Association of kinesin and myosin with pigment granules in crustacean chromatophores
Summary
Chromatic adaptation in crustaceans results from the differential distribution of colored pigment granules within their chromatophores consequent to cell signaling by neurosecretory peptides. However, the force transducing, mechanochemical protein motors responsible for granule translocation, and their molecular mechanisms of action, are not well understood. The present study uses immunocytochemical techniques and a motility assay in vitro to demonstrate that protein motors from the kinesin and myosin superfamilies are stably associated with membrane-bounded pigment granules in the red, ovarian chromatophores of the freshwater, palaemonid shrimp, Macrobrachium olfersii. Monoclonal antibodies against conventional kinesin heavy chain, and an anti-myosin whole serum, labeled pigment-containing fragments prepared from homogenates of chromatophores with fully dispersed or aggregated pigments: this finding infers a permanent association between the protein motors and the pigment granules, and suggests that such motors may be regulated while bound to their cargos. The pigment aggregator appears to be a myosin since the anti-myosin whole serum attenuated hormonally triggered pigment aggregation in the motility assay in vitro, and induced pigment hyper-dispersion in some chromatophores. Western blots of the chromatophore-containing, ovarian tissue homogenate demonstrated protein bands consistent with myosin II and myosin XII, either of which may be the pigment aggregator. This study provides the first direct evidence for myosin and kinesin protein motors directly and stably associated with pigment granules in crustacean chromatophores, and may represent the first successful isolation of myosin class XII.
Introduction
The capacity of numerous animal species to adaptively alter their outward appearance is a polyphyletic trait, selected during the course of evolution. Prominent among such capabilities is the capacity to alter coloration patterns, important in predator/prey interactions, intraspecific communication, protection from ultraviolet radiation and thermoregulation. The fascinating phenomenon of color change in an individual organism, which then becomes either more or less conspicuous, constitutes an obvious point for investigation.
Color change in animals can be divided into two broad categories: chromogenic color change and chromomotor color change. Chromogenic changes are persistent and involve an increase or decrease in the production of the color-producing pigment, and number and/or type of pigment-bearing cells. Chromomotor change is transient, rapidly reversible, and results from the redistribution of existing pigments within pigmentary effectors to produce an outward change in color.
The neotropical, freshwater, palaemonid shrimp, Macrobrachium olfersii, like many other decapod crustaceans, possesses specialized pigmentary effectors that provide rapid color changes. These multicellular chromatosomes consist of groups of up to 20, individual, unicellular chromatophores, organized with their cell bodies clustered at the hub of the stellate chromatosome (McNamara, 1981). The chromatophore extensions project radially outward from the chromatosome center, and ramify into dendritic processes. A typical crustacean chromatosome can span 200 μm or more in diameter, and may exhibit one or a variety of pigments or color combinations, including black, white, red, blue, orange, brown, green and yellow.
Crustacean chromatosomes are located throughout the integumental epithelium and on the surfaces of internal organs like the nervous and digestive systems, and ovaries. They function in concert, their pigments aggregating or dispersing, providing overall adaptive color change. Specific neuropeptide hormones induce antagonistic aggregation or dispersion of the particular colored pigments that constitute the chromatosome. One such neurosecretory hormone, the red pigment concentrating hormone (RPCH), was first described by Josefsson (1967), and its amino acid sequence established, allowing synthesis of the oligopeptide (Fernlund and Josefsson, 1972). The red, ovarian chromatosomes of M. olfersii respond to this octapeptide hormone at nanomolar concentrations in vitro, completely aggregating their pigments over a 25-min perfusion period. On withdrawal of RPCH from the perfusate, the chromatosome pigments return to a dispersed, steady state (McNamara and Ribeiro, 1999).
Other chemical messengers regulate pigment transport in crustacean chromatophores. Besides the general pigment aggregating hormone, PCH (RPCH), and the pigment dispersing hormones, alpha- and beta-PDH, crustacean cardioactive peptide and melatonin regulate pigment transport in M. potiuna red, epidermal chromatophores (Nery et al., 1997, 1999). This diversity of pigment-translocation inducing agonists, and their receptors, suggests that distinct signaling pathways converge on the same physiological response, i.e. pigment migration (McNamara and Ribeiro, 2000; Ribeiro and McNamara, 2002).
What might be the mechanical apparatus that regulates red pigment mobility within individual ovarian chromatophores in M. olfersii? Typical effectors of intracellular transport include many motor proteins and cytoskeletal components. Numerous reports document the roles of myosins, kinesins and dyneins, along with their corresponding cytoskeletal architecture, in chromatophore pigment translocation in a variety of vertebrate species, from amphibians to mammals (Gross et al., 2002; Liu et al., 1998; Nascimento et al., 1997; Reilein et al., 2003; Rodionov et al., 1991; Rodionov et al., 1998; Tuma and Gelfand, 1999). However, knowledge of the granule translocation mechanism in invertebrate chromatophores is lacking, even in the Crustacea, a group that has received considerable attention (Lambert and Fingerman, 1978; McNamara and Ribeiro, 1999; McNamara and Taylor, 1987; Robison and Charlton, 1973).
In the present study, we establish the existence of such protein motors in crustacean chromatophores for the first time, and directly demonstrate the presence of myosin and kinesin associated with the membrane-bounded pigment granules in the red, ovarian chromatophores of M. olfersii. We also show that the presence of these protein motors on the pigment granules is independent of the aggregated or dispersed state of the chromatophore pigment, which suggests a stable association between the protein motors and their pigment granule cargo.
Results
The Coomassie-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel of the ovarian capsule homogenate containing red chromatosomes from M. olfersii (Figure 1) reveals the complex protein profile of this tissue. The Western blot of the homogenate, when probed with the anti-myosin whole serum, exhibits two bands, which correspond to two myosin heavy chains >210 kDa (Figure 1). The blot was purposely over-developed, revealing the molecular size markers, to better establish the relative positions of the bands in the sample lane, and to aid in comparing the Western and Coomassie-stained gels. A non-linear regression (R2 = 0.95, not shown), plotting the molecular weights of the Western blot bands (both standards and unknowns) against their migration distance through the gel (Rf), places the unknown lighter myosin at 240 kDa, and the heavier myosin at 307 kDa.
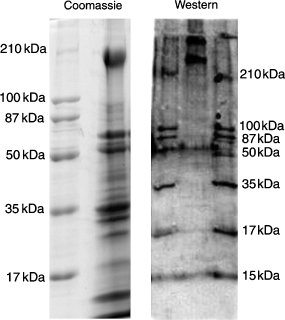
(A) Coomassie-stained, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel showing the protein profile of the ovarian tissue homogenate containing red chromatosomes from Macrobrachium olfersii. (B) Western blot, using the anti-myosin whole serum, revealing two myosin heavy chains >210 kDa (upper center lane) in the same ovarian tissue homogenate. The blot has been overdeveloped to emphasize the molecular mass markers, unequivocally locating the anti-myosin positive bands and facilitating comparison with the Coomassie-stained SDS-PAGE gel. Respective molecular mass markers are indicated beside each preparation.
Double-labeled preparations, using anti-myosin whole serum and anti-kinesin heavy-chain, of pigment-containing fragments prepared from homogenates of M. olfersii ovarian chromatosomes, fixed with pigment in the dispersed state, demonstrate that protein motors from the myosin and kinesin superfamilies co-localize with the visible pigmented areas (Figure 2A). Analysis of these pigment-containing fragments reveals a significantly more intense fluorescence signal from the kinesin and myosin in the pigmented areas compared with the negative controls without primary antibodies (Figure 2B).
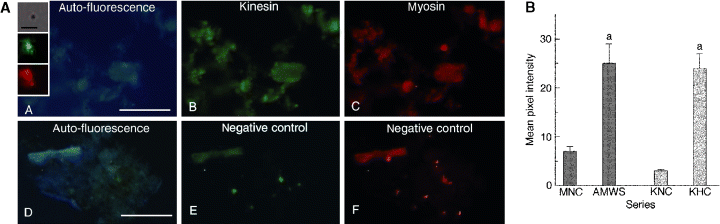
(A) Kinesin and myosin co-localize in chromatophore fragments prepared from homogenates of red, ovarian chromatosomes fixed with fully dispersed pigments. (A) DAPI channel, showing autofluorescent signal from the pigmented areas. (B) FITC channel, demonstrating kinesin associated with the pigmented areas. (C) TRITC channel, revealing myosin associated with the pigmented areas. Panels D, E and F are the corresponding negative controls for each channel. Scale bar = 50 μm. (Insert) Kinesin and myosin signals are detectable on single pigment granules. From top to bottom: DIC image of a single pigment granule, or a very small granule aggregate; FITC signal from the same granule, showing kinesin; TRITC signal from the same granule, demonstrating myosin. Scale bar = 5 μm. Inserts have been post-processed using Adobe PhotoShop® (Adobe, San Jose, CA, USA) for display purposes only. (B) Quantitative analysis of fluorescence intensities from myosin and kinesin associated with a chromatophore fragment homogenate prepared from fully dispersed, red, ovarian chromatosomes of Macrobrachium olfersii. Data are mean (±SEM, n = 14–15) pixel intensities obtained after 2 h incubation with anti-myosin whole serum + goat, anti-rabbit IgG-TRITC (dark bars), or anti-kinesin heavy chain + goat, anti-mouse IgM (μ-chain)-FITC (light bars). MNC, myosin negative control; AMWS, anti-myosin whole serum; KNC, kinesin negative control; KHC, anti-kinesin heavy chain. aP ≤ 0.001 compared with corresponding negative controls. Pixels in the raw images were quantified using NIH Image software.
Myosin and kinesin also co-localize with the pigmented areas visible in fragments of homogenates from chromatophores fixed with aggregated pigment (Figure 3A). Statistical analysis shows a significantly more intense kinesin and myosin signal in the pigmented areas compared with the negative controls without primary antibodies (Figure 3B).
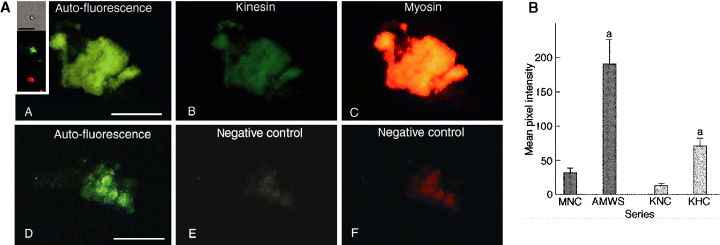
(A) Kinesin and myosin co-localize with pigmented areas in preparations of homogenates of red, ovarian chromatosomes fixed with fully aggregated pigments. (A) DAPI channel, revealing an auto-fluorescent signal from pigmented areas. (B) FITC channel, demonstrating kinesin associated with pigmented areas. (C) TRITC channel, showing myosin associated with pigmented areas. Panels D, E and F are the corresponding negative controls for each channel. Scale bar = 25 μm. (Insert) Kinesin and myosin signals are detectable on single pigment granules isolated from chromatosomes with aggregated pigment. From top to bottom: DIC image of a single pigment granule or a very small granule aggregate; FITC signal from the same granule, revealing kinesin; TRITC signal from the same granule, demonstrating myosin. Scale bar = 5 μm. Inserts have been post-processed using Adobe PhotoShop® for display purposes only. (B) Quantitative analysis of fluorescence intensities from myosin and kinesin associated with a chromatophore fragment homogenate prepared from the fully aggregated, red, ovarian chromatosomes of Macrobrachium olfersii. Data are mean (±SEM, n = 5–11) pixel intensities obtained after 2 h incubation each with anti-myosin whole serum + goat, anti-rabbit IgG-TRITC (dark bars), or anti-kinesin heavy chain + goat, anti-mouse IgM (μ-chain)-FITC (light bars). MNC, myosin negative control; AMWS, anti-myosin whole serum; KNC, kinesin negative control; KHC, anti-kinesin heavy chain. aP ≤ 0.01 compared with corresponding negative controls. Pixels in the raw images were quantified using NIH Image software.
In a more refined assay, both kinesin and myosin are detectable from single pigment granules or very small granule aggregates, extracted from homogenates of chromatophores fixed with either dispersed (Figure 2A, insert) or aggregated pigments (Figure 3A, insert). No fluorescence signal was detected from the individual pigment granules in negative control preparations. Further, there was no signal from myosin II (non-muscle) or myosin V antibodies associated with the pigment granules in any chromatophore preparation (data not shown).
After exposure to the anti-myosin whole serum, some chromatosomes show pigment hyper-dispersion, in which the pigment granules disperse away from the chromatophore cell bodies, migrating to the cell periphery and leaving unpigmented cytoplasm in the perikarya (arrows, Figure 4A, top panel). The cell bodies of normally dispersed, control chromatosomes do not exhibit unpigmented areas (Figure 4A, bottom panel).
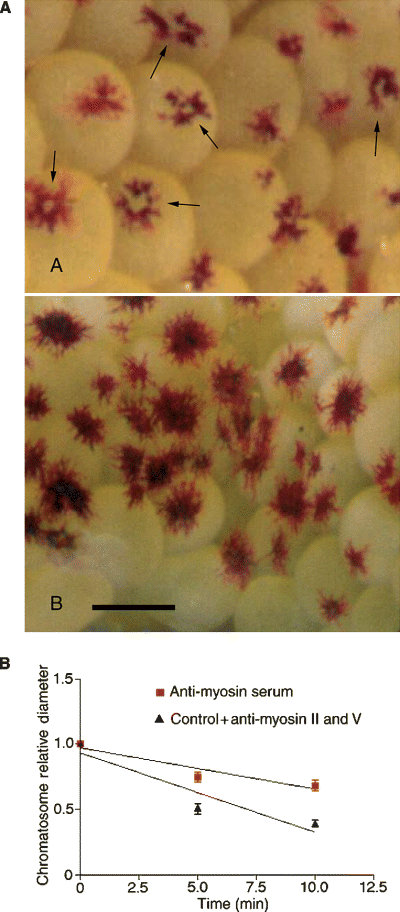
(A) Hyper-dispersion in live red, ovarian chromatosomes from Macrobrachium olfersii treated with anti-myosin whole serum. Top panel: hyper-dispersed chromatosomes, exhibiting a central, pigment-free area (arrows) subsequent to pigment migration from the chromatophore perikarya to the cell extensions. Lower panel: normally dispersed, control chromatosomes. Scale bar = 2 mm. (B) Comparison of RPCH-triggered, pigment aggregation rates in red ovarian chromatophores loaded with antibodies against anti-myosin whole serum, myosin V or myosin II using streptolysin-O. Only the anti-myosin whole serum significantly reduced aggregation rate (multiple regression analysis, P = 0.028). Aggregation rates for anti-myosin V and anti-myosin II are not different from control values (normal goat serum), and have been combined into a single overall control rate.
The in vitro kinetic assay shows that only the anti-myosin whole serum significantly attenuates the rate of RPCH-triggered pigment aggregation. Since all the other responses were similar to the control conditions [streptolysin-O (SLO) alone and SLO plus goat serum], the data were pooled into one single control rate (Figure 4B).
Discussion
Fluorescence studies of whole pigment cells can be challenging, and we employed homogenization of the ovarian capsule as the preferred technique. This process produces pigment-containing chromatophore fragments and free pigment granules, while diminishing the background signal associated with the non-chromatophore ovarian tissue. Even so, additional issues derive from the optical properties of the pigment itself, particularly autofluorescence. To counter this, computerized quantitation and statistical methods are necessary to allow comparison of fluorescence intensities, and thus to unequivocally establish the association of both myosin and kinesin with the pigment fragments. Pigment autofluorescence is clearly shown in the 6′-diamidino-2-phenylindole (DAPI) channels of 2, 3, and a greater DAPI signal should be associated with aggregated pigment, as the granules are more concentrated per unit area than in dispersed pigment. As DAPI fluorescence markers were not used in this study, the DAPI signal seen in these images may derive from pigment autofluorescence in the negative controls, and autofluorescence plus bleed-over from strong tetramethylrhodamine isothiocyanate (TRITC) and fluorescein isothiocyanate (FITC) signals in the experimental groups. Note, however, that the pigment autofluorescence seen in the DAPI channels is not an indicator of the levels encountered in the other channels. Further, since each of the 12 panels shown in 2, 3 represents a different condition of antibody labeling and pigment state (either aggregated or dispersed), they cannot be compared directly. Such panels are single examples of the entire data set that was statistically analyzed for each channel and pigment state, e.g. for the FITC channel, the experimental ‘dispersed pigment’ group was directly compared only with the corresponding ‘dispersed pigment’ negative control group (Figure 2B, AMWS versus MNC) and so on. Statistical tests were not performed on the isolated pigment granules.
The double-labeling antibody experiments, showing that pigment in the red, ovarian chromatophores of M. olfersii possess at least two distinct protein motors disclose consistent findings, given that functional associations between actin-based and microtubule-based protein motors and pigment granules have been reported in the chromatophores of a variety of animal species, including fish and frog (Gross et al., 2002; Rodionov et al., 1998). That the labeled protein motors co-localize completely with the pigment in fragments from chromatophores fixed in either the dispersed or aggregated states suggests that the kinesin and myosin molecules are associated with the pigment granules in a stable manner, and thus are likely to be regulated while remaining bound to their pigment cargo. Rogers et al. (1999) have reported that some degree of motor protein regulation takes place via a reversible association between myosin V and melanosomes in the melanophores of Xenopus leavis. However, this reversible association is cell-cycle regulated, rather than hormonally signaled, since myosin V dissociates from melanosomes during mitosis.
The present findings, that both microtubule-based and actin-based protein motors are associated with pigment granules in M. olfersii red ovarian chromatophores concurs with mounting evidence that pigment translocation requires cooperation between actin filaments and microtubules (Rodionov et al., 1998; Rogers and Gelfand, 1998). Even when a single motor/cytoskeletal system is disturbed, pigment translocation and/or distribution is adversely affected. The pigment aggregator in the red, ovarian chromatophores of M. olfersii appears to be a myosin. The in vitro motility assay shows that only the anti-myosin whole serum effectively attenuated RPCH-triggered pigment aggregation, a result consistent with our data from fluorescence microscopy showing that the pigment is labeled only by the anti-myosin whole serum. Interestingly, some chromatosomes treated with anti-myosin whole serum exhibit pigment hyper-dispersion (Figure 4A). This phenomenon has been reported by Nilsson et al. (1996) and others, and is attributed to the disruption of one of the elements required for normal pigment aggregation. The pigment hyper-dispersion seen in M. olfersii chromatosomes supports the notion that myosin is the pigment aggregator, and provides independent evidence of effective antibody penetration into live chromatosomes using the SLO technique.
The myosin motor in question may be myosin XII. The Western blot analysis (Figure 1) reveals two bands that correspond to myosin heavy chains >210 kDa. While both bands are of molecular mass greater than the heaviest molecular marker used, reasonable deductions can be made concerning their identities. There are only two known myosin heavy chain classes >210 kDa: one is myosin II heavy chain, of approximately 240 kDa, expressed in all muscle tissues and in non-muscle cells; the other is the myosin XII heavy chain. While not previously isolated, the predicted mass of the myosin XII heavy chain is 307 kDa (Baker and Titus, 1997). Further, the presence of a myosin II in this blot is expected, as crustacean ovarian tissue includes both striated and smooth muscle fibers. This agrees with our fluorescence microscopy findings showing that the anti-myosin serum labels the surrounding non-chromatosome ovarian tissue (not shown). Thus, the remaining 307-kDa band in the Western blot appears to match the fluorescence microscopy data, which demonstrate that the anti-myosin whole serum intensely labels the ovarian chromatophore pigment (2, 3). It is plausible, therefore, that this signal derives from myosin XII.
Ultrastructural studies of red, ovarian chromatophores from M. olfersii show the chromatophore extensions to contain both microtubules and microfilaments (Ribeiro and McNamara, 2001). The number of microtubules is reduced compared with the red, epidermal chromatophores (McNamara and Taylor, 1987), usually <10 per extension. The numerous, short microfilaments appear as semi-parallel arrays aligned along the extension long axis or somewhat randomly throughout the extension cytoplasm. This arrangement also suggests that an actin-dependent myosin motor alone might be responsible for retrograde pigment granule transport (aggregation), while the microtubule-dependent motor, conventional kinesin, may facilitate anterograde transport (dispersion), since both the actin microfilaments and the microtubules are oriented mainly parallel to the chromatophore extension. This elegant arrangement may depend on the uniform polarity of the microfilament arrays, as yet unestablished. That butanedione monoxime, a myosin-ATPase inhibitor, inhibits complete pigment aggregation in M. olfersii red, ovarian chromatophores (McNamara and Ribeiro, 1999) further corroborates the notion that myosin plays an important role in aggregation.
The anti-myosin serum used here was developed in rabbit against myosin purified from bovine uterine smooth muscle, and was chosen for its potential specificity against more than one myosin class. In positive-control tests, myosin labeling was amply distributed among the chromatosomes in the shrimp ovarian tissue (not shown). The findings with the anti-myosin serum suggest that this actin-associated motor protein plays an important role in pigment translocation in these chromatophores. Myosins constitute an integral part of the pigment translocation mechanism in mouse (Liu et al., 1998; Nascimento et al., 1997; Provance et al., 1996, 2002), fish (Rodionov et al., 1998), and frog melanocytes (Gross et al., 2002).
To further characterize the myosin associated with the ovarian chromatophore pigments, we probed chromatosome preparations for myosin V and myosin II (non-muscle). The anti-myosin V antibodies were produced by cloning the cDNA sequence corresponding to head-domain amino acids 5–752 of chick brain myosin V into a bacterial expression system, and inoculating the purified expressed product into rabbits (Espreafico et al., 1992). The polyclonal antibodies were then affinity-purified with chick brain myosin V, and subsequently screened against various guinea-pig tissues known to contain myosin V, or not. Electrophoretic analyses consistently revealed a single 190-kDa band in tissue where myosin V was known to be present (brain) but was absent from heart and lung tissue (Coling et al., 1997). The anti-myosin II polyclonal antibodies used here are less well characterized, and were produced against non-muscle myosin II purified from chick brain. These antibodies were also affinity-purified and do not cross-react with myosin V; they have been used to localize myosin II in bovine chromaffin cells (Rose et al., 2003).
Kinesin was discovered in 1985 and shown to be the effector of fast axonal transport in squid giant neurons (Vale et al., 1985). The anti-kinesin antibodies were derived using bovine brain conventional kinesin as the antigen; mouse hybridomas were used to produce this monoclonal anti-kinesin IgM. This primary antibody has been used with success in a variety of species, including sea urchin (Sigma technical information). In positive-control tests in M. olfersii, this antibody selectively labeled presumptive neurons in the same fibrous ovarian tissue capsule that contains the red chromatosomes (not shown). In other tests, this anti-kinesin antibody has been used to screen rat cerebral extract where it recognizes 130- and 140-kDa bands (Sigma technical information), which is not surprising as two kinesin heavy chains, the products of distinct genes, originally termed ‘ubiquitous’ kinesin heavy chain (uKHC) and ‘neuronal’ (nKHC), are expressed in rat brain (Vignali et al., 1997).
Further investigation is necessary to confirm the specific motors responsible for pigment aggregation and dispersion in this system. However, our findings do suggest that conventional kinesin may be the protein motor involved in granule dispersion, assuming that the chromatophores exhibit conventional microtubule polarity. Further, myosin XII or perhaps myosin II may be responsible for pigment aggregation. Together, these findings establish a role for kinesin and myosin in the intracellular transport of pigment granules in the freshwater shrimp, M. olfersii.
Methods
Reagents
All reagents were purchased from the Sigma-Aldrich® Corporation (St Louis, MO, USA) unless otherwise stated. Primary antibodies: anti-myosin whole anti-serum, developed in rabbit against myosin light and heavy chains purified from bovine uterine smooth muscle; polyclonal anti-myosin II (non-muscle) and polyclonal anti-myosin V (head-domain) (kindly provided by Prof. Roy E. Larson, Faculty of Medicine of Ribeirão Preto, University of São Paulo, São Paulo, Brazil); monoclonal anti-kinesin heavy chain (KHC) immunoglobulin M (IgM). Secondary antibodies: goat anti-mouse IgM (μ-chain specific) FITC; goat anti-rabbit IgG TRITC. Other reagents: normal goat serum, paraformaldehyde, saponin, bovine serum albumin, sodium azide, Vectashield® (Vector Laboratories, Burlingame, CA, USA) mounting medium for fluorescence microscopy. Physiological shrimp saline: NaCl (180 mM), KCl (5 mM), MgCl2 (1 mM), CaCl2 (5.5 mM), dextrose (2 mM), sodium bicarbonate (2.5 mM), HEPES (5 mM). Phosphate-buffered saline (PBS)/bovine serum albumin (BSA): 50 mM phosphate-buffered saline, pH 7.4, plus 0.1% bovine serum albumin and 0.05% sodium azide.
Specimen collection and ovary dissection
Female specimens of the freshwater shrimp, M. olfersii, were collected from the Paúba River in São Sebastião, on the coast of São Paulo State, Brazil (MMA/IBAMA/DIREN permit no. 18/2002). In the laboratory, the shrimp were maintained in large, aerated tanks, subject to a natural photoperiod (≈12 h light:12 h dark), and were fed a diet of lettuce, carrot, ground beef, chicken and fish.
Prior to dissection, pigment aggregation was induced in some shrimp by placing them on a white background for approximately 30 min. In other experiments, pigment dispersion was induced by placing the shrimp on a black background for about 30 min. The fully aggregated or fully dispersed state of the chromatophore pigments was verified before dissection.
Whole ovaries were rapidly dissected from the shrimp under physiological saline. The ovarian connective tissue capsule, which encloses the clutch of eggs, and contains numerous, large, red chromatosomes, was then ventrally transected and the eggs were gently removed. This fibrous capsule of chromatosomes and ovarian tissue was then fixed immediately in 4% paraformaldehyde in physiological shrimp saline for 45 min.
Immunolabeling
The previously fixed tissue comprising samples of chromatophores with either fully aggregated or dispersed pigment was washed in PBS/BSA buffer, and homogenized for 30 s in a small, hand-held, teflon-glass homogenizer (Wheaton, Millville, NJ, USA), containing 200 μl PBS/BSA buffer plus 0.25% saponin. The homogenization process produced chromatophore fragments partially depleted of pigment, together with a supernatant containing suspended pigment granules. The homogenates were centrifuged at 15 000 g for 10 min at 4°C, which pelleted the fragments. Individual pigment granules, released during the homogenization process, remained in the supernatant. These supernatants were removed and reserved in separate tubes.
Pellet samples were resuspended in 100 μl PBS/BSA buffer and divided into ‘control’ and ‘experimental’ groups of equal volumes (50 μl) for each of the two original pigment states (either aggregated or dispersed), as were the supernatants. Each sample of chromatophore fragments or isolated pigment granules was blocked with goat serum and stored overnight at 4°C. The following morning, primary antibodies against kinesin heavy chain (1:500) (raised against bovine brain conventional kinesin, IgM isotype) and anti-myosin whole serum (1:50) (raised against bovine ovarian smooth muscle), or anti-myosin V head domain (raised against chick brain myosin V) (1:200) or anti-myosin II non-muscle (raised against chick brain myosin II) (1:200) were added to the ‘experimental’ groups alone and were incubated for 2 h. The ‘negative control’ groups did not receive primary antibodies. Those groups containing chromatophore fragments were centrifuged twice at 15 000 g for 10 min and resuspended in PBS/BSA buffer. All groups, i.e. those containing either chromatophore fragments or isolated pigment granules, in both the ‘negative control’ and ‘experimental’ series, then received equal concentrations of labeled secondary antibody: (1:200) goat anti-mouse IgM FITC, and goat anti-rabbit IgG TRITC, and further incubated for 2 h.
The samples containing the chromatophore fragments were then centrifuged twice at 15 000 g and 4°C, and resuspended in 50 μl PBS/BSA buffer. An aliquot of 20 μl from each sample was then mixed with 20 μl Vectashield® mounting medium, and slide mounts were prepared for examination using an Olympus® (Melville, NY, USA) BX60 epi-fluorescence microscope.
Size exclusion chromatography
To separate unbound antibody from the isolated pigment granules in the supernatants, entire samples were applied individually to a gel-filtration chromatography column containing Sepharose® (GE Healthcare/Amersham Biosciences, Uppsala, Sweden) 4B beads (approximately 4.5 ml packed bed volume), and 0.5-ml fractions of column effluent were collected. A pinkish fraction was shown to contain pigment granules microscopically. Ten microliter aliquots from each granule-containing fraction were combined with Vectashield® mounting medium, and slide mounts were prepared for epifluorescence microscopy.
Image acquisition and analysis
Images were acquired using an Optronics® (Goleta, CA, USA) 75OL CCD camera attached to an Olympus® BX60 epifluorescence microscope. Eight-bit fluorescent images were background subtracted and intensity quantified using NIH Image freeware, available at http://rsb.info.nih.gov/nih-image. The images of chromatophore fragments were traced by hand and quantified automatically. Only one fragment per slide (shrimp) was chosen at random, and each fragment contained at least 100 contiguous pixels.
Statistical analyses
All transformed fluorescence intensities (divided by CCD exposure time, either 1 or 0.5 s) of pigment images taken from the series containing fragments from chromatophores with dispersed (n = 14–15) or aggregated (n = 5–11) pigments were normally distributed: thus, an unpaired Student's t-test was used to compare each ‘experimental’ and ‘control’ series. Welch's correction, which does not assume equal variances between compared groups, was applied in all cases. Statistical analysis was performed using GraphPad Instat® statistical software (San Diego, CA, USA). Differences with P-values ≤0.05 were considered significant.
In vitro motility assay
Antibodies were introduced into live chromatosomes using the SLO technique (Boyle and Lieberman, 1999). Pigment aggregation in response to RPCH was then tested in the chromatosomes loaded independently with each antibody. Whole ovaries exposed to SLO plus normal goat serum (‘control’ group) or SLO plus anti-myosin V, or anti-myosin II non-muscle, or anti-myosin whole serum (‘experimental’ groups) were mounted in an observation chamber containing physiological saline and observed using a stereomicroscope. Red pigment concentrating hormone (10 nM) was added to the saline, and sequential photographic images were taken at 5-min intervals recording the pigment aggregation process. The maximum diameter of one chromatosome per ovary was quantified at each interval during aggregation using NIH Image. From seven to 10 replicates were used for each antibody and control experiment. The data were normalized, and the linear portions of the aggregation curves (from T = 0 to 10 min) were compared using a multiple regression analysis (GraphPad Prism®).
SDS-PAGE and Western blot analyses
Fresh samples of ovarian tissue capsules from M. olfersii containing red chromatosomes were homogenized in a small, hand-held, Wheaton homogenizer in 100 μl of buffer containing 100 mM PBS pH 7.4 and SDS (4%). This homogenate was mixed with an equal volume of double strength Laemmli buffer, and placed in a water bath at 100°C for 5 min. The samples were then loaded into a continuous-gradient polyacrylamide gel (5–20%) and electrophoresed at a constant current of 20 mA for 1 h. Coomassie staining was performed using aqueous 20% colloidal Coomassie Brilliant Blue G-250 for 16 h; the gel was then de-stained and fixed in 20% aqueous methanol (3 × 2 h each change).
The anti-myosin whole serum was used as the primary antibody in a Western blot analysis to identify the myosin classes expressed in the ovarian capsule tissue, since this serum labeled pigment granules in the fluorescence studies, and attenuated pigment aggregation in the kinetic assay in vitro. An unstained SDS-PAGE gel was placed in a transfer chamber and the separated proteins were transferred to a nitrocellulose membrane which was blocked with a buffer [Tris-buffered saline (TBS)] consisting of Trizma® Base (Sigma Aldrich, St. Louis, MO, USA) 50 mM (pH 7.4), NaCl (0.9%), non-fat powdered milk (3%) and Tween® (Sigma Aldrich) (0.5%) for 1 h. The membrane was then exposed to the anti-myosin whole serum (2 μg/ml) for 1 h, washed four times with TBS, exposed to horseradish peroxidase (HRP)-conjugated secondary antibodies, and developed using an FCL chemiluminescence kit from Amersham Biosciences® (Uppsala, Sweden). The blot was purposely overdeveloped to visualize the molecular mass markers, allowing unequivocal localization of the anti-myosin positive bands and easy comparison with those in the Coomassie-stained SDS-PAGE gel.
Acknowledgements
The authors thank Prof. Roy Edward Larson and Dr Norberto Garcia-Cairasco (Faculdade de Medicina de Ribeirão Preto, USP) for providing the anti-myosin II and V antibodies, and for access to fluorescence imaging hardware, respectively. This work represents part of a doctoral dissertation submitted by RTB to the graduate course in Comparative Biology (DB-FFCLRP/USP), financed by a CAPES scholarship. JCM acknowledges CNPq for a research scholarship (no. 303282/84-3), FAPESP (no. 2000/04588-2) and CAPES for research funding, and the Centro de Biologia Marinha, USP for support during collecting expeditions.