A Transgenic Mouse Model with Inducible Tyrosinase Gene Expression Using the Tetracycline (Tet-on) System Allows Regulated Rescue of Abnormal Chiasmatic Projections Found in Albinism
Present address: Patricia Giraldo, Centro Nacional de Investigaciones Oncológicas (CNIO), Melchor Fernández Almagro 3, 28029 Madrid, Spain. Estela Giménez and Alfonso Lavado contributed equally to this work.
Abstract
Congenital defects in retinal pigmentation, as in oculocutaneous albinism Type I (OCA1), where tyrosinase is defective, result in visual abnormalities affecting the retina and pathways into the brain. Transgenic animals expressing a functional tyrosinase gene on an albino genetic background display a correction of all these abnormalities, implicating a functional role for tyrosinase in normal retinal development. To address the function of tyrosinase in the development of the mammalian visual system, we have generated a transgenic mouse model with inducible expression of the tyrosinase gene using the tetracycline (TET-ON) system. We have produced two types of transgenic mice: first, mice expressing the transactivator rtTA chimeric protein under the control of mouse tyrosinase promoter and its locus control region (LCR), and; second, transgenic mice expressing a mouse tyrosinase cDNA construct driven by a minimal promoter inducible by rtTA in the presence of doxycycline. Inducible experiments have been carried out with selected double transgenic mouse lines. Tyrosinase expression has been induced from early embryo development and its impact assessed with histological and biochemical methods in heterozygous and homozygous double transgenic individuals. We have found an increase of tyrosinase activity in the eyes of induced animals, compared with littermate controls. However, there was significant variability in the activation of this gene, as reported in analogous experiments. In spite of this, we could observe corrected uncrossed chiasmatic pathways, decreased in albinism, in animals induced from their first gestational week. These mice could be instrumental in revealing the role of tyrosinase in mammalian visual development.
Abbreviations –
-
- CMV
-
- cytomegalovirus
-
- dpc
-
- days post coitum
-
- DiI
-
- 1,1′,di-octadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate
-
- IPTG
-
- isopropyl beta-d-thiogalactopyranoside
-
- OCA
-
- oculocutaneous albinism
-
- PBS
-
- phosphate buffer saline
-
- RPE
-
- retinal pigment epithelium
-
- rtTA
-
- reverse tetracycline-controlled transcriptional activator
-
- UTR
-
- untranslated region
Introduction
Mutations in the tyrosinase gene are associated with oculocutaneous albinism type I (OCA1), which is characterized by hypopigmentation (1). In addition, the absence of melanin in certain types of cells, such as the retinal pigment epithelium (RPE), which occurs in OCA1 individuals, triggers diverse abnormalities in the development of the visual system. At maturity there is a marked reduction in rod photoreceptor numbers, an under-development of central retinal regions and an abnormal pattern of chiasmatic connections between the eye and the brain [reviewed in (2)]. All these abnormalities are corrected in transgenic animals carrying the entire tyrosinase gene [(3, 4); reviewed in (5)].
To investigate the role of tyrosinase in the development of the mammalian visual system, various animal models have been produced and analysed (6, 7). However, none of these transgenic animals allowed an accurate temporal regulation of tyrosinase gene expression. Therefore, to activate its expression at precise developmental time-points, potentially different from the endogenous program, we established a transgenic mouse model with inducible expression of the tyrosinase gene. We decided to use the tetracycline binary system (8), with the reverse transactivator protein (rtTA), whose binding to tet-operator sequences present in the inducible promoter is triggered by the antibiotic or related molecules (i.e. doxycycline), an strategy known as TET-ON (9).
Tight spatial and temporal gene expression with the tetracycline system has been achieved in cells and transgenic mice in a wide range of biological, biomedical and biotechnological areas (10, 11), including pigment cell research (12) and neurosciences (13–15).
In spite of its known limitations (16–20), the tetracycline system represents a valid strategy that can be applied to study the effects of expressing a gene of interest in ectopic places and/or at specific temporal points. Active research in the field of inducible gene expression will eventually establish a reproducible protocol. In the meantime, researchers need to apply available techniques, at a given time, and explore and document its use, success and troubles, to improve the design of future experiments.
Materials and methods
Description of Plasmids
For the generation of plasmid pTRETYR (∼6.3 kb), a XhoI–EcoRI 0.45 kb DNA fragment from plasmid pTRE (Clontech, Palo Alto, CA, USA), carrying the minimal doxycycline-inducible cytomegalovirus (CMV) promoter PhCMV*−1 (8) was fused, with a EcoRI–EcoRI 2 kb DNA fragment from plasmid pmcTyr1 (21), carrying a full-length cDNA for the mouse Tyr gene, and cloned in plasmid pHD2WOP (22), which provided the splice site and polyadenylation signal from SV40 small-t antigen. Plasmid pTRETyBS (∼6.3 kb) was generated by fusing PhCMV*−1 from pTRE (Clontech), as above, to a blunt-ended HindIII–EcoRI 2 kb DNA fragment from plasmid pTyBS (23), carrying a full-length cDNA for the mouse Tyr gene and 71 bp from its promoter and 5′ untranslated region (UTR), and cloned in plasmid pHD2WOP. Plasmid pTRETyBSnew (∼6.3 kb) is essentially identical to pTRETyBS with the exception of a 5′HindIII–Afl III 71 bp fragment, which was removed from the Tyr cDNA piece. Plasmid pHSTyrTET (∼14 kb) was obtained by fusing the EcoRI–EcoRI 3.7 kb DNA fragment, carrying the LCR from the mouse Tyr gene [plasmid pTyr14:E6 (24)], a SalI–XhoI 6.1 kb DNA fragment with the promoter and 5′ upstream regulatory sequences from the mouse Tyr gene [plasmid pTYRCAT(–6.1) (25)] [combined in plasmid pHS6.1, Lluis Montoliu, unpublished, used in (26)], and a blunt-ended EcoRI–BamHI 1 kb DNA fragment containing the coding region for the reverse transactivating chimeric protein (rtTA) from plasmid pTet-On (Clontech), in plasmid pHD2WOP. Plasmid DNAs were purified, using Qiagen Plasmid Maxi Kit (Qiagen, Hilden, Germany). All plasmids were confirmed by restriction enzyme and sequence analyses. Detailed description of plasmids is available upon request.
Generation and Analysis of Transgenic Mice
Plasmids pTRETyr, pTRETyBS, pTRETyBSnew and pHSTyrTET were linearized with restriction enzyme EagI and their corresponding 3.2, 3.2, 3.2 and 11.5 kb TRETyr, TRETyBS, TRETyBSnew, HSTyrTET inserts, respectively, excised and isolated from vector sequences (Fig. 1). Transgenic mice were generated by standard procedures using the albino inbred mouse strain FVB/N (Harlan Iberica, Barcelona, Spain) (6). We identified seven founder mice for TRETyr, four for TRETyBS, three for TRETyBSnew and 13 for HSTyrTET transgenes. Out of them, six, three, three and four lines transmitted the transgene through the germ-line, respectively. Transgenic mouse colonies were bred and expanded in albino outbred NMRI (Harlan) mice, to avoid the retinal degeneration mutation (Pdebrd1), carried by the FVB/N genotype (27). Transgenic founder mice were identified by PCR, and confirmed by Southern blot analyses, as described (6). PCR analyses used oligonucleotides-pairs TT3 [5′GACTACAGCTACCTCCAAGAGTCAGATCC-3′]/TT4 [5′GTTTGTCCAATTATGTCACACCACAGAAG-3′] (for TRETyr, TRETyBS and TRETyBSnew transgenes) and Tyr-Seq5′ [5′-CTGGGCGAGTTTACGGGTTG-3′]/rtTA [5′-ACCCGGGTCGAGTAGGCG-3′] (for HSTyrTET transgenes), resulting in 558 and 350 bp PCR products, respectively. Conditions for PCR were: 2 min at 94°C, followed by 35 cycles: 30 s at 94°C, 30 s at 65°C and 2 min at 72°C, and, finally, 10 min at 72°C. Transgenic lines were established and their copy-number (ranging from 1–∼250 copies) evaluated in F1 heterozygous individuals by slot-blot analysis (28). No correlation was found between transgene copy-number and expression levels. Irises and whole-mounted retinae were prepared as previously described (7).
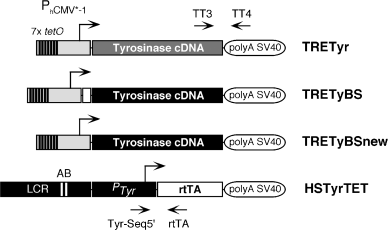
Transgenic constructs used in this work. Schematic view of TRETyr, TRETyBS, TRETyBSnew and HSTyrTET transgenes. A bent arrow indicates the transcription start site and direction of transcription for each gene. PhCMV*−1 corresponds to the minimal promoter from the human cytomegalovirus (CMV) fused with seven copies (shown as seven thin black rectangles) of the binding sequence (tetO) for the tet repressor (8). PTyr corresponds to −6.1 kb 5′ upstream regulatory sequences and promoter from the mouse Tyr gene (25). LCR corresponds to the 3.7 kb EcoRI fragment containing the mouse tyrosinase LCR, located at −15 kb of the gene (3711 bp, EMBL Database X76647). A and B identify the LCR-core, with putative binding sequences for nuclear factors (28). TT3, TT4, Tyr-Seq5′ and rtTA show the relative position of corresponding oligonucleotides. The full-length tyrosinase cDNAs used in this work correspond to pmcTyr1 [(21), shown as a grey box in TRETyr] and pTyBS [(26), shown as a black box in TRETyBS and TRETyBSnew]. The coding region for the transactivator rtTA protein corresponds to plasmid pTET-ON (Clontech). Not drawn to scale.
For induction experiments, doxycycline (Sigma, St Louis, MO, USA) was administered, ad libitum, dissolved in drinking water (2 mg/ml) containing 5% sucrose from the start of the experiment (1 week after the vaginal plug or around the perinatal period) until the day of processing the animals (between 6 and 8 weeks after birth). All experiments complied with local and European legislation concerning vivisection and the experimentation and use of genetically modified organisms.
Quantification of Melanin and Tyrosinase Enzymatic Activity
Melanin content in whole eye extracts was measured by spectrophotometry as described previously (29). Tyrosinase enzymatic activities were recorded following described assays (30, 31). For statistical analyses, where indicated, t-Student paired tests were applied (StatView and SPSS).
Anterograde Labelling of Chiasmatic Axonal Projections from Retinal Ganglion Cells
Axonal pathway tracing was done as reported (32). In brief, 18.5 days post coitum (dpc) mouse embryos were removed from mothers and sacrificed by decapitation. Their eyelids opened and the heads placed in 4% paraformaldehyde in phosphate buffer saline (PBS) at 4°C for 14–16 h. In each case, the left eye was then opened, the lens removed and small crystals of 1,1′,di-octadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI) (Molecular Probes, Eugene, OR, USA) placed in the optic nerve head and the eye cup filled with 1% agarose in PBS. These were then left for 10 d at 37°C in 0.5% paraformaldehyde in PBS.
Results
To develop a tyrosinase inducible gene-expression system in transgenic mice a series of constructs were prepared (Fig. 1), according to the reported TET-ON general scheme (9). Transgenic mice were generated and analysed with all constructs. All TRETyr transgenic mice were phenotypically albino. To test the induction of tyrosinase gene expression in TRETyr transgenic mice, experimental crosses were established with a reporter transgenic line carrying an ubiquitous TET-OFF [tTA (8)] transactivator protein (M3, J.J. Lucas and J. Avila, unpublished data). None of the resulting F1 double transgenic mice displayed any sign of pigmentation. A number of mutations were found in the tyrosinase cDNA sequence present in pmcTyr1/pTRETyr plasmids, resulting in amino acid substitutions in the deduced protein sequence: W236C, C244F, V346G, S361I, D444Y and S450I. Most of these mutations were independently sufficient to inactivate tyrosinase enzymatic function and/or its cytoplasmic processing [F. Solano, personal communication (33); http://www.cbc.umn.edu/tad].
We decided to use the mouse tyrosinase full-length cDNA present in pTyBS plasmid (23) as an alternative source for a functional tyrosinase protein. Two new constructs were prepared, TRETyBS and TRETyBSnew, with different 5′ UTR (Fig. 1), and used for the generation of transgenic mice. All TRETyBSnew transgenic mice were albino. None of the inducible protocols and experimental matings produced any trace of pigment and therefore these mice were discarded. Finally, TRETyBS transgenic mouse lines showed traces of basal pigmentation, at variable levels, in the retina, around the optic nerve head, and were selected for subsequent analyses.
HSTyrTET transgenic mice were selected according to the analysis of experimental crosses with the reporter L7 transgenic line (9) (not shown). Transgenic TRETyBS mouse lines were crossed with the selected transgenic HSTyrTET mouse line and F1 double transgenic were identified for induction experiments. Tyrosinase gene expression activation was measured as an increase in eye or retinal pigmentation (observed in whole-mount retinae). Pigmentation levels did not change in all but one induced combination, where a limited increase in eye-pigmentation (eyes displayed a ruby-like colour), but not in skin, was observed. These double-transgenic mice were therefore used in subsequent experiments and analysed both in hemizygousity and homozygousity (Fig. 2I).

Whole-mounted retinae and irises from adult homozygous double-transgenic TRETyBS/HSTyrTET mice. (I) Starting from left, an albino outbred NMRI mouse, two doxycycline-induced homozygous double-transgenic TRETyBS/HSTyrTET individuals and two uninduced double-transgenic controls. Darker red eye colour is visible in induced mice. (II) Whole-mounted retinae and irises from uninduced (A–L) and induced (M–U) adult homozygous double-transgenic TRETyBS/HSTyrTET individuals. Each row represents a single mouse. There are three control and three induced mice shown. Within each row, from left to right: peripheral retina (A, E, I, M, Q, U), central retina (B, F, J, N, R, V), low-magnification of a whole-mounted retina (C, G, K, O, S, X) and irises (D, H, L, P, T, Y). Scale bars for peripheral and central retinal regions = 30 μm. Scale bars for whole-mounted retinae = 100 μm. Scale bars for irises = 500 μm. (III) Whole-mounted retinae and irises from representative albino NMRI (A, B) and YRT2-transgenic [wild-type pigmented (6)] individuals (C, D). Scale bars as above. The fully pigmented RPE of a YRT2-transgenic mouse, without the attached pigmented choroid, has been reported before (7).
Variability in eye colour was also observed and confirmed, as different retinal pigmentation levels, in whole-mount retinae and irises preparations (Fig. 2II). Occasionally, some uninduced double-transgenic homozygous individuals showed higher retinal pigmentation levels than TRETyBS parental transgenic line (Fig. 2II E–G). However, in contrast, most of induced double-transgenic homozygous mice displayed higher pigmentation levels at the RPE (Fig. 2II M–O, Q–S). Remarkably, the pigmentation levels found in irises were rather low and uniform (Fig. 2II D, H, L, P, T, Y). In all cases, retinal and iris pigmentation levels observed in experimental individuals were above those characteristic of albino NMRI mice, totally devoid of pigment (Fig. 2III A, B), and below YRT2-transgenic (5, 6) fully-pigmented (wild-type) animals (Fig. 2III C, D).
To measure the observed retinal pigmentation, a number of tyrosinase enzymatic tests and melanin recordings were carried out. First, hemizygous double-transgenic TRETyBS/HSTyrTET individuals were induced from 1 week after mating, or from the perinatal period (1–2 d before delivery), and their results compared with uninduced hemyzygous double-transgenic controls. In spite of the high degree of variation observed in these mice, tyrosinase melanogenic activity was significantly increased in induced mice from the first gestational week, as compared with uninduced parental TRETyBS and double-transgenic control mice (Fig. 3A). In contrast, in hemizygous double-transgenic mice induced perinatally, even higher variability was observed and most animals did not increase tyrosinase melanogenic activity, as compared with uninduced individuals (Fig. 3A).
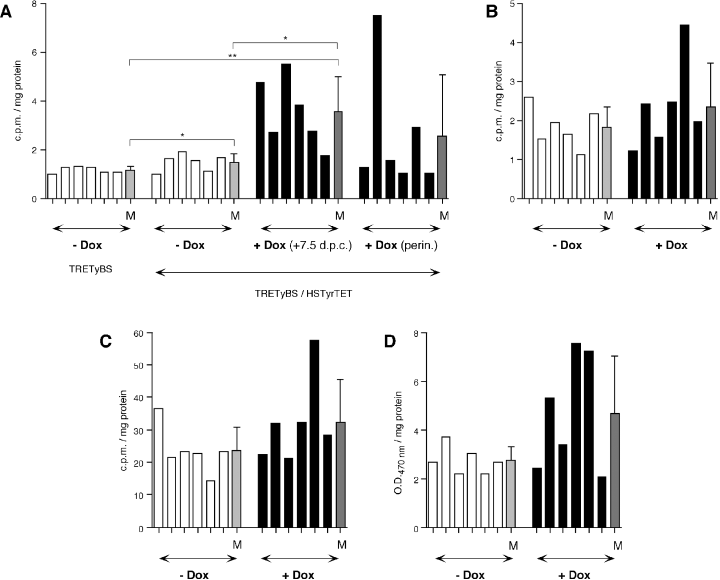
Measurement of tyrosinase enzymatic activity and melanin contents in whole-eye extract from adult hemizygous and homozygous double-transgenic TRETyBS/HSTyrTET mice (A) Measure of tyrosinase melanogenic activity in parental TRETyBS hemizygous transgenic mice, hemizygous double-transgenic TRETyBS/HSTyrTET individuals, uninduced (−Dox, white bars) or induced (solid bars) from the first gestational week (7.5 dpc) or perinatally (perin.). Each bar represents a mouse (n = 6). At the end of each group the mean value (±SD) is represented as light-grey (−Dox) or dark-grey (+Dox) bars. Statistically significant differences are indicated as follows: *P < 0.1; **P < 0.01. (B) Measure of tyrosinase melanogenic activity in homozygous double-transgenic TRETyBS/HSTyrTET individuals (n = 6). Bar colours as above. Observed differences are not statistically significant (P = 0.4664). (C) Measure of tyrosinase hydroxylase activity in homozygous double-transgenic TRETyBS/HSTyrTET individuals (n = 6). Bar colours as above. Observed differences are not statistically significant (P = 0.3181). (D) Colorimetric measurement of ocular melanin contents in homozygous double-transgenic TRETyBS/HSTyrTET individuals (n = 6). Bar colours as above. Observed differences are just not statistically significant (P = 0.1051).
Second, to assess tyrosinase gene expression in homozygous double-transgenic mice similar experiments were carried out. In spite of the intense degree of variability observed in these enzymatic measurements (that prevented from establishing statistical significance), increased values of tyrosinase melanogenic activity (Fig. 3B), tyrosinase hydroxylase activity (Fig. 3C) and overall eye melanin content (Fig. 3D) were documented in some induced individuals (Fig. 3B–D). The measurable eye melanin content of TRETyBS parental mice (background levels) was ∼2.3 times higher than recorded values for albino NMRI mice and ∼4 times lower than in YRT2-transgenic wild-type pigmented (5, 6) animals. Light absorbance (OD470 nm) values in eye-extracts from albino mice, devoid of melanin, represents less than 10% of the recorded absorbance value for YRT2-transgenic wild-type pigmented animals. Correspondingly, tyrosinase enzymatic measurements of experimental individuals were above those recorded for albino mice, and below (20–40%) those obtained for wild-type pigmented animals (not shown).
To investigate the potential rescue of visual abnormalities associated with albinism in these transgenic animals we first assessed the number of photoreceptors (4, 34) in hemizygous double-transgenic mice induced from the first gestational week. Significant differences in photoreceptor numbers were not found between experimental and control groups, although the former did occasionally have slightly higher photoreceptor numbers.
Second, we investigated the optic tract in homozygous double-transgenic mice induced from the first gestational week, compared with their corresponding uninduced control animals. In this experiment, we decided to use only homozygous instead of hemizygous double-transgenic mice, as we reasoned that low tyrosinase expression levels and variability observed in hemizygous for photoreceptor counts could also prevent from detecting significant differences in chiasmatic pathways. Albino mammals show a deficit of ipsilateral-projecting (uncrossed) axons from retinal ganglion cells in favour of contralateral-projecting (crossed) fibres which was corrected in tyrosinase-transgenic mice (3). By using anterograde labelling experiments we could visualize ipsilateral-labelled nerve fibres in seven of 11 (∼63%) induced animals. In contrast, in only one of eight (∼12.5%) uninduced animals uncrossed fibres could be demonstrated (Fig. 4). For comparison, wild-type pigmented YRT2-transgenic mice (3, 6) and albino NMRI mice displayed ipsilateral labelled axons in 100 and 15% analysed chiasms, respectively (representative individuals are shown in Fig. 4I, J). These results suggested that early induction of tyrosinase could rescue the chiasmatic abnormality, which is characteristic of albino individuals, in most induced mice.
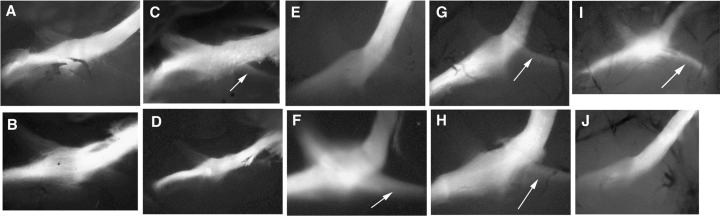
Whole mounted optic chiasms from adult homozygous double-transgenic TRETyBS/HSTyrTET mice. DiI crystals have been placed unilaterally in the left eye of fixed heads of 18.5 dpc mouse foetuses from control uninduced (A–D) and induced (E–H) homozygous double-transgenic TRETyBS/HSTyrTET individuals, and incubated for 10 d. Four representative chiasms are shown from each group along with representative chiasms from YRT2-transgenic [wild-type pigmented (5, 6)] (I) and albino NMRI (J) individuals. The presence of ipsilateral axonal fibres (indicated with an arrow) is obvious in C, F, G, H and I mice. The magnitude of this projection is markedly decreased in uninduced homozygous double-transgenic animals (i.e. C) or barely detectable (A–E), similar to that of albino control mice (J). Chiasms are observed through the skull basement, after careful removal of the palate.
Discussion
We have generated a transgenic animal model with inducible tyrosinase gene expression using the tetracycline (TET-ON) system. Several combinations of constructs were explored and analysed. The tyrosinase cDNA present in plasmid pmcTyr1 (21)/TRETyr was found to carry several inactivating mutations. The presence of short 5′ UTR [containing the presumptive the start of transcription and an identified E-box (23, 35, 36)] in TRETyBS construct was found critical to reveal any tyrosinase transgene expression in our inducible constructs.
The level of transactivation achieved with the four germ-line transmitting HSTyrTET transgenic mouse lines, firstly assessed in combination with the L7 reporter transgenic mice and secondly established with transgenic mice carrying the TRETyBs construct, was relatively weak. A number of explanations can be provided for this, including: 1) the likely selection occurring in utero for transgenic founders either displaying low levels of expression of the toxic rtTA protein (17), or being mosaic (most, ∼69%, of HSTyrTET lines did not transmit the transgene through the germ-line), 2) the reported difficulty in generating transgenic mouse lines expressing high levels of rtTA (TET-ON), recognized as one of the major limitations of this system, as compared with equivalent mouse lines made with tTA (TET-OFF) (37), 3) the fact that the coding region for the rtTA protein derives, in part, from a prokaryotic genome, shown to impair adequate transcription in mammalian genomes (38). In this regard, the artificial combination of tyrosinase regulatory elements, including the LCR and the promoter of the mouse tyrosinase gene, shown to work in other experimental designs at different levels, not necessarily reproducing the endogenous pattern for the tyrosinase gene (39), could have compromised the actual expression levels of the HSTyrTET construct, therefore only those lines with limited and more restricted (only RPE cells, but not neural crest-derived melanocytes) rtTA gene expression have eventually been detected and established. Variegation phenomena were commonly observed in pigmented RPE cells of induced and uninduced animals, suggesting a suboptimal transcription of the construct, that is characteristic of standard-type transgenic animals (5, 40).
Basal uninduced tyrosinase expression was also observed (2, 3) in TRETyBS transgenic mice (mainly confined at central regions of the retina) indicating background residual activity, as reported in similar experiments (16–20, 37). To compensate for the deficits associated with standard TET-ON inducible schemes, observed here, new chimeric transactivation proteins have been produced either containing just an optimization of the codon usage (‘mammalianized’, 16, 20), or a combination of codon optimization and new mutations proposed to reduce the basal activity of rtTA while increasing its inducibility (41), whose activity is being explored in vitro and in vivo (41, 42).
Relatively high variability was also observed among individuals on induction of tyrosinase gene expression (2-4), a situation also reported in similar experiments (11, 13–15). A number of aspects could contribute to explain this variability. First, it is known that functionally active local concentrations of doxycycline cannot be readily obtained in tissues such as brain, or associated tissues (13–15, 37). Thus, it is not predictable to reach equivalent steady-state levels of the drug in all animals at the same time in a given organ [i.e. we exposed pregnant females from 7.5 dpc, to ensure tyrosinase gene activation by 10.5 dpc, its normal stage (7)]. Second, the drug is included in the drinking water, administered ad libitum to mice, therefore its actual consumption per animal cannot be assumed to be equivalent. We assessed the use of doxycycline included in food pellets and did not observe differences regarding inducibility and/or variability in the response achieved (unpublished results). Third, this is a binary experimental model, initially made on an inbred mouse strain FVB/N but subsequently mobilised to an outbred mouse strain (NMRI), to avoid the presence of the retinal degeneration phenotype (27), found in the former strain. Therefore, experiments are made with double transgenic, either hemizygous or homozygous, on a mixed outbred genetic background, in which the segregation of modifier genes occurs (43).
In spite of all problems we could establish a transgenic animal with inducible tyrosinase gene expression with the TET-ON system. Most induced animals developed higher pigmentation levels in their eyes (Fig. 2). Histological examination of their retinae reflected the common variability observed in this experiment. Nonetheless, most induced animals displayed higher pigmentation levels in their RPE cells (Fig. 2). However, corresponding changes in pigmentation in irises, colonised by neural crest-derived melanocytes, were not observed, in agreement with an overall lack of pigmentation in skin and hair melanocytes.
Generally, induced animals tended to display higher tyrosinase enzymatic activities in whole-eye protein extracts. However, variability observed usually prevented from detecting significant differences in the analyses, with the exception of the tyrosine hydroxylase activity test run with double transgenic hemizygous animals (Fig. 3A). Here, there was a clear result regarding the timing required for induction. The comparison of induction experiments initiated at 7.5 dpc with those started around the perinatal period (when most rods are being produced) revealed more consistent induced profiles achieved with the former, whereas perinatally-induced animals failed to reproducibly trigger tyrosinase gene expression.
We assessed the functional rescue of visual abnormalities associated to albinism (2). First, we counted the number of photoreceptors at central retinal locations and did not observe significant differences between induced and uninduced experimental groups. However, this could only be analysed in hemizygous animals and differences in photoreceptor counts between albino and pigmented wild-type animals are known to be, at most, in the range of 20–30% (4). Therefore, combining the variability and the limited tyrosinase gene expression achieved in induced animals this result was not unexpected. In addition, although it appears to exist a relationship between ocular pigmentation and photoreceptor rod counts, there is a certain amount of pigmentation required to result in significant increase in rod numbers (34).
Second, we assessed the correction of the uncrossed chiasmatic projections, generally decreased in albino mammals, in favour of the corresponding crossed chiasmatic projections (3). Anterograde labelling experiments, demonstrated that most of the induced double transgenic mice displayed ipsilateral projections, in comparison with their corresponding uninduced control littermates, which remained with the characteristic albino phenotype and only displayed the uncrossed pathway in a minority of the animals analysed (Fig. 4). These results demonstrated, with our inducible transgenic mouse model, the effectiveness of triggering tyrosinase gene expression from the first gestational week onwards, resulting in a partial rescue of the chiasmatic albino abnormality. Furthermore, our results are in good agreement with previous studies demonstrating that low levels of RPE pigmentation were sufficient to allow the establishment of the uncrossed chiasmatic pathway (44).
Other strategies have been recently reported to regulate tyrosinase gene expression in vivo, in inducible experiments. The prokaryotic lac operator-repressor system was efficiently transferred to mice and shown to regulate tyrosinase gene expression, and hence pigmentation, by the lactose analogue isopropyl beta-d-thiogalactopyranoside (IPTG) (45). These transgenic mice were subsequently used to analyse the establishment of correct chiasmatic pathways. Upon early induction of tyrosinase gene expression production of normal uncrossed fibres was observed, whereas later induction periods did not correspond with similar chiasmatic correction levels (46). Those results are in agreement with data reported in this work, indicating that triggering tyrosinase gene expression from early embryo development allows the correction of the uncrossed chiasmatic pathway found in albino individuals.
In conclusion, the transgenic animal model reported here allowed the regulated induction of tyrosinase gene expression, resulting in the correction of albino visual abnormalities following an early embryonic activation of the gene. These mice could be instrumental in establishing the role of tyrosinase in normal development of mammalian visual system.
Acknowledgments
Acknowledgements– This work was supported by funds from the Spanish Ministry of Science and Technology Bio97-0628, Bio2000-1653 and Laboratorios Dr Esteve S.A. to LM, and from The Wellcome Trust and The British Council to GJ. The authors are grateful to H. Bujard and A. Servadio for L7 transgenic mice; J.J. Lucas and J. Avila for M3 transgenic mice; P. Overbeek for pTyBS plasmid; F. Beermann, G. Schütz, L. Chin, A. Servadio, L. Enjuanes, F. Solano and J.C. García-Borrón for useful comments, and S. Montalbán and M. Cantero for technical assistance.




