Inhibitory effect of melatonin on diquat-induced lipid peroxidation in vivo as assessed by the measurement of F2-isoprostanes
Abstract
Abstract: Melatonin is a powerful antioxidant and free radical scavenger. A large body of in vivo and in vitro evidence shows that melatonin effectively inhibits membrane lipid peroxidation; this damage was based on the measurement of malondialdehyde and/or 4-hydroxynonenal levels. In the current study, for the first time using a more sensitive and specific biomarker, i.e. F2-isoprostanes, we investigate the effect of melatonin on diquat-induced lipid peroxidation in Fischer 344 rats. When diquat (40 mg/kg body weight) was intraperitoneally injected into rats, the levels of liver F2-isoprostanes were significantly increased at 1, 3, and 6 hr while plasma free F2-isoprostanes concentrations were augmented at 3, 6, and 12 hr after administration of the toxin. In addition, the plasma alanine aminotransferase activity level was measured as a parameter of hepatoxicity; the activity of this enzyme was augmented at 3, 6, and 12 hr after diquat administration when compared with levels of this constituent in untreated control rats. Pretreatment with melatonin (20 mg/kg) 30 min before diquat administration resulted in a significant reduction in both tissue and plasma F2-isoprostanes levels, and plasma alanine aminotransferase activity. These findings, using a sensitive and specific index of lipid peroxidation, show that the hepatoxicity of diquat, at least partially, is a consequence of reactive oxygen species-induced lipid damage. Melatonin's protective effects likely relate to its direct free radical scavenging ability and/or due to other antioxidative processes induced by the indole.
Introduction
Phospholipids in the cell membrane are a major site of attack by reactive oxygen species (ROS) as membranes contain high concentrations of polyunsaturated fatty acids. The allylic hydrogens of polyunsaturated fatty acids are extremely sensitive to attack by free radicals; the resulting damage leads to the generation of peroxyl radicals and to lipid peroxidation. Peroxyl radicals that are generated under these conditions are capable of abstracting allylic hydrogens from neighboring fatty acids to produce additional lipid hydroperoxides, thereby generating a chain reaction of lipid breakdown [1, 2]. Lipid peroxidation is highly devastating because it reduces membrane fluidity making it easier for phospholipids to exchange between the two monolayers and thereby increasing the permeability of the membrane bilayer to substances that do not normally cross the membrane. Lipid peroxidation can also lead to inactivation of membrane-bound receptors or enzymes, which, in turn, may impair normal cellular function. In addition, generation of secondary reactive aldehyde compounds such as 4-hydroxynonenal may contribute to and amplify cellular damage because of their ability to covalently modify critical bimolecules [2, 3]. Thus, measurement of products of lipid breakdown has been commonly used to assess oxidative stress/injury.
The majority of published studies estimating the oxidative destruction of lipids have relied on the measurement of thiobarbituric acid reactive substances (TBARS) [2]. However, the TBARS assay, which is commonly used to measure malondialdehyde (MDA), is not specific to MDA [4]. In addition, both MDA and 4-hydroxynonenal (which is also formed during lipid peroxidation) are very reactive, and therefore, unstable compounds which can form adducts.
A more reliable means for measuring oxidative damage to lipid is the estimation of levels of prostaglandin F2-like compounds (termed F2-isoprostanes) that are formed in vivo from the free radical catalyzed peroxidation of arachidonic acid independent of the cyclooxygenase enzyme [5]. In contrast with MDA, F2-isoprostanes are highly stable compounds which are quantified using gas chromatography/mass spectrometry (GC/MS). In recent years, numerous publications document the reliability and sensitivity of the GC/MS measurement of F2-isoprostanes as an index of oxidative damage to cellular lipids [4, 6].
Melatonin, the principle secretory product of the pineal gland in mammals [7], is also present in bacteria, fungi, algae, insects, reptiles, fish, amphibians, and birds [8–12]. Over the past decade, melatonin has been shown to be a highly effective antioxidant and free radical scavenger [13, 14]. Melatonin reportedly scavenges toxic reactive species including the hydroxyl radical (ḃOH) [15, 16], the superoxide radical (O ) [17, 18], the peroxynitrite anion (ONOO−) [19, 20], the peroxyl radical (LOO) [21], nitric oxide [22, 23] and singlet oxygen (1O2) [24, 25]. Moreover, being highly lipophilic as well as hydrophilic, melatonin readily penetrates all known morphophysiological barriers and accumulates in all tissues and subcellular compartments [26, 27]. A number of in vitro and in vivo reports claim that melatonin effectively protects membrane lipids from oxidative damage, induced by a variety of free radical-generating agents and processes in a variety of tissues [28–38].
) [17, 18], the peroxynitrite anion (ONOO−) [19, 20], the peroxyl radical (LOO) [21], nitric oxide [22, 23] and singlet oxygen (1O2) [24, 25]. Moreover, being highly lipophilic as well as hydrophilic, melatonin readily penetrates all known morphophysiological barriers and accumulates in all tissues and subcellular compartments [26, 27]. A number of in vitro and in vivo reports claim that melatonin effectively protects membrane lipids from oxidative damage, induced by a variety of free radical-generating agents and processes in a variety of tissues [28–38].
To further document the protective actions of melatonin in reducing free radical-mediated lipid oxidation, in the present study, by examining the levels of F2-isoprostanes in Fischer 344 rats treated with a free radical-generating agent diquat, we investigated the ability of melatonin to limit fatty acid deterioration in vivo.
Materials and methods
Chemicals
Melatonin was purchased from Sigma (St Louis, MO, USA) and [2H4]8-Iso-PGF2 was from Cayman Chemical (Ann Arbor, MI, USA). Serum alanine aminotransferase (ALT) kit was from Teco Diagnostics (Anaheim, CA, USA). Diquat was obtained from Chem Service (West Chester, PA, USA). All other chemicals used were of analytical grade and were purchased from commercial sources.
Animals and experiments
Four-month-old male Fischer 344 rats were obtained from Shanghai SLAC Laboratory Animal Company (Shanghai, China). The animals were adapted to an intermittent 12 hr light and 12 hr dark cycle for a week before experimentation. A total of 60 rats were divided into 10 groups: group 1 served as vehicle control that was given 0.9% NaCl; group 2 was given 20 mg/kg melatonin by intraperitoneal (i.p.) injection; rats of groups 3, 5, 7, and 9 were i.p. injected with 40 mg/kg diquat and killed at 1, 3, 6, and 12 hr; rats of groups 4, 6, 8, and 10 were given 20 mg/kg melatonin, 30 min before diquat administration and killed at the same time points as groups 3, 5, 7, and 9. Blood was collected from the inferior vena cava of anesthetized animals into prechilled tubes containing ethylenediaminetetraacetic acid and immediately centrifuged to separate the plasma. A volume of 100 μL of fresh plasma was used for the ALT assay; the remainder of the plasma was frozen and stored in liquid nitrogen until analyzed. Livers were removed and immediately frozen in liquid nitrogen for storage until F2-isoprostanes assay. Experiments were conducted after approval by the appropriate animal care committee.
Alanine aminotransferase assay
Plasma ALT activity was measured using a commercially available kit and the manufacturer's instructions were followed. ALT activities are expressed as IU/mL.
F2-isoprostanes assay
The levels of F2-isoprostanes in liver samples were determined using GC/negative-ion chemical ionization/MS as previously described [39]. Briefly, tissues (150–200 mg) were homogenized in ice-cold Folch solution (chloroform:methanol 2:1) containing 5 mg/100 mL butylated hydroxytoluene. Lipids were then extracted and hydrolyzed with 15% KOH. After acidification with HCL, the F2-isoprostanes were extracted with a C18 Sep-Pak and a silica Sep-Pak column (Waters Corp., Milford, MA, USA), converted to pentafluororobenzyl esters, and purified by thin-layer chromatography (TLC). The purified F2-isoprostanes were derivatized to trimethysilyl ether derivatives and quantified by GC/MS using [2H4]8-Iso-PGF2-α as an internal standard. The amounts of F2-isoprostanes are expressed as nanogram of 8-Iso-PGF2 per gram tissue.
The plasma free F2-isoprostanes levels were determined using the same method described above except for the lipid extraction and base hydrolysis steps: 1 mL plasma was added to high-performance liquid chromatography-grade water (pH 3), mixed with [2H4]8-Iso-PGF2-α as an internal standard. After going through a C18 Sep-Pak and a silica Sep-Pak column, F2-isoprostanes were esterified with pentafluororobenzyl bromide and then subjected to TLC. The purified F2-isoprostanes were then derivatized to trimethysilyl ether derivatives and quantified by GC/MS. The amount of free F2-isoprostanes in plasma was expressed as picogram of 8-Iso-PGF2-α/mL.
Results
The levels of F2-isoprostanes in the liver increased significantly at 1 hr after 40 mg/kg diquat injection and reached the maximum at 3 hr (Fig. 1). When 20 mg/kg melatonin was given 30 min before the diquat injection, the diquat-induced hepatic F2-isoprostanes levels were significantly reduced at 1 hr (6.19 ± 0.44 versus 4.46 ± 0.45), 3 hr (8.76 ± 0.57 versus 6.06 ± 0.59), and 6 hr (5.89 ± 0.34 versus 4.83 ± 0.41) (P ≤ 0.05 in each case).
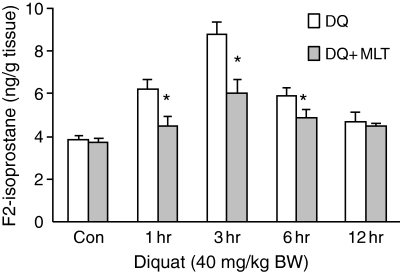
The effect of melatonin (20 mg/kg) on diquat (40 mg/kg)-induced F2-isoprostanes in liver. Results are given as mean ± S.E.M. (n = 6) and *P ≤ 0.05 versus diquat only at the same time.
Fig. 2 shows that the plasma free isoprostanes levels, as an index of total endogenous production of lipid peroxidation from all tissues, significantly increased at 3 hr (61.5 ± 3.92), 6 hr (86.2 ± 5.11), and 12 hr (67.8 ± 4.16) after diquat treatment compared with untreated control rats (36.8 ± 2.45). A quantity of 20 mg/kg melatonin significantly reduced diquat-induced F2-isoprostanes levels in plasma at 3 hr (61.5 ± 3.92 versus 48.0 ± 2.34) and 6 hr (86.2 ± 5.11 versus 64.2 ± 5.2) (P ≤ 0.05 in each case).
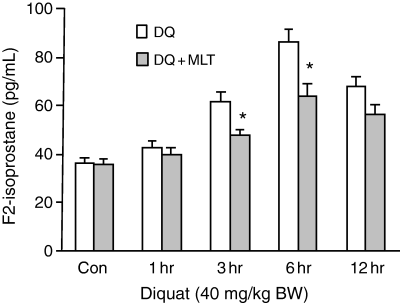
The effects of melatonin on diquat-induced free F2-isoprostanes level in rat plasma. Results are given as mean ± S.E.M. (n = 6) and *P ≤ 0.05 versus diquat only at the same time.
A single dose of 20 mg/kg melatonin not only dramatically inhibited the levels of F2-isoprostanes, but also significantly reduced plasma ALT activity (a parameter of hepatoxicity) which was increased at 3, 6, and 12 hr after diquat treatment. The reductions at 3 hr (69.6 ± 4.95 versus 52.1 ± 3.2; P ≤ 0.05) and 6 hr (123.1 ± 8.56 versus 88.6 ± 6.02; P ≤ 0.01) were statistically significant (Fig. 3).
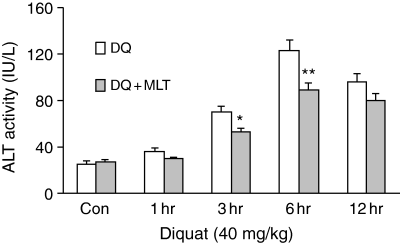
Melatonin's effect on diquat-induced plasma alanine aminotransferase levels in Fischer 344 rats. Results are given as mean ± S.E.M. (n = 6) and *P ≤ 0.05, **P ≤ 0.01 versus diquat only at the same time.
Discussion
Diquat (1,1′-ethylene-2,2′-dipyridylium) is a hepatotoxic bipyridyl herbicide, which is widely used in the USA and other parts of the world [40, 41]. In vitro and in vivo studies have shown that diquat-induced hepatic injury is due to its free radical generation through redox cycling of the herbicide [41–44]. In animals, diquat is readily converted by one electron reduction to free radicals which react rapidly with dioxygen (O2). This reaction regenerates the native bipyridyl and converts O2 to the O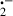 . Thus, in the presence of sufficient supply of reducing equivalents such as nicotinamide adenine dinucleotide phosphate, a small amount of bipyridyl can generate large numbers of O
. Thus, in the presence of sufficient supply of reducing equivalents such as nicotinamide adenine dinucleotide phosphate, a small amount of bipyridyl can generate large numbers of O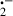 and singlet oxygen; O
and singlet oxygen; O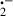 is subsequently converted to the highly toxic ḃOH [41, 42, 45]. These ROS interact with polyunsaturated fatty acids forming fatty acid-free radicals and, with further oxidation, lipid hydroperoxide radicals. The hydroperoxide radicals then maintain the formation of new fatty acid radicals while being converted to lipid peroxides in a chain reaction resulting in extensive lipid peroxidation [45].
is subsequently converted to the highly toxic ḃOH [41, 42, 45]. These ROS interact with polyunsaturated fatty acids forming fatty acid-free radicals and, with further oxidation, lipid hydroperoxide radicals. The hydroperoxide radicals then maintain the formation of new fatty acid radicals while being converted to lipid peroxides in a chain reaction resulting in extensive lipid peroxidation [45].
F2-isoprostanes are prostaglandin-like compounds resulting from free radical-catalyzed peroxidation of arachidonic acid [5]. They are initially esterified to phospholipids and subsequently released by phospholipase [46]. Free F2-isoprostanes can be quantitated in plasma and other biological fluids using MS. Phospholipid esterified F2-isoprostanes can be similarly determined after extraction from tissues and base hydrolysis. The quantitation of free F2-isoprostanes in plasma and esterified F2-isoprostanes has proven to be a valuable tool for measuring lipid peroxidation in a number of tissues [4, 47].
In the present study, 1 hr after administration of diquat, the levels of esterified hepatic F2-isoprostanes in rats significantly increased and reached a maximum at 3 hr. However, the plasma ALT levels, an index of hepatoxicity, increased at 3 hr and reached their peak at 6 hr. This indicates that free radical-induced lipid peroxidation is the cause of diquat-induced toxicity and not a result of it. Meanwhile, plasma free F2-isoprostanes, an index of whole body endogenous production of lipid peroxidation, first increased at 3 hr after diquat. This is likely due to the action of phospholipase. Esterified F2-isoprostanes require time to release from the membranes into the blood. Also, the incremental increases of F2-isoprostanes levels in plasma were significantly higher than in liver which suggests that, besides liver damage, diquat also induces lipid peroxidation in other organs as well.
Melatonin, at a single dose of 20 mg/kg, efficiently inhibited diquat-induced lipid peroxidation as well as the associated hepatotoxicity as indicated by the fact that melatonin reduced circulating ALT levels. Melatonin's protective effects may be related to any of the following actions of the indoleamine: (i) melatonin directly scavenges a variety of toxic oxygen and nitrogen-based reactants including the highly toxic ḃOH [15, 16] and its precursor hydrogen peroxide (H2O2) [48], ONOO− and its precursor NO [19, 20], the LOOḃ [21], and the singlet oxygen (1O2) [24, 25]. Diquat-induced hepatoxicity is believed to be due to redox cycling properties of the molecule. The one-electron reduction/oxidation of this bipyridium compound generates large numbers of O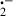 as well as 1O2, H2O2, and ḃOH [42, 45]. (ii) In addition to its direct free radical detoxifying activity, melatonin also functions as an indirect antioxidant by stimulating the mRNA levels and the activities of superoxide dismutase (SOD) [49, 50], glutathione peroxidase [51], and glutathione reductase [52, 53]. Elevated activities of these enzymes have an important protective role in reducing diquat-induced lipid peroxidation and molecular damage [54, 55]. (iii) Besides these direct and indirect beneficial actions, it was recently reported that melatonin has a high metal binding affinity [56]. In the study in question, melatonin was shown to bind Fe3+, thus preventing it from being reduced to Fe2+ which promotes the formation of the ḃOH via the Fenton reaction. Diquat has been shown to be a highly potent generator of O
as well as 1O2, H2O2, and ḃOH [42, 45]. (ii) In addition to its direct free radical detoxifying activity, melatonin also functions as an indirect antioxidant by stimulating the mRNA levels and the activities of superoxide dismutase (SOD) [49, 50], glutathione peroxidase [51], and glutathione reductase [52, 53]. Elevated activities of these enzymes have an important protective role in reducing diquat-induced lipid peroxidation and molecular damage [54, 55]. (iii) Besides these direct and indirect beneficial actions, it was recently reported that melatonin has a high metal binding affinity [56]. In the study in question, melatonin was shown to bind Fe3+, thus preventing it from being reduced to Fe2+ which promotes the formation of the ḃOH via the Fenton reaction. Diquat has been shown to be a highly potent generator of O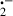 [42]; large amounts of O
[42]; large amounts of O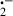 can be rapidly converted to H2O2 by enzyme SOD in vivo. H2O2, although itself not very active, is metabolized to the ḃOH in the presence of transition metals such as Fe2+. Thus, removal of Fe3+ by melatonin would reduce ḃOH generation from its precursor H2O2; (iv) important recent discoveries also show that metabolites of melatonin, which are formed when it scavenges toxic reactants are themselves potent detoxifiers of ROS [57, 58]. Thus, not only the parent molecule, melatonin, but also its metabolites sequentially neutralize toxic reactants. This greatly increases the efficacy of melatonin as a protector against oxygen and nitrogen-based reactants [59, 60]. (v) There is also evidence that melatonin promotes intracellular glutathione levels; glutathione is an important antioxidant and the fact that melatonin stimulates its synthesis [61] could contribute to melatonin's protective actions against oxidative damage. (vi) The ability of melatonin to increase the efficiency of electron transport through the mitochondrial respiratory chain likely provides melatonin an advantage as an antioxidant. While functioning in this capacity, melatonin reduces electron leakage and thereby free radical generation [62]; (vii) being highly lipophilic as well as somewhat hydrophilic [26, 27], melatonin easily enters cells and subcellular compartments which prevents oxidative damage to a variety of molecules. This is a feature not shared by most antioxidants which are typically compartmentalized in either the lipid-rich or aqueous environments of the cell [63, 64]. Any or all of these actions of melatonin may have contributed to its ability to reduce diquat-induced lipid peroxidation and hepatoxicity.
can be rapidly converted to H2O2 by enzyme SOD in vivo. H2O2, although itself not very active, is metabolized to the ḃOH in the presence of transition metals such as Fe2+. Thus, removal of Fe3+ by melatonin would reduce ḃOH generation from its precursor H2O2; (iv) important recent discoveries also show that metabolites of melatonin, which are formed when it scavenges toxic reactants are themselves potent detoxifiers of ROS [57, 58]. Thus, not only the parent molecule, melatonin, but also its metabolites sequentially neutralize toxic reactants. This greatly increases the efficacy of melatonin as a protector against oxygen and nitrogen-based reactants [59, 60]. (v) There is also evidence that melatonin promotes intracellular glutathione levels; glutathione is an important antioxidant and the fact that melatonin stimulates its synthesis [61] could contribute to melatonin's protective actions against oxidative damage. (vi) The ability of melatonin to increase the efficiency of electron transport through the mitochondrial respiratory chain likely provides melatonin an advantage as an antioxidant. While functioning in this capacity, melatonin reduces electron leakage and thereby free radical generation [62]; (vii) being highly lipophilic as well as somewhat hydrophilic [26, 27], melatonin easily enters cells and subcellular compartments which prevents oxidative damage to a variety of molecules. This is a feature not shared by most antioxidants which are typically compartmentalized in either the lipid-rich or aqueous environments of the cell [63, 64]. Any or all of these actions of melatonin may have contributed to its ability to reduce diquat-induced lipid peroxidation and hepatoxicity.
It is well known that melatonin limits free radical-induced oxidative damage to various macromolecules. In the present study, using F2-isoprostanes levels as an alternative sensitive and accurate measurement of lipid peroxidation, we confirm that melatonin is an excellent antioxidant, which protects against diquat-induced lipid peroxidation and hepatoxicity. Considering its low acute and chronic toxicity, melatonin's clinical application against oxidative damage because of a variety of biological toxins (such as the herbicide diquat) should be considered.




