Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin
Summary
Cultured human melanocytes differ tremendously in visual pigmentation, and recapitulate the pigmentary phenotype of the donor's skin. This diversity arises from variation in type as well as quantity of melanin produced. Here, we measured contents of eumelanin (EM) and pheomelanin (PM) in 60 primary human melanocyte cultures (51 neonatal and nine adults), and correlated some of these values with the respective activity and protein levels of tyrosinase, and the melanocortin-1 receptor (MC1R) genotype. Melanocytes were classified into four phenotypes (L, L+, D, D+) as depicted by visual pigmentation using light microscopy, and by the pigmentary phenotype of the donor's skin. There were large differences in total melanin (TM) and EM, which increased progressively for L, L+, D and D+ melanocytes. TM content, the sum of EM and PM, showed a good correlation with TM measured spectrophotometrically, and with the activity and protein levels of tyrosinase. Log EM/PM ratio did not correlate with MC1R genotype. We conclude that: (i) EM consistently correlates with the visual phenotype; (ii) lighter melanocytes tend to be more pheomelanic in composition than darker melanocytes; (iii) in adult melanocyte cultures, EM correlates with the ethnic background of the donors (African-American > Indian > Caucasian); and (iv) MC1R loss-of-function mutations do not necessarily alter the phenotype of cultured melanocytes.
Introduction
Cultured human melanocytes show a tremendous diversity of visual pigmentation, and recapitulate the pigmentary phenotype of the skin from which they were derived (Hunt et al., 1995; Scott et al., 2002). We suggest that this diversity is due to variation in type as well as quantity of melanin produced.
The production of melanin pigment is catalyzed by the specific enzyme tyrosinase, which converts l-tyrosine to dopaquinone (Hearing, 1998; Ito, 2003). Dopaquinone is a highly reactive molecule, and is rapidly cyclized to form dopachrome, which is then either decarboxylated to give 5,6-dihydroxyindole or tautomerized to give 5,6-dihydroxyindole-2-carboxylic acid. These dihydroxyindoles are then oxidized to form eumelanin (EM), a dark brown to black pigment. However, in the presence of cysteine dopaquinone binds to form 5-S-cysteinyldopa, along with minor isomers (Ito, 2003). Oxidation of cysteinyldopa isomers in melanocytes leads to the production of pheomelanin (PM), a yellow to reddish melanin.
In reality, most of the melanin pigments present in the hair, skin, and eyes are not homopolymers of a single monomer unit, but rather they are complex heteropolymers made up of both EM and PM building blocks (Ito, 1997; Ito and Wakamatsu, 2003; Liu et al., 2005; Prota, 1992). Thus, the color of human skin, hair, and eyes should reflect not only the type of melanin produced but also the quality of melanin, i.e. EM/PM ratio (Ito and Wakamatsu, 2003). In fact, hair color determined objectively using the L*a*b* color system correlated well with melanin contents (Naysmith et al., 2004). Thus, the log values of EM/PM ratio were inversely related to the color variables b* (yellow–blue), a* (red–green), and to a lesser extent L* (light–dark). Moreover, recent studies on human skin show that total melanin (TM) and EM and PM contents correlated with skin color measured on the L* scale (Alaluf et al., 2002; Hennessy et al., 2005). However, studies on cultured human melanocytes measuring EM and PM contents have been only sporadic with limited numbers of cultures tested (De Leeuw et al., 2001; Hunt et al., 1995; van Nieuwpoort et al., 2004; Scott et al., 2002; Smit et al., 1998).
The melanocortin-1 receptor (MC1R) gene encodes a G-protein coupled receptor that is primarily expressed on melanocytes, where it plays a key role in pigmentation regulation, and some variant alleles are associated with red hair color (RHC) and fair skin, as well as skin cancer risk (Beaumont et al., 2005; García-Borrón et al., 2005; Rees, 2003). Reduction in receptor coupling as well as cell surface localization appears to be the major factor to the genetic association between the MC1R variants and the RHC phenotype. The role of MC1R in pigmentation regulation in cultured melanocytes has been investigated in the present study.
To evaluate the tremendous diversity of visual pigmentation in cultured human melanocytes, we measured contents of EM and PM in 60 primary human melanocyte cultures, and correlated some of these values with visual pigmentation, activity and protein levels of tyrosinase, and with MC1R genotype. Contents of EM and PM (chemical phenotype) were determined by high-performance liquid chromatography (HPLC) microassays (Wakamatsu and Ito, 2002). This represents the largest study on pigmentation in cultured human melanocytes and has led to the conclusion that EM, but not always PM, consistently correlates with the visual pigmentation (visual phenotype).
Results
Primary cultures of human melanocytes
A summary of primary cultures of human melanocytes used in this study is shown in Table 1. We examined 60 primary cultures isolated from 51 neonatal and nine adult skins. Those melanocytes were classified into four types: L (very light), L+ (light), D (fairly dark), and D+ (dark) lines, based on pigmentation depicted by light microscopic observation and the pigmentation of donors’ skin. In this report, we propose to call this classification visual phenotype. Photographs of representative cultures are shown in Figure 1. Most of melanocytes were dipolar in shape regardless of the visual phenotype and differ only in pigmentation.
| n | Classification | Visual pigmentation | Number of melanocyte cultures | ||
|---|---|---|---|---|---|
| Total | Neonatal | Adult | |||
| 1–28 | L | Very light | 28 | 26 | 2 |
| 29–41 | L+ | Light | 13 | 11 | 2 |
| 42–48 | D | Fairly dark | 7 | 7 | 0 |
| 49–60 | D+ | Dark | 12 | 7 | 5 |

Photographs of cultured human melanocytes classified into the four phenotypes on the basis of visual pigmentation (see Table 1).
Correlation of EM and PM contents with visual pigmentation
We measured contents of EM and PM (chemical phenotype) in the 60 primary cultures using our HPLC methods. As shown in Figure 2, there was a great diversity in TM contents measured as the sum of EM and PM among different cultures. Thus, TM levels (mean ± SE) increased progressively and significantly (P < 0.005) from 5.8 ± 0.7, 29 ± 3.6, 50 ± 4.4 to 126 ± 19 μg/106 cells for L, L+, D, and D+ cells, respectively. Distribution of EM and PM values were rather skewed, and thus those values were next compared in log scale. Log EM levels (μg/106 cells) increased steeply and highly significantly (P < 0.0001) from 0.34 ± 0.06, 1.25 ± 0.05, 1.59 ± 0.04 to 1.96 ± 0.05 (Figure 3A), while log PM levels (μg/106 cells) increased more gradually from 0.31 ± 0.08, 0.74 ± 0.14, 0.89 ± 0.15 to 1.26 ± 0.11 for L, L+, D, and D+ cells, respectively (P < 0.01 for L versus other phenotypes, Figure 3B). Thus, log PM levels were more variable than log EM levels. Log EM/PM ratios also showed an increase from 0.03 ± 0.05, 0.52 ± 0.15, to 0.71 ± 0.15 for L, L+, and D cells, respectively (P < 0.005 for L versus other phenotypes, Figure 3C). Log EM/PM ratio did not increase in D+ cells (0.70 ± 0.11) compared with D cells. These results indicate that lighter melanocytes tend to be more pheomelanic in composition than darker melanocytes. However, there were great diversities in the log EM/PM ratios within the same visual phenotypes as well, as illustrated in 2, 3.
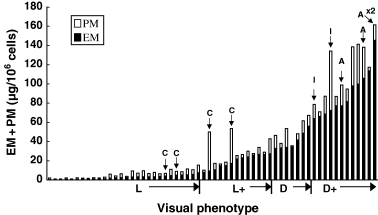
Contents of eumelanin (EM) and pheomelanin in the 60 melanocytes cultures examined. They were classified into the four visual phenotypes based on visual pigmentation (see Table 1) and shown in the sequence of increasing amount of EM in each phenotype group. Bars with arrow denote melanocyte cultures from adult skin biopsies: C, Caucasian; I, Indian; A, African-American; ×2 indicates that actual values are twice those shown.
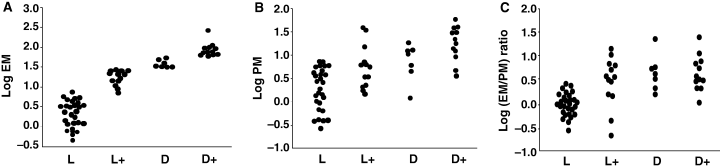
Chemical phenotype in relation to visual phenotype in the 60 cultures. Melanocytes were classified into the four visual phenotypes based on visual pigmentation (see Table 1). (A) Log eumelanin (EM) levels (μg/106 cells) in relation to visual phenotype. P < 0.0001 for any pair of the phenotypes. (B) Log pheomelanin (PM) levels (μg/106 cells) in relation to visual phenotype; P < 0.01 for pairs of L vs L+, D, or D+, and L+ vs D+. (C) Log EM/PM ratio in relation to visual phenotype; P < 0.005 for pairs of L vs L+, D, or D+.
We examined whether TM content, representing the sum of EM and PM, correlates well with TM measured by spectrophotometry. For this purpose, we randomly selected 24 cell cultures having a diverse degree of pigmentation. As shown in Figure 4, TM content measured by HPLC showed a good correlation (R2 = 0.913) with TM content measured spectrophotometrically in the 24 cell cultures.
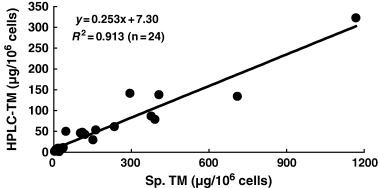
Correlation between total melanin (TM) content as the sum of eumelanin and pheomelanin, and TM content as measured spectrophotometrically.
In nine adult melanocyte cultures (Figure 2), the chemical phenotype correlated well with the ethnic background of the donors: four Caucasians had the least (mean EM levels 8.9 μg/106 cells), two Indians had more (68 μg/106 cells), and three African-Americans had the highest EM (156 μg/106 cells). Interestingly, adult melanocyte cultures appeared to be more pheomelanic as compared with neonatal melanocyte cultures, with a difference in log PM levels between nine adult and 51 neonatal cultures being highly significant (P < 0.0001). Furthermore, between seven adults and 25 neonatal cultures with darker phenotypes (L+, D, and D+), their log EM/PM ratio also showed a significant difference (P < 0.05) as did the log PM (P < 0.0001).
Correlation of TM content with tyrosinase activity and protein level
Total melanin content, measured as the sum of EM and PM, was compared with tyrosinase activity as well as tyrosinase protein level. As shown in Figure 5A, TM content correlated well (R2 = 0.782) with tyrosinase activity in the randomly selected 12 cell cultures with different visual pigmentation, and the differences in TM and tyrosinase activity between the lightest and darkest cultures approached 50-fold. On the other hand, when the same cultures were analyzed for protein levels of tyrosinase by Western blotting (Figure 5B), the differences in tyrosinase level were not as dramatic as those in TM content and tyrosinase activity.
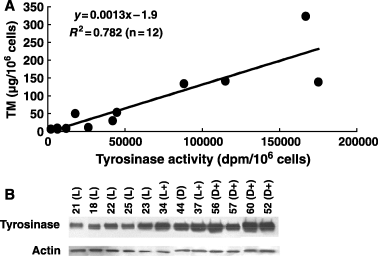
(A) Correlation between tyrosinase activity and total melanin (TM) content as the sum of eumelanin (EM) and pheomelanin (PM). Tyrosinase activities were measured by the in situ tyrosine hydroxylase assay. (B) Protein levels of tyrosinase by Western blotting. The sequence of cell cultures are in the order of tyrosinase activity given in (A), and the number corresponds to that shown in Figure 2 and Table 1 with the classification being shown in parentheses.
Correlation of EM/PM ratio with MC1R variation
We analyzed 19 selected cultures, nine of which were previously investigated in an earlier study (Scott et al., 2002), for MC1R genotype and the ability to respond to α-melanocyte stimulating hormone (α-MSH) with increased cAMP formation, proliferation, tyrosinase activity, reduction in UV-induced apoptosis and hydrogen peroxide generation, and enhanced repair of DNA photoproducts, as described by Scott et al. (2002) and Kadekaro et al. (2005). We found that melanocytes with loss-of-function MC1R due to expression of various MC1R alleles (Table 2) did not show striking differences in their log EM, log PM, or log EM/PM ratio from melanocytes with functional MC1R (P > 0.05). Thus, loss-of-function mutations of MC1R failed to show a correlation with the chemical phenotype. This was particularly true for one culture (no. 32) that had a high EM (and a low PM), despite expressing two strong ‘red hair’ alleles (R151C/D294H). Other melanocytes with loss-of-function MC1R had mostly low EM and PM values, which were comparable with those of melanocytes with functional MC1R.
| Numberb | MC1R genotypec | Classification | Log EMd (μg/106 cells) | Log PMd (μg/106 cells) | Log EM/PMd |
|---|---|---|---|---|---|
| Loss-of-function MC1R (n = 9)c | |||||
| 1 | V60L/V60L | L | −0.32 | 0.21 | −0.53 |
| 2 | R160W/R160W | L | −0.18 | −0.37 | 0.18 |
| 18 | R160W/+ | L | 0.54 | 0.79 | −0.25 |
| 19 | R160W/D294H | L | 0.54 | 0.51 | 0.03 |
| 21 | R142H/+ | L | 0.57 | 0.44 | 0.13 |
| 22 | R160W/D294H | L | 0.61 | 0.46 | 0.15 |
| 24 | V60L/R160W | L | 0.70 | 0.61 | 0.09 |
| 28 | V60L/R160W | L | 0.89 | 0.90 | −0.01 |
| 32 | R151C/D294H | L+ | 1.17 | 0.26 | 0.91 |
| Functional MC1R (n = 10)c | |||||
| 5 | V92M/+ | L | −0.08 | −0.55 | 0.48 |
| 7 | +/+ | L | 0.09 | −0.14 | 0.24 |
| 8 | R163Q/+ | L | 0.10 | −0.36 | 0.46 |
| 10 | R151C/+ | L | 0.14 | −0.17 | 0.31 |
| 11 | V60L/+ | L | 0.16 | 0.18 | −0.01 |
| 15 | V92M/V92M | L | 0.41 | 0.03 | 0.38 |
| 30 | +/+ | L+ | 0.97 | 1.61 | −0.64 |
| 38 | +/+ | L+ | 1.41 | 0.18 | 1.23 |
| 40 | +/+ | L+ | 1.43 | 0.33 | 1.10 |
| 41 | +/+ | L+ | 1.44 | 1.19 | 0.25 |
- aData for the following nine cultures were taken from Table 1 of Scott et al. (2002): nos 2, 5, 7, 8, 11, 15, 32, 38, and 40. The conversion factor of 5 for a sum of 4-AHP and 3-AHP was adapted in the present study (as in the original paper by Ito and Fujita, 1985) instead of 10 that was previously used in Scott et al. (2002) because it is consistent with the factor of 9 for 4-AHP (Wakamatsu and Ito, 2002).
- bThe number corresponds to the sequence presented in Table 1 and Figure 2.
- c+/+ = wild type MC1R; V60L, is weakly associated with red hair, V92M, and R163Q are considered ‘pseudo alleles’ with no significant effect on EM synthesis, while R151C, R160W, and D294H are strong red hair alleles. The function was assessed by the response to α-MSH as described by Scott et al. (2002) and Kadekaro et al. (2005).
- dNo significant differences in log EM, log PM, and log EM/PM values were found between the loss-of-function MC1R and the functional MC1R, by the Mann–Whitney nonparametric U-test: P-values are 0.343, 0.217, and 0.085, respectively.
Discussion
Cultured human melanocytes show a tremendous diversity of visual pigmentation (visual phenotype). The aim of this study was to evaluate the effects of EM and PM contents (chemical phenotype), activity and protein level of tyrosinase, and MC1R mutations on the visual phenotype. It has been debated that human pigmentary phenotypes should be defined based on quantitative measures, rather than arbitrarily, such as the commonly used Fitzpatrick's skin phototypes. The present study represents a step in the right direction, and analysis of additional melanocyte cultures should allow for classification according to EM and TM. In this study, we used a panel of cultured human melanocytes that were all maintained under identical culture conditions, as described in Materials and methods. All cultures were tested while in early passage (passages three to 10).
We found that EM, not PM, consistently increased with visual pigmentation. The ratio of EM/PM, as presented in the log value, varied widely, and sometimes was comparable in the dark (D+) and very light (L) melanocytes (Figure 3C). Nevertheless, we observed that on the average, lighter (L) melanocytes tend to be more pheomelanic in composition than darker (L+, D, and D+) melanocytes. Similar results have been reported for cultured human melanocytes (De Leeuw et al., 2001; Smit et al., 1998) and in isolated melanosomes thereof (van Nieuwpoort et al., 2004). These observations are consistent with our hypothesis proposed for mixed melanogenesis. We proposed that melanogenesis proceeds in three distinctive steps: cysteinyldopa-genesis, pheomelanogenesis, followed by eumelanogenesis. It thus appears that the ratio of EM/PM is determined by tyrosinase activity and cysteine concentration (Chintala et al., 2005; Ito, 2003; Land and Riley, 2000).
Eumelanin, but not always PM, consistently correlates with the visual phenotype. This suggests that EM is more important in determining the degree of visual pigmentation in cultured human melanocytes. This may be attributable not only to the predominance of EM over PM but also to the relative effect on pigmentation. We have shown that absorbance of PM is much lower than that of EM: when dissolved in Soluene-350, 5-S-cysteinyldopa-melanin (PM) had an absorbance at 500 nm about 40% that of 5,6-dihydroxyindole-melanin (EM), and the difference was further accentuated in longer wavelengths (Ozeki et al., 1996). Our previous study, although preliminary, also showed that some melanoma cell lines with high PM contents did not have visible pigment (del Marmol et al., 1993).
Total melanin content, measured by HPLC, showed a good correlation with TM content measured spectrophotometrically (Figure 4). Similar correlations have been reported for human hair and skin samples (Alaluf et al., 2001; Ozeki et al., 1996).
In adult melanocyte cultures, the chemical phenotype correlated well with the ethnic background of the donors: Caucasian had the least, Indian had more, and African-American had the highest EM. A similar result has been obtained in our previous study in which cultured melanocytes from Caucasian donors had significantly less EM and PM compared with Asians (Hunt et al., 1995). It has been described that the in vivo differences in pigmentation of the skin remain evident in melanosomes isolated from cultured melanocytes (De Leeuw et al., 2001; van Nieuwpoort et al., 2004; Smit et al., 1998; Wenczl et al., 1998).
Another interesting finding with cultured adult melanocytes is that they contain significantly higher PM than cultured neonatal melanocytes. At present, the significance of higher PM levels in cultured adult melanocytes may be speculative (van Nieuwpoort et al., 2004; Wenczl et al., 1998). Regarding the high PM levels, it should be pointed out that dysplastic nevus cells contain significantly more PM at the tissue (Salopek et al., 1991), cellular (Smit et al., 1998), and melanosomal level (Pavel et al., 2004). Dysplastic nevus cells are considered by some to be precursor lesions of melanoma (Bennett, 2003; Pavel et al., 2004).
In this study, tyrosinase activity measured as the tyrosine hydroxylase activity closely correlated directly with the TM content (Baker et al., 1995). The tyrosine hydroxylase assay is based on the release of tritium from the 3 and 5 positions of radiolabeled tyrosine in the form of tritiated water. Therefore, the method measures production of dopaquinone (release of 3-T) and the subsequent oxidative polymerization of dihydroxyindole monomers (release of 5-T) formed from dopaquinone and/or conversion to 5-S-cysteinyldopa (release of 5-T). Thus, it should be expected that this method reflect the degree of pigmentation.
The level of tyrosinase protein measured by the Western blotting only roughly paralleled the TM content. It is now clear that the expression of the regulatory proteins, such as tyrosinase, is not the main determinant for the final TM or visual pigmentation. There are many other regulatory mechanisms that would determine protein activity, including post-translation modifications, availability at the proper microenvironment and so forth. Regarding tyrosinase, sorting and final anchoring to the melanosome is very important for its maximal function. Melanosomal pH appears to be another important regulation. It has been proposed that Caucasian melanocytes are acidic and thus pigmentation is suppressed both because of a lower tyrosinase activity and a lower rate of polymerization of melanin precursors at acidic pHs (Ancans et al., 2001; Hirobe et al., 2003; Smith et al., 2004).
Melanocortin-1 receptor is a transmembrane receptor located on the melanocytes. The binding of α-MSH or adrenocorticotropin to MC1R results in an increase of cAMP levels leading to the upregulation of tyrosinase and tyrosinase-related proteins (Abdel-Malek et al., 1995; Rees, 2003; Suzuki et al., 1996). Thus, loss-of-function mutations of MC1R leads to the switch to PM production in follicular melanocytes, giving rise to red hair in human (Naysmith et al., 2004) and yellow hair in mice (Ito and Wakamatsu, 2003; Ozeki et al., 1995). However, human skin had not been measured for melanin types in relation to MC1R mutation, and only one previous study addressed this issue in cultured human melanocytes (Scott et al., 2002). The study hereby presented represents a further attempt to measure EM and PM contents in human cultured melanocytes with known MC1R genotypes. We did not observe any significant differences in the log EM or log PM contents of the nine melanocyte cultures expressing loss-of-function MC1R compared with those of the 10 melanocyte cultures with functional MC1R (Table 2). Interestingly, one culture (no. 32) expressing two MC1R alleles known to be strongly associated with the RHC phenotype had a high EM (and a low PM). These observations confirm that expression of these MC1R alleles may not be enough for the fair skin phenotype and can be expressed in individuals with dark skin, as suggested by epidemiological studies (Kennedy et al., 2001; Matichard et al., 2004; Palmer et al., 2000). Our findings are consistent with previously reported results on the levels of tyrosinase and related proteins in MC1R genotyped primary melanocyte cultures from Queensland neonates, which showed no correlation of these pigmentation markers with MC1R genotype (Leonard et al., 2003). In fact, red haired individuals with black skin were recently identified in four Jamaicans having loss-of-function MC1R (McKenzie et al., 2003). The human MC1R gene is highly polymorphic and certain allelic variants of the gene are associated with the RHC phenotype, melanoma and non-melanoma skin cancer (Bastiaens et al., 2001; Kennedy et al., 2001; Palmer et al., 2000; Rees, 2003). Our previous study indicates that loss-of-function mutations in the MC1R gene may not necessarily affect constitutive pigmentation, but predispose human melanocytes to the DNA damaging effects of UV radiation, which may increase melanoma risk (Kadekaro et al., 2005; Scott et al., 2002). From these considerations, it appears that MC1R mutations do not necessarily alter the skin phenotype but rather relate to increased sensitivity to UV-induced DNA damage.
Finally, the ratio of EM/PM varies tremendously regardless of degree of pigmentation in cultured melanocytes. It has been shown by Duval et al. (2002) that keratinocytes can strongly influence the EM/PM ratio for melanocytes in culture and also will play an important role for pigmentation regulation. It is also possible that regulation of levels of cysteine, a PM precursor, may account for the diversity of the ratio of EM/PM. In this regard, our recent study has demonstrated that subtle gray (sut) mouse pigmentation mutant arose by means of a mutation in the Slc7a11 gene, encoding the plasma membrane cystine/glutamate exchanger xCT and a resulting low rate of extracellular cystine transport into sut melanocytes reduces PM production (Chintala et al., 2005). Thus, Slc7a11 is a major genetic regulator of PM production in hair and melanocytes. It is conceivable that Slc7a11 gene may be involved in the greater production of PM in some melanocyte cultures found in this study.
Conclusions
In conclusion, these results indicate that: (i) cultured human melanocytes vary tremendously in pigmentation; (ii) EM, but not always PM, consistently correlates with the visual phenotype; (iii) lighter melanocytes tend to be more pheomelanic in composition than darker melanocytes; (iv) in adult melanocyte cultures, EM correlates well with the ethnic background of the donors (African-American > Indian > Caucasian); and (v) MC1R loss-of-function mutations do not necessarily alter the phenotype of cultured melanocytes but rather relate to increased sensitivity to UV-induced DNA damage.
Materials and methods
Melanocytes culture
Primary human melanocyte cultures were established from foreskins derived from anonymous newborn males (n = 51) or from skin biopsies (n = 9) obtained from the forearm or breast or abdomen tissue following surgery performed on young adults with known ethnic origin, after informed consent, and maintained as described (Abdel-Malek et al., 1995). Briefly, the culture medium consisted of MCDB 153, supplemented with 3% heat-inactivated fetal calf serum, 1% penicillin/streptomycin/amphotericin, 5 μg/ml of insulin, 1 μg/ml of α-tocopherol, 8 nM of TPA, 0.6 μg/ml of basic fibroblast growth factor, and 13 μg/ml of bovine pituitary extract. Early passage (<10) cultures were used for all experiments to insure minimal genetic drift in vitro. These cell cultures were examined for visual pigmentation by microscopic observation and classified into four types: L, L+, D, and D+. The classification was made without prior knowledge of melanin contents by HPLC assays and spectrophotometry.
Analysis of EM and PM and spectrophotometric TM content
Melanocytes were lyophilized and 0.2–0.5 × 106 cells each were processed for chemical analyses of EM to detect the specific degradation product, pyrrole-2,3,5-tricarboxylic acid (PTCA), after permanganate oxidation (Ito and Fujita, 1985; Ito and Wakamatsu, 1994) and of PM to detect the specific degradation product, 4-amino-3-hydroxyphenylalanine (4-AHP), after hydriodic acid hydrolysis (Ito and Fujita, 1985; Wakamatsu and Ito, 2002). The amounts of EM was obtained by multiplying the PTCA value by a conversion factor of 160 (Ozeki et al., 1996), while the amount of PM was obtained by multiplying the 4-AHP value by a conversion factor of 9 (Wakamatsu and Ito, 2002). The PTCA determinations were performed in duplicate and the 4-AHP determinations were as single measurements. Some data (n = 15) for PM were obtained with our previous method analyzing a combined amount of 4-AHP and 3-amino-4-hydroxyphenylalanine (3-AHP) and a conversion factor of 5 (Ito and Fujita, 1985). For spectrophotometric analysis of TM content, the cells were harvested and counted, and 1 × 106 melanocytes were lysed using 1% Triton-X. Lipid from the cell lysates were removed using an ethanol:ether (1:1) solution. Purified melanin pellets were resuspended in 0.2 M NaOH (1 × 106 cells/ml) and samples were heated at 60°C to solubilize the melanin. Absorbance of the samples was determined at 490 nm using a Bio-Rad Microplate Reader (Model 550; Bio-Rad, Hercules, CA, USA). Melanin content was determined using a standard curve generated from known concentrations of synthetic melanin (Sigma, St. Louis, MO, USA).
Determination of tyrosinase activity
To determine the in situ tyrosine hydroxylase activity of tyrosinase (Abdel-Malek et al., 1992; Pomeranz, 1969), melanocytes were plated onto 60 mm dishes at a density of 2.5 × 105 cells. After 72 h, cells were incubated in the presence of tritium labeled tyrosine for 24 h, and the amount of tritium labeled water that was released by the cells into the media was measured. For each culture, triplicate dishes and two determinations per dish were analyzed.
Immunoblot analysis of tyrosinase
Human melanocytes were plated onto 100 mm dishes at a density of 1.5 × 106 cells. Seventy-two hours later cell extracts were prepared using radioimmunoprecipitation assay buffer containing a cocktail of protease inhibitors. Twelve micrograms of each cell lysate was used for electrophoresis in a 10% polyacrylamide gel. The separated proteins were transblotted onto Immobilon-P membranes that were incubated with the α-hPEP-7 (1:10 000 dilution), a polyclonal antibody raised against the carboxy-terminus of the human tyrosinase (from Richard King and William Oetting, University of Minnesota, Minneapolis, MN, USA), for 18 h at 4°C, followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG at 1:1000 dilution, for 1 h at room temperature. Membranes were also reacted with horseradish peroxidase-conjugated actin antibody at 1:500 dilution, as control for loading. The bands were visualized using Enhanced ChemiLuminescence (Amersham, Arlington Heights, IL, USA) according to the manufacturer's instructions.
Sequencing of MC1R gene
Total RNA was isolated from cultured human melanocytes using the RNA Easy Kit (Oiagen Science, Valencia, CA, USA) and cDNA was obtained by reverse transcription of 100 ng of total RNA using random hexamers as primers (50 μM Oligo(dT)/20 μl final volume). An equivalent volume of 2 μl of cDNA suspension was used for RT-PCR amplification using SuperScript TMIII, Invitrogen Life Technologies (Carlsbad, CA, USA). The entire coding region of the MC1R was amplified in two separate reactions using the following sets of primers: for the first-half of the sequence N-terminal primer (5′-GCAGCACCATGAACTAAGCA-3′) and C-terminal primer (5′- CCAGCATAGCCAGGAAGAAG-3′) and for the second-half of the sequence N-terminal primer (5′-GTGGACCGCCTACATCTCCAT-3′) and C-terminal primer (5′-GGACCAGGGAGGTAAGGAAC -3′). A PCR touchdown cycling profile was used, consisting of one cycle of 95°C for 5 min, followed by 25 cycles of 94°C for 1 min, 62°C for 1 min with a 0.5°C/cycle decrease, and 72°C for 1 min; followed by 10 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 10 min. The PCR products were purified with a QIAquic PCR Purification Kit (Qiagen Science) according to the manufacturer's instructions and sequenced with an automated system (Perkin Elmer/Applied Biosystems intrument models 373A or 377; Perkin Elmer/Applied Biosystems, Boston, MA, USA). For some cultures, the MC1R genotyping was carried out using 25–50 ng of genomic DNA, as described by Duffy et al. (2004).
Statistical analysis
Statistical testing was carried out using JMP 5.0 for Macintosh (SAS Institute, Japan: http://www.jmp.com/japan/corp/index.shtml). Differences were analyzed for statistical significance using the Mann–Whitney nonparametric U-test. P < 0.05 are considered to be significant.
Acknowledgements
This study was supported in part, by a grant-in-aid for Scientific Research (no. 16591122) from the Ministry of Education, Culture, Sports and Technology of Japan (K. Wakamatsu and S. Ito); by RO1 ES-09110 and ES06096 (Z. Abdel-Malek), Johnson & Johnson Skin Research Center Training grant (A.L. Kadekaro and Z. Abdel-Malek), and by Dermatology Foundation Research Grant (S. Leachman).