic factor OPG were associated with a high degree of MT1-mRNA in these cell lines. This finding is consistent with our speculation that high levels of MT1 in bone tumors are related to the proliferative status of these lesions, particularly as high MT1- and RANKL-mRNA expression as well as low MT1-mRNA together with high OPG-mRNA expression was observed in some individual osteosarcoma samples in our study. In any case, MT1-mediated effects on cAMP-regulated pathway might play a role in the regulation of bone cell proliferation [38]. In conclusion, we found an abundant expression of MT1-mRNA in human malignant and non-malignant bone tumors and low expression levels in hOB. These findings provide the basis for further investigations on the role of this receptor in bone homeostasis and pathology. Additional studies in greater numbers of patients with malignant and benign tumors are needed to identify MT1-mediated pathways.




