Ednrb2 orients cell migration towards the dorsolateral neural crest pathway and promotes melanocyte differentiation
Present address: Manijeh Pasdar, Cell Biology Department, University of Alberta, Edmonton, AB, T6G2H7, Canada.
Summary
Endothelin receptors B (Ednrb) are involved in the development of the enteric and melanocytic lineages, which originate from neural crest cells (NCCs). In mice, trunk NCCs and their derivatives express only one Ednrb. In quail, trunk NCCs express two Ednrb: Ednrb and Ednrb2. Quail Ednrb is expressed in NCCs migrating along the ventral pathway, which gives rise to the peripheral nervous system, including enteric ganglia. Ednrb2 is upregulated in NCCs before these cells enter the dorsolateral pathway. The NCCs migrating along the dorsolateral pathway are melanocyte precursors. We analyzed the in vitro differentiation and in ovo migration of mouse embryonic stem (ES) cells expressing and not expressing Ednrb2. We generated a series of transfected ES cell lines expressing Ednrb2. This receptor, like Ednrb, oriented genuine ES cells towards melanocyte lineage differentiation in vitro. The in ovo migration of Ednrb2-expressing ES cells was massively oriented towards the dorsolateral pathway, unlike that of WT or Ednrb-expressing ES cells. Thus, Ednrb2 is involved in melanoblast differentiation and migration.
Introduction
Endothelin receptors (Ednr) are G protein-coupled receptors (GPCR) with seven transmembrane domains (for review see Pla and Larue, 2003). Two Ednrb subtypes exist: Ednrb and Ednrb2. Ednrb has been cloned in various vertebrates, whereas Ednrb2 has been cloned in chicken and quail. Various Ednr regulate blood pressure and neural crest development (Miyauchi and Masaki, 1999).
Neural crest cells (NCCs) are pluripotent cells that delaminate from the dorsal part of the neural tube and migrate extensively in vertebrate embryos (Le Douarin and Kalcheim, 1999). In the trunk, NCCs migrate via (i) the ventral pathway, between the somites and the neural tube and (ii) the dorsolateral pathway, between the somites and the ectoderm. Early NCCs, which emigrate from the neural tube, follow the ventral pathway and give rise principally to neurons and glial cells. Late NCCs proliferate in the migration staging area (MSA), located between the neural tube, somites and ectoderm, for 24 h. They then follow the dorsolateral pathway, giving rise to skin melanocytes. Migration along the dorsolateral pathway is dependent on the melanoblast specification of NCCs (Erickson and Goins, 1995). The molecular mechanisms controlling entry into the dorsolateral pathway in melanoblast migration are unclear.
In the trunk, Ednrb is expressed in all NCCs before and after their delamination from the neural tube (Hou et al., 2004). The expression of this receptor is restricted to NCCs migrating via the ventral pathway in birds, but is maintained in both pathways in mice (Nataf et al., 1996). In birds, Ednrb2 is activated in NCCs located in the MSA just before the initiation of dorsolateral migration. Ednrb2 is expressed throughout melanocyte differentiation (Lecoin et al., 1998). Endothelin-3 (Edn3), the ligand of Ednrb and Ednrb2, is produced in the ectoderm and in gut mesenchyme (Nataf et al., 1998).
In mice, the endothelin ligand/receptor (Edn3/Ednrb) system is required for normal melanocyte and enteric ganglion development (Baynash et al., 1994). Mutations have been found in the genes encoding these proteins in many mammals, including humans, in whom they may cause Waardenburg syndrome type IV [WS4 or Shah-Waardenburg syndrome (OMIM 277580)] or Hirschsprung syndrome (HSCR) (Puffenberger et al., 1994). Patients with HSCR have fewer than normal enteric ganglia and patients with WS4 have pigmentation defects and deafness caused by the absence of melanocytes in the skin and in the inner ear, respectively. In melanocytes, Ednrb is required for the migration of melanoblasts along the dorsolateral pathway (Shin et al., 1999). The precise role of Ednrb in migration and in proliferation/survival has been investigated. The major function of Ednrb seems to be associated with terminal migration (Lee et al., 2003). in vitro, Edn3 is a potent mitogenic molecule for NCCs and stimulates the production of melanocytes (Reid et al., 1996). The targets of Edn3 in the quail are glial and melanocytic unipotent precursors, and common bipotent precursors (Lahav et al., 1998; Le Douarin et al., 2004), in vitro, Edn3 downregulates Ednrb and upregulates Ednrb2 (Lahav et al., 1998).
Wild-type mouse embryonic stem (ES) cells migrate in both the ventral and dorsolateral pathways when grafted into the MSA of chicken embryos (Beauvais-Jouneau et al., 1999) and preferentially differentiate in vitro into melanocytes under specific conditions (Yamane et al., 1999). In this respect, we used mouse ES cells to investigate the role of quail Ednrb2 in migration and differentiation. The expression of Ednrb2 stimulated the production of melanocytes and oriented the migration of cells towards the dorsolateral pathway.
Results
Establishment of Ednrb2- and Ednrb-expressing ES cell lines
RT-PCR and a pharmacological assay detected no Ednr expression in normal WT ES cell lines (Figure 1A,C). ES cells were transfected with expression-selection vectors allowing the constitutive expression of quail Ednrb2, or as a control, mouse Ednrb. ES cell clones were amplified under hygromycin selection, and the expression of Ednrb2 or Ednrb in these cells was assessed by RT-PCR (Figure 1A).
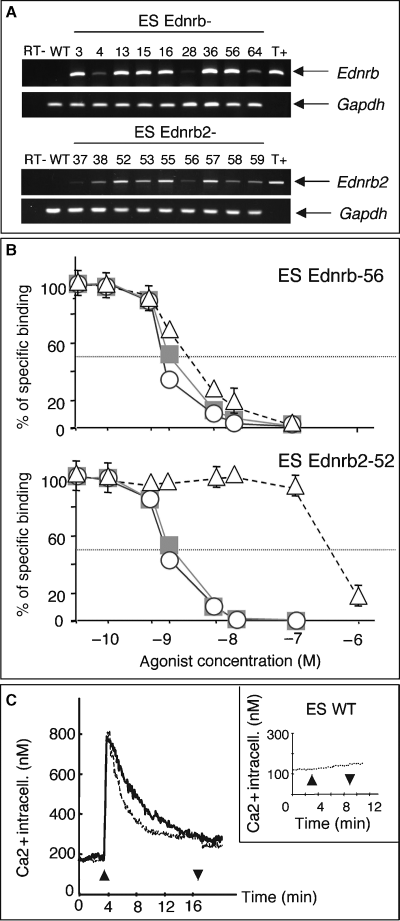
Expression and functionality of Ednrb and Ednrb2 in transfected ES cells. (A) RT-PCR on RNA extracted from 18 ES cell lines stably transfected with Ednrb or Ednrb2. R1 WT ES cells (WT) do not express Ednrb or Ednrb2. The negative controls (RT-) consisted of RNA derived from ES Ednrb-56 and ES Ednrb2-52 cells without reverse transcriptase. The positive controls (T+) consisted of plasmids nos 408 and 327. (B) Assay of competitive binding of Edn1 (circles), Edn3 (squares) and sarafotoxin 6c (triangles) on Ednrb-56 and Ednrb2-52 ES cells incubated with 125I-Edn1. (C) Intracellular calcium concentration determined by Fura-2 fluorescence measurement before and after the addition of 100 nM Edn3 (up arrow) and after the removal of Edn3 (down arrow) in ES Ednrb-56 (thick line), Ednrb2-52 (thin line) and WT (inset) ES cell lines.
We used a pharmacological binding competition assay to test for the presence of Ednr and to evaluate their binding capacities on the transfected ES cell lines (Figure 1B). Ednrb and Ednrb2 have similar affinities for Edn1-3, their classical ligands. Ednrb and Ednrb2 ES cell clones had similar affinities for Edn1 and Edn3 (for Edn1: EC50Ednrb = 0.8 nM, EC50Ednrb2 = 0.9 nM, and for Edn3: EC50Ednrb = 1.0 nM, EC50Ednrb2 = 1.0 nM), whereas Ednrb had a higher affinity for sarafotoxin 6c than did Ednrb2 (EC50Ednrb = 2 nM, EC50Ednrb2 =370 nM). Similar results were previously obtained with different cell types (Lecoin et al., 1998). Scatchard plot analysis demonstrated the presence of 41,000 Ednr on the surface of each ES Ednrb2-52 cell, vs. 47,000 on ES Ednrb-56 cells.
The activity of Ednrb2 and Ednrb in ES cells was evaluated by means of an intracellular calcium assay (Figure 1C). The presence of both receptors significantly increased cytoplasmic calcium concentration after induction with Edn3. No calcium uptake was observed with R1 wild-type ES cells or Ednrb2-AS-18 ES cells containing the antisense Ednrb2 construct. We had therefore produced a series of ES cell lines expressing functional Ednrb or Ednrb2 on their surface.
Ednrb2 and Ednrb promote melanocyte differentiation
Endothelins promote melanocyte production in NCCs in vitro (Lahav et al., 1996). We investigated the role of Ednrb2 and Ednrb in the melanocyte lineage by allowing ES cells producing either Ednrb or Ednrb2 to differentiate in vitro. When cultured in vitro on a layer of ST2 stromal cells with dexamethasone, basic fibroblast growth factor and cholera toxin, in the absence of LIF, ES cells produced large numbers of melanocytes. The number of melanocytes generated by a given genotype was similar between experiments (Yamane et al., 1999). We subjected ES Ednrb-16, ES Ednrb-36, Ednrb2-52, Ednrb2-55, ES Ednrb2-AS-18 and R1 WT ES cells to the melanocyte differentiation assay and the number of colonies containing at least one pigmented cell (melanocyte colonies) was determined. After 30 d in the presence or absence of 40 nM Edn3, Ednrb-16, ES Ednrb-36, Ednrb2-52, or Ednrb2-55, ES cell lines produced significantly more melanocyte colonies than did wild-type or AS ES cells (Figure 2). Thus, Ednrb2 and Ednrb both promote melanocyte differentiation.
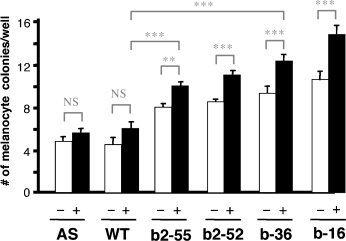
Ednrb and Ednrb2 promote melanocyte differentiation. The number of melanocyte colonies per well was determined on day 30. Results of three independent experiments performed in duplicate are shown. AS (Ednrb2-AS-18), WT (R1), b2-55 (Ednrb2-55), b2-52, b-36 and b-16 ES cell lines were cultured in vitro on a layer of ST2 cells without (−) or with (+) 40 nM Edn3 for 30 d. Some P-values are indicated ***P < 0.001; **P < 0.05; *P < 0.01; NS = non-significant difference.
Ednrb2 orients migration towards the dorsolateral neural crest pathway
As Ednrb2 is expressed in specific NCCs just before these cells enter the dorsolateral pathway (Lecoin et al., 1998), the upregulation of Ednrb2 may be responsible for directing the migration of these cells via this pathway. We tested this hypothesis by labeling Ednrb2-52, Ednrb2-55, Ednrb-36, Ednrb-56 and WT undifferentiated ES cells with a vital fluorescent marker and grafting the labeled cells into the MSA of chicken embryos containing 20–25 somites. The position and the number of cells were determined 18 h later (Figure 3A–C). No significant differences were observed between the two Ednrb2 ES cell clones or between the two Ednrb ES cell clones. Migrating cells were found in 80, 63 and 100% of the embryos grafted with WT, Ednrb and Ednrb2 ES cells, respectively (Figure 4A–C). Thus, expression of Ednrb2 increased the general migration potential of the grafted cells.
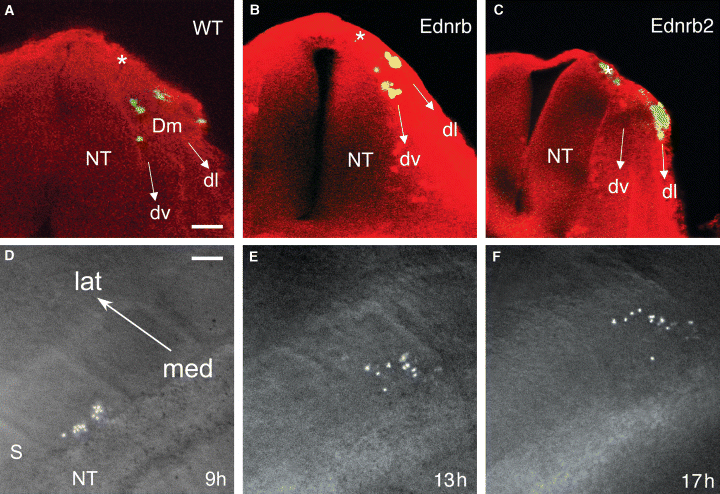
Migration of ES cells expressing Ednr in chicken embryos. (A–C) Transverse sections of embryo trunks fixed 18 h after the grafting of fluorescent green-labeled ES cells of the WT (A); Ednrb-56 (B); Ednrb2-52 (C) ES cell lines. (D–F) Dorsal views of an embryo 9 h (D); 13 h (E) and 17 h (F) after the grafting of Ednrb2-52 ES cells. dl; dorsolateral pathway, Dm; dermomyotome, dv; ventral pathway, lat; lateral, med; medial, NT; neural tube, S; somite, *site of the graft. Scale bars: 25 μm (A–C) and 40 μm (D–F).
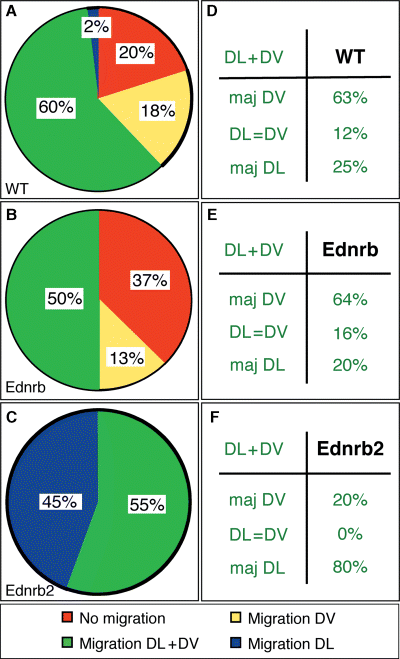
Ednrb2 enhances cell migration and dorsolateral migration. (A–C) Statistical analyses of migration pattern. The percentages of embryos in which no cell migrated (red), cells migrated via both pathways (green), via the ventral pathway exclusively (yellow) and the dorsolateral pathway exclusively (blue) 18 h after grafting are indicated. (D–F) Embryonic distribution of the cells that migrated via both pathways. The percentages of embryos in which most cells migrated either via the ventral (maj DV) or via the dorsolateral (maj DL) pathway or in which similar numbers of cells migrated via both pathways (DL = DV) are shown.
In a given embryo, ES cells may follow the dorsolateral and/or ventral pathways (Beauvais-Jouneau et al., 1999). We found that 2, 0 and 45% of embryos grafted with WT, Ednrb and Ednrb2 ES cells, respectively, contained only cells migrating via the dorsolateral pathway. In embryos in which cells migrated in both pathways, most cells followed the dorsolateral pathway in embryos grafted with Ednrb2 ES cells, whereas most of the cells followed the ventral pathway in embryos grafted with WT or Ednrb ES cells (Figure 4D–F). Thus, Ednrb2 expression oriented the migration of ES cells towards the dorsolateral pathway.
We studied the long-term migration of Ednrb2 ES cells and WT ES cells, by transfecting these cells with a constitutive expression vector containing the LacZ gene. The distance migrated along the dorsolateral pathway was similar in Ednrb2-LacZ and WT-LacZ ES cells at any given time (18, 42 and 66 h) after the graft (data not shown). This is consistent with previous findings that there is no significant difference between the apparent speed of migration of Ednrb2 ES cell and WT ES cells (about 10 μm/h) during the first few hours of in ovo migration (Figure 3D–F). In conclusion, Ednrb2 had no effect on the apparent speed of migration of cells in the dorsolateral pathway.
Discussion
Two Endr are expressed in avian trunk NCCs – Ednrb and Ednrb2 – both of which are involved in proliferation. We show here that Ednrb2 is also involved in melanoblast differentiation and migration.
Edn signaling increases the number of melanocytes in cultures of avian or mouse NCCs (Lahav et al., 1996). In the avian system, the induction of NCC differentiation in vitro has been reported to be correlated with the appearance of cells expressing Ednrb2 and producing melanin (Lahav et al., 1998; Lecoin et al., 1998). We showed in this study that the exogenous expression of Ednrb2 induces the differentiation of melanocytes in vitro. Ednrb had a slightly more potent effect than Ednrb2, but it should be noted that melanocyte production was increased in the absence of exogenous endothelin in the medium. It was recently shown that ST2 cells produce Edn1 (Aoki et al., in press). Edn1 and Edn3 have similar affinities for Ednrb and Ednrb2 (Figure 1). The observed effect may be induced by one of these factors. The addition of exogenous Edn3 significantly increased the production of melanocyte colonies by the various ES cell lines.
WT ES cells do not produce significant amounts of Dct or Mitf, both of which are melanoblast markers. When grafted in chicken, some, but not all ES cells migrating in the dorsolateral pathway displayed molecular differentiation, followed by the expression Dct or Mitf. This suggests that molecular differentiation (reflected in the expression of Dct or Mitf) is not required for the dorso-lateral migration of ES cells (Pla et al., 2004). We also produced ES cells expressing mMitf constitutively. Several independent mMitf ES cell clones were grafted similarly to Ednrb2 or WT ES cells. The exogenous expression of mMitf in ES cells does not affect in ovo ES cell migration (data not shown). We showed in this study that the ectopic expression of Ednrb2 in ES cells increased cell migration and played a determinant role in the selection of the dorsolateral pathway for the migration of ES cells. Avian Ednrb2 is upregulated just before NCCs begin to migrate in the dorsolateral pathway (Lecoin et al., 1998). These findings strongly suggest that Ednrb2 play a causative role in the choice of migration pathway in NCCs. This receptor is functional only when activated. Edn3 is known to be expressed in the ectodermal cells at HH19-20, but not at HH12-13. It is unknown whether Edn3 is expressed at HH15, the time at which ES cells were grafted. At this time point, we observed a clear migration effect in the various ES cell clones expressing Ednrb2. As Ednrb2 is not constitutively active (see Figure 1), we can assume that Edn3 is present at the time of grafting, in the environment of the migrating ES cells. Moreover, endogenous chicken melanoblasts begin to migrate at HH20, whereas ES cells start to migrate 10 h after grafting. ES cells therefore begin to migrate 5–10 h before endogenous melanoblasts, and may be said to migrate precociously. No difference was observed between Ednrb2 and control ES cells in terms of the time at which migration began.
Although Ednrb is also expressed on cells following the dorsolateral pathway in mice, ES cells expressing this receptor were not oriented towards this pathway, migrating instead like WT ES cells. This may be because mouse Ednrb is also expressed in the ventral pathway, suggesting that the signals controlling the choice of migration pathway are independent of Ednrb (Lee et al., 2003). Indeed, Ednrb signaling is required for the late stages of melanoblast migration in mice, but not for the initial choice of pathway (Lee et al., 2003).
Avian and mouse NCCs display molecular similarities and differences (Pla and Larue, 2003), with differences in the expression patterns of Ednrb and Kit. No ortholog of Ednrb2 has been identified in the mouse and human genomes. Kit, a transmembrane tyrosine-kinase protein, is upregulated in the MSA before migration in mice, whereas it is upregulated via the dorsolateral pathway after the onset of migration in chickens (Wehrle-Haller and Weston, 1995). Kit may provide the initial signal leading to dorsolateral migration in mice, whereas this role might be played by Ednrb2 in birds. If this were the case, then mouse cells lacking Kit would not display impairment of early migration in a bird environment, as previously reported (Beauvais-Jouneau et al., 1999).
Other proteins, such as ephrins and chondroitin sulfates, are involved in the migration of melanoblasts. However, the connection, if indeed there is one, between Ednrb2 and ephrins/chondroitin sulfates is currently unclear. Ephrin receptor tyrosine kinases and their ligands are involved in the control of dorsolateral migration in chicken (Santiago and Erickson, 2002). During early NCC development, ephrin signaling blocks dorsolateral migration. However, this signaling subsequently promotes melanoblast migration via the dorsolateral pathway. The mechanism underlying this change in response to ephrin signaling is unknown. The stage at which ephrin function is reversed is correlated with the stage at which Ednrb2 is upregulated in the MSA of the avian embryo (Lecoin et al., 1998; Santiago and Erickson, 2002). Ednrb2 signaling may modify the cellular response to ephrin signals, converting a repulsive response into an attractive one. WT and Ednrb2-52 ES cells produce mRNA molecules for ephrin receptors, such as EphA4, EphB2 and EphB3, in similar amounts to NCCs and melanoblasts (data not shown and Santiago and Erickson, 2002). Post-translational differences may occur, but have yet to be demonstrated. Little is known about the types of cell that produce chondroitin sulfate, an extracellular molecule, but melanoblasts are highly unlikely to be involved in this production (Oakley et al., 1994).
Although the mechanism of action of Ednrb2 is still not understood, we show here that this protein has a specific function in selection of the dorsolateral pathway of migration for NCC and, like Ednrb, induces melanocyte differentiation.
Materials and methods
Cell culture and cell lines
Mouse ES cells were grown on 0.1% (w/v) gelatin in high-glucose DMEM supplemented with 15% heat-inactivated FCS (Perbio Science Ltd, Cheshire, UK), 2 mM glutamine (Gibco-BRL, Gaithersburg, MD, USA), 2000 U/ml mouse LIF (Gibco-BRL), and 0.1 mM β-mercaptoethanol (Sigma), with penicillin and streptomycin.
Establishment of Ednrb2- and Ednrb-expressing ES cell lines
pCAGGS-Hyg was produced by inserting a 1.9 kb ClaI-HindIII fragment containing the hygromycin resistance gene driven by the PGK1 promoter (a gift from A. Berns) into the HindIII site of pCAGGS (Niwa et al., 1991). We used the quail Ednrb2 and mouse Ednrb cDNAs (Lecoin et al., 1998). The 4.2 kb XhoI fragment of the quail Ednrb2 cDNA and the 1.6 kb BssHII fragment of the mouse Ednrb cDNA were inserted into the XhoI site of pCAGGS-Hyg in the sense or antisense orientation to produce pCAGGS-Hyg-Ednrb2 (no. 327), pCAGGS-Hyg-Ednrb2-AS (no. 328), pCAGGS-Hyg-Ednrb (no. 408) and pCAGGS-Hyg-Ednrb-AS (no. 409). R1 ES cells were transfected with these constructs, using Lipofectamine (Gibco BRL) (Nagy et al., 1993), according to the manufacturer's instructions. ES cells were selected in medium containing 200 μg/ml hygromycin B (Calbiochem, San Diego, CA, USA) for 14 d, and surviving colonies were isolated and expanded. The expression of Ednr genes was investigated in about 80 ES cell clones for each construct, by RT-PCR with the following oligomers: LL126 (5′ GGT GAC ACT GAT CTG GGC AG) and LL127 (5′ GTT GGT GTG ATG CTC AGA GC) for quail Ednrb2 and LL86 (5′ AAA GAG GTA ATG ACG CCA CCC) and LL87 (5′ ACG AAC ACG AGG CAC GAC ACA) for mouse Ednrb, generating 582 and 209 bp fragments, respectively. PCR was standardized with the oligomeric primers for Gapdh (LL82: 5′ GCT GAG TAT GTC GTG GAG TC and LL83: 5′ TTG GTG GTG CAG GAT GCA TT), which generate a 191 bp fragment. The cycling conditions were as follows: three long cycles (1 min at 94°C, 1 min at 58°C, 1 min at 74°C) and 30 short cycles (45 s at 94°C, 30 s at 58°C, 30 s at 74°C).
Pharmacological studies of endothelin receptors
One day before the experiment (d-1), ES cells were seeded in 24-well plates (5 × 105 cells/well). On d0, ES cells were washed twice with binding medium (50 mM Tris-HCl pH7.6, 125 mM NaCl, 6.5 mM MgCl2, 1 mM EDTA, 1 mg/ml BSA, 20 mM Hepes) and incubated with 50 pM (125I-Tyr)Edn1 (Amersham Biosciences, Piscataway NJ, USA) plus various concentrations of competitor (10−10 to 10−6 M) – human Edn1 (Sigma, E7764), human Edn3 (Sigma, E9137) or sarafotoxin 6c (Calbiochem) – for 1 h at RT. Cells were washed twice with ice-cold binding medium and lysed in 0.1 N NaOH. Cell-bound radioactivity was determined with a gamma counter (Beckman Coulter LS-3801, Fullerton, CA, USA).
Intracellular calcium assays
On d-1, ES cells were seeded on slides coated with 0.3 % (w/v) gelatin in PBS. On d0, cells were loaded with 5 μM Fura-2 (Molecular Probes, Eugene, OR, USA) by incubation at RT for 1 h. Fluorescence was measured under an inverted fluorescence microscope (Nikon-PTI Photoscan II System; Kontron Instruments, Zurich, Switzerland) whilst superfusing with ES medium with or without various concentrations of Edn3 (0.6 ml/min) at 37°C, as previously described (Marchetti et al., 1995).
In vitro differentiation of ES cells into melanocytes
We followed Yamane's original protocol (Yamane et al., 1999). Briefly, on d0, various numbers of ES cells were seeded on a confluent layer of stromal ST2 cells. The differentiation medium {alpha-MEM supplemented with 10% native FCS [1:1 (v:v of JRH:Perbio)], 10−7 M dexamethasone (Sigma), 20 pM bFGF (R&D Systems), 10 pM cholera toxin (Sigma) and in some cases 40 nM Edn3 (Sigma)} was changed every 3 d. We defined embryoid bodies as the masses of ES cells differentiating on the ST2 layer. We found that the number of embryoid bodies per dish affected the differentiation of ES cells into melanocytes. We determined the number of embryoid bodies per well on d7 and d10. Analyses were then performed with similar and optimal numbers of embryoid bodies – about 60 per well (Pla et al., 2004).
Grafting of fluorescent undifferentiated ES cells into the MSA of chicken embryos and analysis of migration
Mouse ES cells were cultured in the presence of LIF [2000 U/ml (GibcoBRL)] to prevent differentiation before grafting. They were fluorescently labeled and grafted as previously described (Beauvais-Jouneau et al., 1999). Briefly, undifferentiated ES cells were labeled by incubation with CFSE (10 μM; Molecular Probes) for 45 min at 37°C in an atmosphere containing 10 % CO2 and were then cultured in suspension for 1 h to allow aggregates of 20–40 cells to form. These aggregates were grafted into the MSA at the fifth last somite in chicken embryos that had developed 20–25 somites. After various lengths of time at 37°C in a humidified atmosphere, the graft was isolated, fixed and treated with rabbit antiserum recognizing laminin (a gift from H. Feracci, CNRS-Institut Curie, Paris, France; 1/500 dilution in PBS containing 5% FCS) and then with secondary antibody (1/50 dilution of Texas Red-conjugated anti-rabbit Ig; Amersham). Embryo slices two-somites thick were examined with a confocal scanning microscope (Leica Lasertechnic, Heidelberg, Germany). We analyzed 45 embryos grafted with R1 ES cells, 19 grafted with Ednrb clone 56 (Ednrb-56) ES cells, 8 grafted with Ednrb-36 ES cells, 22 grafted with Ednrb2-55 ES cells and 13 grafted with Ednrb2-52 ES cells.
Acknowledgements
We would like to thank Catherine Chollet and Jeanine Marchetti for assistance with calcium influx experiments (U367 INSERM), Hervé Kempf (Collège de France) for assistance with pharmacological experiments, Hélène Ferracci (UMR 144 CNRS-Curie) for providing various supplies, Ana-Maria Vallès, Laure Lecoin (UMR 146 CNRS-Curie) and Jean-Loup Duband (UMR 7622 CNRS-Paris VI) for helpful discussions. We thank Marika Pla and Christo Goridis for critical reading of this manuscript. PP and OS received fellowships from the French Ministry of Education, Research and Technology, and from the Institut Curie, respectively. This research was financed by Ligue contre le cancer, GEFLUC and ARC.