Determination of eumelanin in human urine
Summary
Normal and malignant melanocytes produce melanins and melanin-related metabolites, most of which are retained in the cells but some are secreted into the blood and then excreted in the urine. In this study, we developed a method to measure levels of eumelanin in urine samples and evaluated its clinical significance in comparison with the melanin-related metabolites 6-hydroxy-5-methoxyindole-2-carboxylic acid (6H5MI2C) and 5-S-cysteinyldopa (5-S-CD), and with pheomelanin, measured after degradation as 4-amino-3-hydroxyphenylalanine (4-AHP). The method is based on the production of pyrrole-2,3,5-tricarboxylic acid (PTCA) on permanganate oxidation of eumelanin, followed by quantification by liquid chromatography. For 118 urine samples from 10 control subjects, mean urinary excretions of PTCA, 6H5MI2C, 5-S-CD and 4-AHP were 19, 67, 37 and 59 μmol/mol creatinine respectively. In melanoma patients (n = 45), the mean urinary excretions of PTCA, 6H5MI2C, 5-S-CD, and 4-AHP were 91, 926, 4070 and 3530 μmol/mol creatinine respectively. Median level of PTCA in melanoma patients was elevated 2.1-fold compared with control subjects. The degrees of elevation for 6H5MI2C, 5-S-CD, and 4-AHP were 1.8-, 22- and 6.2-fold respectively. Thus, although urinary PTCA is of little clinical value in following the progression of melanoma, urinary 4-AHP appears to be of considerable value in this respect.
Introduction
Normal melanocytes as well as their malignant derivatives, the melanoma cells, produce melanins and melanin-related metabolites, most of which are retained in the cells but some are secreted into the blood and then excreted into the urine. It is thus expected that the levels of these melanin-related compounds in serum or urine may reflect the degree of pigmentation (Ekelund et al., 1985; Westerhof et al., 1987) and the progression of melanoma (Hartleb and Arndt, 2001).
The production of melanin pigment is catalyzed by the specific enzyme tyrosinase, which converts l-tyrosine to dopaquinone (Hearing, 1998; Ito, 2003). Dopaquinone is a highly reactive molecule, and in the absence of sulfhydryl compounds, it gives rise to dopachrome through rapid cyclization and redox reactions. Dopachrome is then either decarboxylated to give 5,6-dihydroxyindole (DHI) or tautomerized to give 5,6-dihydroxyindole-2-carboxylic acid (DHICA). DHI and DHICA are then oxidized to form eumelanin, a dark brown to black pigment. However, a significant portion of DHICA leaks into the blood stream and is excreted in the urine after O-methylation to the isomeric compounds 5-hydroxy-6-methoxyindole-2-carboxylic acid (5H6MI2C) and 6-hydroxy-5-methoxyindole-2-carboxylic acid (6H5MI2C; Wakamatsu et al., 1990; Westerhof et al., 1987). These indolic eumelanin metabolites are considered to reflect tyrosinase activity in normal melanocytes and melanoma cells (Ekelund et al., 1985; Wakamatsu et al., 1990; Westerhof et al., 1987). In fact, urinary excretion of 6H5MI2C has been evaluated as a marker of melanoma progression (Yamada et al., 1992).
In the presence of cysteine, however, dopaquinone rapidly reacts with cysteine to form 5-S-cysteinyldopa (5-S-CD) along with minor amounts of cysteinyldopa isomers (Ito, 2003). Oxidation of cysteinyldopa isomers in melanocytes leads to the production of pheomelanin, a yellow to reddish melanin, but a significant portion of cysteinyldopa escapes from melanocytes, especially melanoma cells (Wakamatsu et al., 1990). It is thus possible to estimate the progression of melanoma by measuring the concentration of 5-S-CD in the blood or urine (Agrup et al., 1979; Hartleb and Arndt, 2001; Horikoshi et al., 1994; Kärnell et al., 2000; Wakamatsu et al., 2002c).
We have recently shown that in addition to 6H5MI2C and 5-S-CD, melanoma patients secrete high levels of pheomelanin into the blood and urine (Takasaki et al., 2003; Wakamatsu et al., 2003). The measurement of pheomelanin is based on the formation of 4-amino-3-hydroxyphenylalanine (4-AHP) on reductive hydrolysis of pheomelanin with hydriodic acid (Ito and Fujita, 1985; Wakamatsu et al., 2002b). Serum pheomelanin level was found to be elevated in metastatic melanoma patients but is less sensitive than serum 5-S-CD in indicating presence of distant metastases (Wakamatsu et al., 2003). However, no report has appeared measuring the concentration of eumelanin in blood or urine from melanoma patients.
Eumelanin can be analysed by permanganate oxidation to the specific degradation product, pyrrole-2,3,5-tricarboxylic acid (PTCA; Ito and Fujita, 1985) followed by quantitation of PTCA by high performance liquid chromatography (HPLC). In the present study, we applied this specific HPLC method to measure eumelanin (PTCA) in urine samples and evaluated its clinical significance in melanoma. We examined the correlation of PTCA to the melanin-related metabolites 6H5MI2C and 5-S-CD and to pheomelanin (as quantified by the analysis of the degradation product 4-AHP) in melanoma patients as well as control subjects and compared the increase of urinary PTCA levels in melanoma patients with those of the other melanoma markers. We also evaluated the clinical significance of PTCA levels in serum in a limited number of melanoma cases.
Results
Method for determining urinary levels of eumelanin (PTCA)
To determine urinary levels of eumelanin, we intended to use principally the same method as used for eumelanin analyses in hairs, skins or cells (Wakamatsu and Ito, 2002a). However, in early trials to develop the method, we encountered difficulty in separating the PTCA peaks from interfering, large peaks in HPLC chromatogram. This difficulty was overcome by shifting to a chromatographic column with improved selectivity and the change of column temperature (see the Materials and methods). Figure 1 shows typical chromatograms of a standard and KMnO4 oxidation products of urine samples from a control subject and from a melanoma patient. Pyrrole-2,3,5-tricarboxylic acid peaks were well separated from other peaks, despite of the fact that the peaks were small in most cases. In order to examine whether the small peaks were in fact PTCA or not, we collected the peak fraction with the same retention time as PTCA in HPLC and performed Liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) analysis. We observed the [M-H]+ (m/z 198.1) peak corresponding to PTCA, and [M-H-H2O]+ (m/z 180.0), [M-H-CO2]+ (m/z 154.1) and [M-H-2CO2]+ (m/z 110.1) peaks, which correspond to product ions as proved by the MS/MS spectrum, and thus the HPLC peak with the same retention time as the standard PTCA was identified as PTCA.
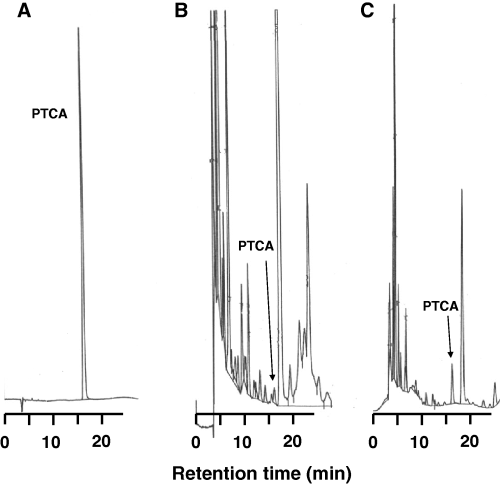
HPLC chromatograms of standard and permanganate oxidation products. (A) Standard of PTCA. Injected amount 91 ng. Sensitivity: 32 mV/full scale. (B) Oxidation product of urine sample from a control subject. Urinary PTCA level is 124 nmol/l. Sensitivity: 8 mV/full scale. (C) Oxidation products of urine sample from a melanoma patient. Urinary PTCA level is 1330 nmol/l. Sensitivity: 32 mV/full scale.
The possibility that ordinary urinary components might affect the recovery of PTCA was examined by adding Sepia melanin to a pooled urine sample. The recovery of PTCA after permanganate oxidation was 8.0 ± 1.6 μg/mg Sepia melanin (n = 5), which is similar to reported values (Liu et al., 2004). This indicates that urinary components do not affect the yield of PTCA.
Comparison of urinary PTCA, 6H5MI2C, 5-S-CD, and 4-AHP between control subjects and melanoma patients
Figure 2 shows the excretions of PTCA, 6H5MI2C, 5-S-CD, and 4-AHP in urine from control subjects and Swedish melanoma patients. The data are positively skewed and are therefore shown in logarithmic scales. The median excretions are given in the figure, and the arithmetic means ± SD are given below.
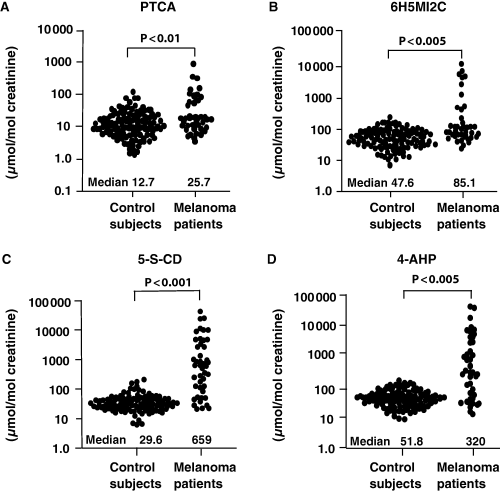
Distribution of urinary levels of (A) PTCA, (B) 6H5MI2C, (C) 5-S-CD, (D) 4-AHP in control subjects and in melanoma patients. Values are expressed in μmol/mol creatinine.
For the 118 samples from 10 control subjects, the urinary mean levels of PTCA, 6H5MI2C, 5-S-CD and 4-AHP were 19 ± 19, 67 ± 54, 37 ± 28 and 59 ± 36 μmol/mol creatinine respectively.
Levels of urinary PTCA showed a seasonal variation (Figure 3). The highest value of 39 ± 12 μmol/mol creatinine was found in July and the lowest value of 8.9 ± 2.4 μmol/mol creatinine in December (P < 0.001). Each subject showed a large fluctuation in urinary levels of PTCA in the course of the 1-yr period.
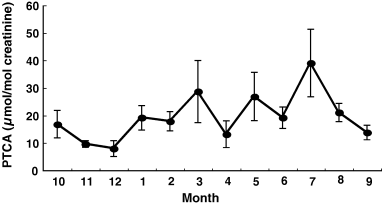
Seasonal variation of urinary levels of PTCA in control subjects. The data are expressed as mean ± SE.
For the 45 urine samples from Swedish melanoma patients, urinary levels of PTCA, 6H5MI2C, 5-S-CD and 4-AHP were 91 ± 185, 926 ± 2160, 4070 ± 8690 and 3530 ± 8550 μmol/mol creatinine respectively.
The median urinary level of PTCA in melanoma patients was significantly elevated 2.1-fold compared with control subjects. Likewise, the median levels of 6H5MI2C, 5-S-CD, and 4-AHP in melanoma patients were significantly elevated 1.8-, 22-, and 6.2-fold compared with control subjects respectively.
Correlations between urinary levels of PTCA, 6H5MI2C, 5-S-CD, and 4-AHP
Correlation coefficients (r2) between the four markers are summarized in Table 1. In control urine, statistically significant correlation was not found among the four markers. In the urine of Swedish melanoma patients, the pair 5-S-CD and 4-AHP showed the highest coefficient, followed by the pair 6H5MI2C and PTCA when calculated on logarithmic data (logarithmic data are more insensitive to extreme values than linear data). These correlations were expected because of the relations between 5-S-CD and 4-AHP in pheomelanin biosynthesis and between 6H5MI2C and PTCA in eumelanin biosynthesis. A high correlation of linear data was obtained between PTCA and 5-S-CD because three samples with high values of PTCA and 5-S-CD lay closely on the same regression line.
| Comparison | Correlation coefficient [r2 (P-values)] | ||
|---|---|---|---|
| Control subjects (n = 118)a | Melanoma patients (n = 45) | ||
| Normal scale | Normal scale | Log scale | |
| PTCA versus 6H5MI2C | 0.156 (n.s.) | 0.142 (n.s.) | 0.558 (<0.001) |
| PTCA versus 5-S-CD | 0.001 (n.s.) | 0.748 (<0.001)b | 0.261 (<0.01) |
| PTCA versus 4-AHP | 0.071 (n.s.) | 0.041 (n.s.) | 0.177 (n.s.) |
| 6H5MI2C versus 5-S-CD | 0.138 (n.s.) | 0.094 (n.s.) | 0.344 (<0.001) |
| 6H5MI2C versus 4-AHP | 0.118 (n.s.) | 0.000 (n.s.) | 0.155 (n.s.) |
| 5-S-CD versus 4-AHP | 0.005 (n.s.) | 0.292 (<0.01) | 0.634 (<0.001) |
- n.s., not significant.
- a118 urine samples from 10 subjects collected over 1 yr period.
- bA good correlation on normal scale was obtained because four samples with high values for PTCA and 5-S-CD lay closely on the same regression line.
Contents of eumelanin and pheomelanin can be calculated with conversion factors of 160 g eumelanin/g PTCA (Ozeki et al., 1996) and 9 g pheomelanin/g 4-AHP (Wakamatsu et al., 2002b), the molecular weight of 199 for PTCA and the molecular weight of 196 for AHP. Figure 4 shows a relation of linear data between urinary levels of eumelanin and pheomelanin in the Swedish melanoma patients. In 27 (60%) of the 45 Swedish melanoma cases, the excretion of pheomelanin was found to be higher than the excretion of eumelanin. On the average, these melanoma patients excreted twice as much pheomelanin compared with eumelanin. One patient excreted extremely high levels of eumelanin while two patients excreted even higher levels of pheomelanin.
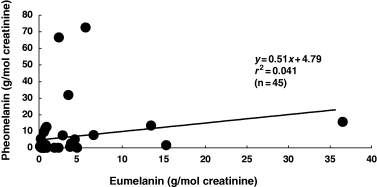
Relation between urinary levels of eumelanin and pheomelanin in 45 melanoma patients.
Comparison of serum PTCA and 5-S-CD between control subjects and melanoma patients
It was found that serum PTCA could also be analysed with a similar method. A preliminary study was therefore carried out to evaluate the clinical significance of serum PTCA in melanoma. Serum levels of 5-S-CD in control subjects (n = 10) and melanoma patients (n = 10) used for this comparison were 4.0 ± 1.7 and 995 ± 957 nmol/l respectively. Serum levels of PTCA in those two groups were 40 ± 22 and 88 ± 73 nmol/l respectively. When compared in the median level, melanoma patients showed a 20-fold increase in serum 5-S-CD (73 versus 3.6) while the increase was only 2.0-fold in serum PTCA (69 versus 35).
Discussion
Clinical significance of serum or urinary 5-S-CD level in following the progression of melanoma has well been established in several studies (Agrup et al., 1979; Hartleb and Arndt, 2001; Horikoshi et al., 1994; Kärnell et al., 2000; Wakamatsu et al., 2002c). In contrast, serum or urinary 6H5MI2C level appears to be less sensitive than the corresponding 5-S-CD in detecting distant metastases (Horikoshi et al., 1994; Yamada et al., 1992). Also, serum pheomelanin (4-AHP) level is of less diagnostic value in melanoma diagnosis than serum 5-S-CD (Wakamatsu et al., 2003). Among other melanoma markers, S100B protein has been most thoroughly evaluated and acknowledged. A comparison study showed that among serum S100B and urinary 5-S-CD and 6H5MI2C, serum S100B protein was found to be the best prognostic marker, but urinary 5-S-CD was as nearly good as S100B (Kärnell et al., 1997). In the present study, we enrolled Japanese control subjects and Swedish melanoma patients. One would argue that there are large variations in urinary excretion of melanin-related metabolites among various ethnic origins differing in the degree of pigmentation. However, the differences between Japanese and Caucasians are expected to be approximately 2-fold for urinary levels of 6H5MI2C and much less for urinary 5-S-CD (Westerhof et al., 1987).
In the present study, we developed a HPLC method to quantify eumelanin (PTCA) in urine. Using this method, we have found that the urinary excretion of PTCA and 6H5MI2C in melanoma patients compared with control subjects is not so high as with 5-S-CD followed by 4-AHP. This indicates that urinary PTCA (and 6H5MI2C) is of little clinical value in following the progression of melanoma. On the contrary, urinary 4-AHP appears to be of considerable value in this respect.
The excretion of melanin in urine of melanoma patients is known as melanuria. Melanuria is, however, a rare finding in melanoma patients (Saghari et al., 2002). It is usually seen in patients with diffuse melanosis and primary or metastatic melanoma to the genitourinary tract. The present method to detect eumelanin in urine is highly sensitive as even urine samples with straw-yellow colour can give clear peaks of PTCA in HPLC chromatograms. However, the specific measurement of urinary eumelanin in the form of a degradation product PTCA did not appear to improve the clinical significance of ‘melanuria’. On the contrary, the result shown in Figure 2 indicates that urinary pheomelanin (4-AHP) appears to be of considerable value in following melanoma progression. This appears to reflect the findings that (i) urinary 4-AHP levels in melanoma urine showed good correlation with urinary 5-S-CD levels (Takasaki et al., 2003) and (ii) in 60% of melanoma cases the excretion of pheomelanin is higher than the excretion of eumelanin.
At least two principal factors may influence the correlations between intermediate metabolites (e.g. 5-S-CD), endpoint metabolites (e.g. 6H5MI2C) and chemical degradation products of eumelanin (e.g. PTCA) and pheomelanin (e.g. 4-AHP). These factors are (i) the type of melanin (eumelanin, pheomelanin and mixture of these) and (ii) the production rate of melanin from the melanoma metastases and cell necrosis followed by excretion into the urine. Considering the first factor the high correlation between 6H5MI2C and PTCA can be expected from the fact that 6H5MI2C is an O-methylated derivative of DHICA, and PTCA is a degradation product of DHICA-derived melanin (Ito and Fujita, 1985). Similarly 5-S-CD is an intermediate product from the synthesis of pheomelanin and 4-AHP is a degradation product of pheomelanin. It might therefore be expected that these two compounds should be correlated. Considering the second factor, there might also be a correlation between the intermediate product 5-S-CD (released from the cytoplasm of the pigment cell) and PTCA in particular if the production of eumelanin is high in the melanosome. A high correlation on linear scale was obtained mainly because of three samples with extremely high values for PTCA and 5-S-CD lay closely on the same regression line which would indicate a high production of melanin. As their urinary pigment may be of different character – eumelanin, pheomelanin and mixtures of these – we think that such urinary pigment, being water soluble, would be an interesting material for further characterization of human melanin structure. In this connection, it should be pointed out that a majority (60%) of melanoma cases excreted pheomelanin-rich pigment. Pheomelanin is rather water soluble under neutral to alkaline conditions (Ozeki et al., 1995).
Serum levels of PTCA were also examined in control subjects and melanoma patients. The result shows that serum PTCA levels do not increase even in patients with very high levels of serum 5-S-CD, indicating that serum PTCA is not useful in following the progression of melanoma.
Finally, it is well known that levels of urinary and serum (plasma) 5-S-CD show seasonal variation with higher values in summer and lower values in winter (Nimmo et al., 1988; Rorsman et al., 1976; Wakamatsu and Ito, 1995). Interestingly, urinary PTCA levels also show seasonal variation (Figure 3). However, the large fluctuation in each control subjects makes the urinary PTCA of little value as a marker for the degree of pigmentation. Seasonal variation is also seen in levels of urinary and serum 6H5MI2C in our ongoing study (unpublished results).
Materials and methods
Subjects
Control urine samples were obtained from 10 control Japanese subjects aged 25–59 yr (38 ± 15). Their one-time urine samples were collected randomly in the middle of every month starting from October 2003 to September 2004. Approximately 7 ml of urine was taken to plastic tubes containing 200 μl of acetic acid. A total of 120 urine samples were used for this study, but two samples were excluded because serum samples taken on the same occasions gave abnormally 5-S-CD high values. Melanoma urine samples were obtained from 45 Swedish patients with various degrees of progression. The 45 samples correspond to the 51 samples described in Takasaki et al. (2003). These urine samples were stored frozen at −70°C until analysis. 6H5MI2C and 5-S-CD in urine are rather stable under these conditions (Kågedal et al., 1992). Determinations of urinary 5-S-CD and 4-AHP in Swedish melanoma patients were carried out in Sweden (Takasaki et al., 2003).
Control serum samples were obtained from the same 10 Japanese subjects as for the urine samples in September 2004. Serum samples from melanoma patients were obtained from 10 Japanese patients at stage IV who had serum 5-S-CD levels exceeding 100 nmol/l. Cut-off value of serum 5-S-CD is 10 nmol/l (Wakamatsu et al., 1991, 2002c).
Determination of urinary levels of 6H5MI2C, 5-S-CD, and pheomelanin (4-AHP)
Determination of urinary 6H5MI2C was performed using HPLC, mostly as previously described (Wakamatsu et al., 1991). A modification was to use a fluorescent detector, JASCO FP-2020 (JASCO Co., Tokyo, Japan), instead of the originally described electrochemical detector. The excitation wavelength was 315 nm and the emission wavelength 390 nm. Determination of urinary 5-S-CD was performed using HPLC with electrochemical detection, as previously reported (Kågedal et al., 1989; Wakamatsu and Ito, 1994). Determination of urinary 4-AHP was performed using HPLC with electrochemical detection as previously reported (Takasaki et al., 2003; Wakamatsu et al., 2003). Levels of analytes were calculated against creatinine as described by Kärnell et al. (2000).
Determination of urinary levels of eumelanin (PTCA)
For analysis of urinary PTCA, 200 μl urine was mixed with 700 μl 0.1 M H2SO4 and 100 μl homogenate of mouse livers (Ito and Fujita, 1985; Ito and Wakamatsu, 1994). To the mixture was added 3% KMnO4 in 20-μl portions under vigorous mixing. Ten minutes later, excess KMnO4 was decomposed with 100 μl 10% Na2SO3. The colourless mixture was extracted with two portions of approximately 7 ml ether. The combined ether extract was evaporated with aspirator, and the residue was dissolved in 200 μl water. Forty microlitre of the solution was injected into the HPLC system as described below. Serum PTCA was determined with 100 μl serum.
Pyrrole-2,3,5-tricarboxylic acid was analysed with an HPLC system consisting of a JASCO 980-PU liquid chromatograph, a Shiseido C18 column (Capcell pak C18; 4.6 × 250 mm; 5 μm particle size), and a JASCO 875-UV detector. The mobile phase used was 0.1 M potassium phosphate buffer, pH 2.10:methanol, 99:1 (vol/vol). The analyses were performed at 50°C at a flow rate of 0.7 ml/min. The UV detector was set at 269 nm. We usually injected 91 ng of PTCA as a standard.
A possibility that PTCA might be derived from 6H5MI2C was examined by oxidizing 6H5MI2C with permanganate. The yield was 5.0 μg PTCA from 1 mg 6H5MI2C. It is therefore confirmed that unless 6H5MI2C is present at extremely high levels, the contribution of 6H5MI2C to PTCA value is negligible. Likewise, the contribution of 5-S-CD and pheomelanin are also negligible (Ito and Fujita, 1985), based on the yields of PTCA: 0.09 and 0.17 μg/mg from 5-S-CD and pheomelanin respectively. The recovery of PTCA was examined by oxidizing with permanganate a pooled urine sample added with a 20 μg/ml final concentration of Sepia melanin (from Sigma Chemical Company, Toyko, Japan).
The peak fraction with the same retention time as PTCA in HPLC was collected from a control urine sample and the eluates were pooled and evaporated in a desiccator and subjected to the LC/MS/MS analysis using an electronspray ionization/ion trap mass spectrometer (LCQ Deca XP, Thermoelectron, Tokyo, Japan). The analysis was carried out directly by the MS/MS at negative charge.
Statistical analysis
Differences were analysed for statistical significance using the Mann–Whitney U-test. P-values <0.05 are considered to be significant. Pearson correlation coefficients were calculated to measure the association between PTCA, 6H5MI2C, 5-S-CD and 4-AHP.
Acknowledgements
This study was supported in part, by a Grant-in-Aid for Cancer Research (15–10) from the Ministry of Health, Labour, and Welfare of Japan and in part by grants from the Swedish Cancer Society (Project 2357-B01-16XAA), from The Health Research Council in the Southeast of Sweden, and from the University Hospital, Linköping.