The stimulatory effect of mu- and delta-opioid receptors on bovine pinealocyte melatonin synthesis
Abstract
Abstract: Mammalian pinealocytes synthesize and secrete melatonin. The synthesis of melatonin is regulated by several biogenic amine, amino acid and peptide transmitters. In our previous study, the delta- and mu-opioid receptors have been identified and characterized in bovine pinealocytes. In order to elaborate the function of different types of opioid receptors in regulating melatonin synthesis, we used a selective mu-opioid receptor agonist, Tyr-[d-Ala2, N-methyl-phe4, glycol5] (DAMGO), a selective delta-opioid receptor agonist, Enkephalin [d-Pen2, d-Pen5], (DPDPE) and a selective kappa-opioid receptor agonist, ((+)-(5α, 7α, 8β)-N-methyl-N-[7- (1-pyrrolidinyl)-1-oxaspiro [4,5] dec-8-yl]-benzene acetamide) (U69593) to investigate the activity of N-acetyltransferase (NAT) activity and melatonin secretion. The results of the present study show that both DAMGO and DPDPE stimulated NAT activity and increased the level of melatonin in cultured bovine pinealocytes. These stimulatory effects were blocked by naloxone, an opioid receptor antagonist. However, the kappa-opioid receptor agonist U69593 was unable to alter either the activity of NAT or the level of melatonin. In order to clarify the mechanism of how the activation of mu- and delta-opioid receptors in bovine pinealocytes leads to an increase in NAT activity, cyclic AMP levels were measured after bovine pinealocytes were treated with morphine, DAMGO and DPDPE. The results indicated that these stimulatory effects acted via induction of cAMP production. This study reveals that the stimulatory effect of opioid receptor on melatonin synthesis is mediated via the activation of adenylate cyclase system.
Introduction
Mammalian pinealocytes are neuroendocrine cells of the pineal gland that synthesize and secrete melatonin into the blood circulation [1]. There is also the intriguing possibility that the pineal gland secretes melatonin into cerebrospinal fluid [2]. The synthesis of melatonin is regulated not only by norepinephrine but also by other biogenic amine, amino acid and peptide transmitters [3, 4]. The opioid peptides are one of the peptide transmitters that regulate pineal functions. The presence of opioid peptides derived from three precursors, namely, pro-opiomelanocortin, pro-enkephalin, and pro-dynorphin has been detected in mammalian pineal gland by radioimmunoassay [5, 6, 7, 8]. Moreover, by utilizing immunohistochemical techniques [9, 10] the presence of opioidergic nerve fibers has been demonstrated in the pineal gland of guinea pigs [11], humans [12], cows [13], European hamsters [14] and tree shrews [15]. In addition, the opioid peptides have been localized in the pinealocytes of the pineal gland of European Hamsters [14] and in the intrapineal neuronal-like cells of guinea pigs [11] and humans [12].
Previous studies have suggested that opioids alter the synthesis of melatonin. For example, the subcutaneous injection of des-tyrosine-β-endorphin increased melatonin levels [16] and morphine stimulated the release of melatonin from rat pineal glands [17]. However, morphine significantly decreased the plasma melatonin level in pigs exposed to light [18]. Melatonin was shown to stimulate the release of endogenous opioid peptide in rat periaqueductal gray. Then the relationship between opioid and melatonin related to the central opioid system of the analgesic action has been suggested [19].
Our previous studies have demonstrated that the activation of opioid receptors by adding morphine to pineal cultures enhanced the activity of N-acetyltransferase (NAT) and augmented the formation of melatonin, and the stimulatory action was blocked by naloxone [20, 21]. By using [3H]diprenorphine, it was demonstrated that these opioid receptors were located on the pinealocytes, and the majority of them were δ opioid receptors, whereas the minority were μ opioid receptors [20]. However, Aloyo [21] indicated that only the δ opioid receptor existed in the pineal gland. More recently, we have used the selective radioligand, [3H]DAMGO, [3H]DPDPE, [3H]U69593 and [3 H]orphanin FQ (OFQ), together with a number of the opioid ligands, and found the presence of δ and μ but not κ or ORL1 receptors in bovine pinealocytes [22, 23]. The presence of two different types of opioid receptors prompted us to further elucidate the function of each receptor in bovine pinealocytes in regulating melatonin synthesis.
Materials and methods
Chemicals
[3H]acetyl coenzyme A (specific activity 2.83 Ci/mmol), and [3H]melatonin (specific activity: 75.7 Ci/mmol), and [125I] cAMP RIA kit were purchased from New England Nuclear (Boston, MA, USA). DPDPE (Enkephalin, [d-Pen2, d-Pen5]), DAMGO (Tyr-[d-Ala2, N-methyl-phe4, glycol5]), ((+)-(5α, 7α, 8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro [4,5] dec-8-yl]-benzene acetamide) U69593, morphine, naloxone, bovine serum albumin, tryptamine hydrochloride, tricine, dextra-coated charcoal isobutyl-1-methylxanthine and melatonin hydrochloride were purchased from Sigma (St Louis, MO, USA). Dulbecco's modified Eagle medium (DMEM), fetal calf serum, penicillin, streptomycin, fungisone and other chemicals related to bovine pinealocyte culture were purchased from Gibco, Grand Island Biological Company (Grand Island, NY, USA). Sheep anti-melatonin antiserum was purchased from Stockgrand Ltd (Guildford, Surrey, UK). All other chemicals and reagents were of the highest commercially available purity, purchased mainly from Sigma, E. Merck (Darmstadt, Germany), and May and Baker Ltd (Dagenham, UK). All solutions containing the drugs were freshly prepared for each experiment.
Animals
Fresh bovine pineal glands were dissected from the brains of 6–12 months old cows (Bos taurus) between 10:00 and 11:00 hr from a local slaughterhouse immediately after the animals were slaughtered. The glands were sliced and kept in ice-cold Dulbecco's modified Eagle medium (DMEM) containing 10% fetal calf serum (pH 7.4) while being transported to the laboratory.
Method for culturing pinealocytes
The preparation of pinealocytes was performed according to our previous procedure [20, 24]. Ten fresh bovine pineal glands were dissected free of adhering connective tissues, blood vessels, and the pineal stalks. The pineal glands were minced with a sterile razor blade for 15 min, suspended in DMEM and then triturated with a Pasteur pipette for 15 min. All steps were carried on ice. The minced tissues were resuspended with DMEM and sediment on ice for 7 min. The suspensions were then filtered through a circle nylon mesh (pore size: 41 μm; diameter: 13 cm). Then the cell suspensions were centrifuged at 800 × g at 4°C for 5 min and pellets were collected. Collected cell numbers were then counted.
The pinealocytes were incubated in the culture medium, which was made in DMEM and Ham's F12 (1:1 V/V), with 1% fetal calf serum, 250 IU/mL penicillin and 25 μg/mL streptomycin (in case of cAMP determination, 5 × 10−4 M isobutyl-1-methylxanthine was included in the media). Each well contained 5 × 106 pinealocytes in 1 mL medium. For routine procedures, the pinealocytes were acclimatized by preincubating them at 37°C in a 5% CO2incubator for 2 hr prior to testing the effects of the drugs. The pinealocytes were preincubated for 30 min in the presence of the antagonist prior to the addition of the agonist. The period of incubation for testing the effects of opioid receptor agonists was 2 hr. At the end of the treatment, all pinealocytes were collected in 1.5 mL microcentrifuge tubes and centrifuged at 4000 × g, 4°C for 5 min. The pellets were collected and frozen immediately on dry ice for assay of NAT activity, and cAMP level whereas the cultured media were collected and stored at −80°C for melatonin determination.
Assay of NAT in pinealocytes
The activity of NAT in bovine pinealocytes was determined according to a technique of Deguchi and Axelrod as adapted by Govitrapong et al. [20, 21]. The pinealocytes were homogenized in 40 μL of 0.1 M sodium phosphate buffer (pH 6.5) containing [3H]acetyl CoA (specific activity 2.83 Ci/mmol, 0.1–1.0 μCi/assay tube). An aliquot of 30 μL of the homogenate was transferred into a test tube, which already contained 10 μL of tryptamine in 0.1 M sodium phosphate buffer (pH 6.5), giving a final concentration of 10 mM in the reaction mixture. The mixture was then incubated at 37°C for 20 min and stopped by adding 1 mL of water-saturated chloroform. Thereafter the solution was vortexed for 15 s and centrifuged at 1000 rpm for 5 min. The aqueous layer was aspirated and the extracted chloroform layer was washed twice with 0.2 mL of 0.1 M sodium phosphate buffer. An aliquot of 0.5 mL of the chloroform extract was transferred into a scintillation vial and dried in a hood. Radioactivity was counted in 5 mL of scintillation fluid by a liquid scintillation spectrometer. The activity of NAT was expressed as fmol of N-[3H]acetyltryptamine formed/mg protein/hr.
Melatonin determination
The melatonin level in the cultured media was determined by radioimmunoassay. The cultured media were diluted with assay buffer (0.1 M tricine containing 0.9% NaCl and 0.1% gelatin). Samples were incubated with the specific antisera against melatonin (G/S/704-6438), at a final dilution of 1:4000 and the [3H]melatonin for 18 hr at 4°C. Dextran-coated charcoal was used to separate bound from free tracer. The reaction mixture was centrifuged at 3000 rpm for 15 min at 4°C. Seven hundred microliters of the supernatant was aliquoted into a scintillation vial that contained scintillation fluid. Radioactivity was counted by a liquid scintillation spectrometer. The amount of melatonin was then calculated and the levels were normalized against the total pinealocyte protein concentration.
Cyclic AMP determination
The pinealocytes were incubated in the culture medium. After treated cell with morphine or DPDPE or DAMGO for 2 hr, cells were collected by centrifugation (10 000 × g, 5 min). The supernatant was aspirated, and cells were placed on dry ice. The frozen cell pellets were added with 5 mM acetic acid (200 μL), then sonicated and boiled at 95°C for 5 min immediately after collection. The suspension was centrifuged (10 000 × g, 5 min) and the supernatants (100 μL) were taken for intracellular cAMP determination. The samples were acetylated before analyzing cAMP by a commercial [125I]cAMP RIA assay kit (New England Nuclear).
Protein determination
The protein concentrations of the pinealocyte homogenates were determined according to a procedure developed by Bradford et al. [25] using bovine serum albumin as a standard.
Statistical analysis of data
All data are present as mean ± S.E.M. When average data are given in percentage of control value, they are means of five to eight independent experiments. The data are expressed as mean ± S.E.M. derived from experiment indicated and were analyzed by ANOVA, and the statistical evaluation of the results was performed by using the Tukey–Kramer Multiple Comparisons test and Mann–Whitney test.
Results
Bovine pinealocytes were treated with morphine in concentrations ranging from 1 to 100 μM . After pinealocytes were incubated for 2 hr, the activity of NAT and the level of melatonin were increased significantly. These effects were concentration dependent. Morphine (1, 10 and 100 μM) significantly stimulated the activity of NAT of the bovine cultured pinealocytes by 1.6-, 1.8- and 2.4-fold, respectively, when compared with control groups (Fig. 1, upper panel). Morphine (1, 10 and 100 μM) also enhanced the melatonin secretion by 1.9-, 2.4- and 3.3-fold, respectively (Fig. 1, lower panel). The stimulatory effect of morphine was abolished by 100 μM of naloxone (Fig. 2).

Effect of morphine on the activity of N-acetyltransferase (upper panel) and the level of melatonin (lower panel) in cultured bovine pinealocytes. The bovine pinealocytes were preincubated at 37°C for 2 hr in 5% CO2 incubation chamber prior to addition of 1, 10 and 100 μM morphine. After further incubation for 2 hr, pinealocytes were collected and centrifuged at 4000 × g, 4°C, 5 min. The pellets were frozen immediately on dry ice for assayed of NAT activity (upper panel) according to the method described in Materials and methods section. The cultured media were kept and stored at −80°C until assays for melatonin secretion (lower panel) according to the method described in Materials and methods section. Each value represents the mean ± S.E.M. of seven separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with control.
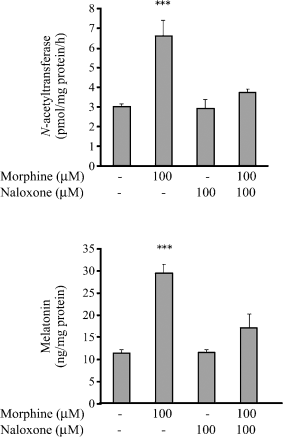
Effect of naloxone on the activity of N-acetyltransferase (upper panel) and the level of melatonin (lower panel) in cultured bovine pinealocytes stimulated by morphine. The bovine pinealocytes were preincubated at 37°C for 2 hr in 5% CO2 incubation chamber prior to addition of drugs. The bovine pinealocytes were preincubated for 30 min in the presence of 100 μM naloxone prior to the addition of 100 μM morphine and further incubated for 2 hr. All pinealocytes were collected and centrifuged at 4000 × g, 4°C, 5 min. The pellets were frozen immediately on dry ice for assayed of NAT activity (upper panel) according to the method described in Materials and methods section. The cultured media were kept and stored at −80°C until assays for melatonin secretion (lower panel) according to the method described in Materials and methods section. Each value represents the mean ± S.E.M. of seven separate experiments. ***P < 0.001 when compared with control.
Bovine pinealocytes were treated with 10 and 100 μM DAMGO. After pinealocytes were incubated for 2 hr, the activity of NAT and the level of melatonin were increased significantly, in a concentration-dependent manner. DAMGO (10 and 100 μM) significantly stimulated the activity of NAT in the cultured bovine pinealocytes by 1.9- and 2.4-fold when compared with the control group (Fig. 3, upper panel). In addition, 10 and 100 μM DAMGO enhanced the melatonin secretion by 1.5- and 1.9-fold, respectively (Fig. 3, lower panel). Naloxone (100 μM) partly blocked the effects of DAMGO (10μM) on the activity of NAT and the melatonin secretion.

Effect of mu-opioid agonist (DAMGO) on the activity of N-acetyltransferase (upper panel) and the level of melatonin (lower panel) in cultured bovine pinealocytes. The bovine pinealocytes were preincubated at 37°C for 2 hr in 5% CO2 incubation chamber prior to addition of drugs. The bovine pinealocytes were preincubated for 30 min in the presence of 100 μM naloxone prior to the addition of 10 μM DAMGO and further incubated for 2 hr. All pinealocytes were collected and centrifuged at 4000 × g, 4°C, 5 min. The pellets were frozen immediately on dry ice for assayed of NAT activity (upper panel) according to the method described in Materials and methods section. The cultured media were kept and stored at −80°C until assays for melatonin secretion (lower panel) according to the method described in Materials and methods section. Each value represents the mean ± S.E.M. of seven separate experiments. **P < 0.01, ***P < 0.001 when compared with control.
The bovine pinealocytes were treated with 10 and 100 μM DPDPE. After pinealocytes were incubated for 2 hr, the activity of NAT and the melatonin secretion increased significantly. The effects were concentration dependent. DPDPE (10 and 100 μM) significantly stimulated the activity of NAT of the cultured bovine pinealocytes by 1.5- and 2.5-fold when compared with the control group (Fig. 4, upper panel). In addition, 10 and 100 μM DPDPE enhanced the melatonin secretion by 1.7- and 2.3-fold, respectively (Fig. 4, lower panel). Furthermore, naloxone (100 μM) completely blocked the effects of DPDPE (10 μM) on the activity of NAT and the melatonin secretion.
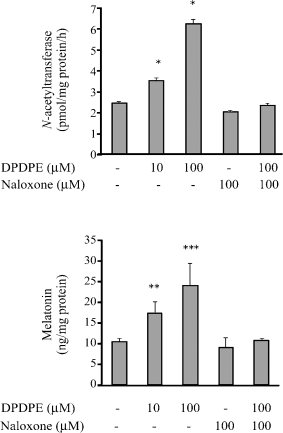
Effect of delta-opioid agonist (DPDPE) on the activity of N-acetyltransferase (upper panel) and the level of melatonin (lower panel) in cultured bovine pinealocytes. The bovine pinealocytes were preincubated at 37°C for 2 hr in 5% CO2 incubation chamber prior to addition of drugs. The bovine pinealocytes were preincubated for 30 min in the presence of 100 μM naloxone prior to the addition of 10 μM DPDPE and further incubated for 2 hr. All pinealocytes were collected and centrifuged at 4000 × g, 4°C, 5 min. The pellets were frozen immediately on dry ice for assayed of NAT activity (upper panel) according to the method described in Materials and methods section. The cultured media were kept and stored at −80°C until assays for melatonin secretion (lower panel) according to the method described in Materials and methods section. Each value represents the mean ± S.E.M. of seven separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with control.
The bovine pinealocytes were treated with 10 and 100 μM U69593. After pinealocytes were incubated for 2 hr, the activity of NAT and the melatonin secretion did not change (Fig. 5).
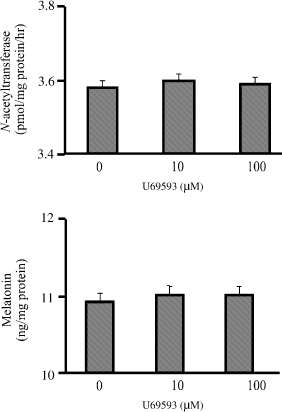
Effect of kappa-opioid agonist (U69593) on the activity of N-acetyltransferase (upper panel) and the level of melatonin (lower panel) in cultured bovine pinealocytes. The bovine pinealocytes were preincubated at 37°C for 2 hr in 5% CO2 incubation chamber prior to addition of drugs. The bovine pinealocytes were preincubated for 30 min in the presence of 100 μM naloxone prior to the addition of 10, 100 μM U69593 and further incubated for 2 hr. All pinealocytes were collected and centrifuged at 4000 × g, 4°C, 5 min. The pellets were frozen immediately on dry ice for assayed of NAT activity (upper panel) according to the method described in Materials and methods section. The cultured media were kept and stored at −80°C until assays for melatonin secretion (lower panel) according to the method described in Materials and methods section. Each value represents the mean ± S.E.M. of seven separate experiments.
Treatment with 10 and 100 μM morphine significantly increased the cAMP level by 1.6- and 2.9-fold, respectively (Fig. 6, upper panel), 10 and 100 μM DAMGO significantly increased the cAMP level by 1.2- and 1.3-fold, respectively (Fig. 6, middle panel), and 10 and 100 μM DPDPE significantly enhanced the cAMP level by 1.5- and 1.7-fold, respectively (Fig. 6, lower panel). Naloxone (100 μM) blocked the stimulatory effects of 10 μM of morphine, DAMGO and DPDPE (Fig. 6).
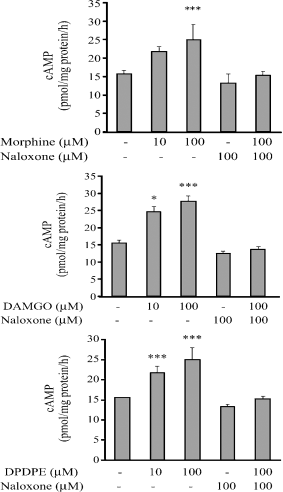
Effect of morphine (upper panel), DAMGO (middle panel), DPDPE (lower panel) on the level of cAMP level in cultured bovine pinealocytes. The bovine pinealocytes were preincubated at 37°C for 2 hr in 5% CO2 incubation chamber prior to the addition of drugs. The bovine pinealocytes were preincubated for 30 min in the presence of 100 μM naloxone prior to the addition of 10 μM different opioid agonists and further incubated for 2 hr. All pinealocytes were collected and centrifuged at 4000 × g, 4°C, 5 min. The pellets were frozen immediately on dry ice for assayed of cAMP. Each value represents the mean ± S.E.M. from at least seven separate experiments. *P < 0.01,***P < 0.001 when compared with control.
Discussion
In order to elaborate the function of different types of opioid receptors in the bovine pineal gland, we used a selective mu-opioid receptor agonist (DAMGO) and a selective delta-opioid receptor agonist (DPDPE) to investigate the activity of NAT. The results of this study showed that both DAMGO and DPDPE stimulated NAT activity and increased the level of melatonin in cultured bovine pinealocytes. These stimulatory effects were blocked by naloxone, an opioid receptor antagonist. However, the kappa-opioid receptor agonist U69593 was unable to alter either the activity of NAT or the level of melatonin. This view supports our previous results of the existence of mu- and delta- but not kappa-opioid receptors in bovine pinealocytes by using radioligand binding [22, 24]. In addition, morphine, DAMGO and DPDPE stimulated intracellular cAMP formation and the stimulatory effect was abolished by naloxone. However, the concentrations (micromolar range) of the stimulatory effects of opioid agonists far exceed the dissociation equilibrium constant (Kd) values (nanomolar range) of the opioid receptors [24]. This is due to cultured media contained variety of chemicals such as fetal calf serum, and various ions, especially sodium ion [26] which reduce the binding of the opioid agonists to the receptors.
At least two regulatory mechanisms control melatonin synthesis. In rodents, transcriptional regulation plays an essential role in the switch-on of NAT at night. Norepinephrine controls NAT transcription through a cAMP mechanism involving activation of protein kinase A and phosphorylation of cAMP response element binding protein (CREB). Phosphorylated CREB (pCREB) activates expression via cAMP response elements (CREs) in the NAT promoter and remarkably increases NAT mRNA levels [27]. The increase in NAT mRNA content is followed by the increases in NAT protein levels and activity [28]. Translation of NAT mRNA results in production of NAT protein, which has two fates: destruction by proteasomal proteolysis or protection/activation. In all vertebrates, cAMP-dependent phosphorylation prevents destruction by causing NAT to bind to 14-3-3 proteins, forming a reversible complex in which NAT is activated and protected from proteolysis [29].
Traditionally, opioid receptor activation results in an inhibition of neurotransmission. This inhibition of neurotransmission is caused by coordinated alteration at the cellular level, including the closing of voltage-sensitive Ca2+ channels, hyperpolarization of the cell via stimulation of K+ efflux and inhibition of cAMP formation [30]. However, opioids also stimulate neurotransmitter release in various cell types [31] including dopamine from the rat basal mesolimbic structure in vivo [32] and other dopamine neuron [33], acetylcholine from the rabbit retina [34] substance P, calcitonin gene-related peptide, cholecystokinin, and adenosine from dorsal root ganglion neuronal culture [35]. The stimulatory effects of opioids involved not only the disinhibition of interneurons but also a direct stimulation of target neurons themselves [31]. Considerable evidence has accumulated to indicate that opioids and opiates can stimulate adenylate cyclase in brain membrane. Activation of the μ-opioid receptor with an increase in cAMP production, rather than a decrease in cAMP production has been demonstrated in a trasgene study [36]. An article on the effects of the endormorphins on different adenylate cyclase isozymes reported that similar to morphine, adenylate cyclase type I and V were inhibited, while type II was stimulated by acute endormorphine exposure [37].
Generally, opioid receptor activation has been shown to be pertussis-toxin-sensitive, implicating membranes of the Gi and Go type in mediating opioid action. Different number of the adenylate cyclase family responded quite differently to receptor-mediated activation of the Gi/Go-protein, such as that produced through opioid receptors. Whereas the activity of type I, V and VI adenylate cyclases is inhibited by Giα subunit, the activity of type II and VII adenylate cyclases has been shown to be insensitive to Giα. However, type II and VII adenylate cyclases are stimulated by the βγ-subunits of the Gi/Go-protein when these enzymes are coordinately activated by Gs [36]. Our previous data showed that acute morphine injection (i.p. 30 mg/kg) caused a significant increase in G protein mRNA level in rat pineal gland, but not in other brain regions [38]. An increase in G subunit would activate a variety of intracellular signaling pathways, for example, adenylate cyclase types II, IV, VII and phospholipase C. In addition, the Gβγ may play an important role in signaling from G protein-coupled receptors to the mitogen-activated protein kinase (MAPK) pathway. A MAPK signal transduction cascade was induced through activation of this receptor by enkephalin or morphine [39].
Our present data showed that morphine, DPDPE and DAMGO stimulated the intracellular cAMP formation and this stimulatory effect was abolished by naloxone. It is reasonable to suggest that the increase in cAMP level may lead to activation of the cAMP-dependent protein kinase, then it will translocate to the nucleus and phosphorylate nuclear proteins, thereby affecting an intrinsic balance of regulatory molecules that control gene expression, perhaps resulting in an increase in NAT synthesis. Alternatively, the increase in cAMP may activate cAMP-dependent inhibition of proteasomal proteolysis of NAT resulting in increase in NAT protein levels. However, the precise mechanism whereby activation of opioid receptor leads to an increase in cAMP formation and results in elevation of NAT activity need to be further delineated.
The present data indicate that both mu- and delta-opioid receptors have an independent function in regulating melatonin synthesis and the stimulation acts via adenylate cyclase system. Taken together with the previous reports that the opioid peptides are present in nerve fibers and the existence of intrapineal opioidergic neuronal-like cells in pineal of some species [9] lead us to suggest that, besides, the sympathetic noradrenergic innervation that regulates melatonin synthesis, opioidergic systems also play an crucial role in regulating NAT enzyme and controlling melatonin synthesis. In some species such as European hamster [14], pinealocytes made presynaptic contact with other pinealocytes and never received a presynaptic innervation; therefore, the intrapineal opioidergic cell might be the anatomical substrate for a paracrine in regulating pineal function. However, in some species whose opioid system has not been clearly identified or absence, the pineal opioid receptor may act as a target for endocrine function. A full comprehensive of the precise localization of opioidergic innervations and the receptors, with respect to other neuronal systems, i.e. noradrenergic sympathetic, will provide additional clarification on the functional interaction of pineal.
Acknowledgements
This study was supported by the National Institute of Health/National Institute of Neurological Disorder and Stroke No. 1RO1-NS40160-04.




