Expression of COUP-TF-interacting protein 2 (CTIP2) in human atopic dermatitis and allergic contact dermatitis skin
Abstract
Abstract: Chicken ovalbumin upstream promoter-transcription factor-interacting protein 2 (CTIP2) is a transcriptional regulator that is highly expressed in skin during mouse development, as well as in proliferating cells of adult mouse skin. We investigated expression of CTIP2 along with proliferation marker Ki-67 in normal human skin, and in skin from atopic dermatitis (AD), and in allergic contact dermatitis (ACD) samples by immunohistochemistry (IHC). We discovered for the first time that CTIP2 was expressed in proliferating basal and suprabasal layer in normal human epidermis. CTIP2 expression was dramatically increased in the epidermis from the AD and ACD samples compared with normal samples, and was labelled in both proliferating basal and suprabasal layers. Altogether our results suggest that CTIP2 expression could be linked to disease progression and/or maintenance in AD and ACD patients.
Background
Atopic dermatitis (AD) is an eczematous and highly pruritic, chronic inflammatory skin disease (1–5). A recent, population-based survey in the US recorded a prevalence of 10.7% for non-specific eczema disease and 6% for AD (6). Allergic contact dermatitis (ACD) is caused by cell-mediated hypersensitivity to chemical haptens and is relatively well understood. Although considerable research effort has been directed at AD in the past, this complex disease has challenged a clear understanding of pathogenesis (1,7). A number of factors, such as interactions between susceptibility genes, host environment, altered epidermal proliferation and/or differentiation, skin barrier defects, susceptibility to infection and immunological factors, contribute to the aetiology of AD (1,5,8–12).
Chicken ovalbumin upstream promoter-transcription factor (COUP-TF)-interacting protein 2 (CTIP2), also known as Bcl11b, is a C2H2 zinc finger protein that has been shown to regulate transcription through interaction with COUP-TF nuclear receptor proteins as well as through direct, sequence-specific DNA binding (13,14). p57KIP2, a gene encoding a cyclin-dependent kinase inhibitor, has been identified as a direct transcriptional target of CTIP2 in neuroblastoma cells (15). CTIP2 is expressed early during mouse development as well as in adult animals, and in both cases, expression is most predominant in the skin/epithelial structures, as well as in the CNS and thymus (16,17). CTIP2 expression was detected in the outermost ectodermal cell layer of a developing mouse foetus as early as embryonic day 10.5 (E10.5). In adult mouse skin, CTIP2 expression was found in cells of the basal layer, hair follicles, and in some cells of the suprabasal layers (16). In CTIP2-null mice, the epidermal proliferation/differentiation program was altered and epidermal permeability barrier formation was impaired, leading to thinning of the epidermis (18). Thus, CTIP2 appears to be a critical regulator of skin development in mice. To the best of our knowledge, there has been no study that describes the expression patterns of this important protein in human skin.
Question addressed
What is the expression pattern of CTIP2 in normal human epidermis (NHE) and in skin of hyperproliferative diseases, such as AD and ACD?
Experimental design
Patient samples
Paraffin sections of skin biopsies from 12 AD and 4 ACD samples (all from different patients) were obtained from Oregon Health & Science University, Department of Dermatology, Portland, Oregon. The biopsy specimens were from banked tissue, all taken from lesions of clearly diagnosed, AD adult patients (all of whom also had clinical allergic respiratory disease) or from 48 hrs., contact allergy test sites. IgE was not routinely used for diagnosis of AD, and hence the levels were not available in these patients. The ACD reactions were generated by topical treatment with 2, 4, dinitrochlorobenzene. Matched-skin biopsies from five healthy adults were used as controls. All sample acquisition protocols were approved by the Institutional Review Board at OHSU.
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded sections were used. IHC was performed as described elsewhere (16,19) [see Appendix for detailed protocol].
Results
To characterize CTIP2 expression patterns, we performed IHC staining on histological sections of normal, healthy human skin and on skin biopsies of AD and ACD patients. We observed nuclear staining for CTIP2 mainly in the cells of basal layer and some cells of suprabasal layer of the NHE (Fig. 1a). CTIP2 was expressed in both basal and suprabasal layers of AD and ACD skin in three independent IHC experiments. No staining was observed in sections stained without primary antibody or with mouse IgG alone (data not shown). Interestingly, CTIP2 expression was dramatically increased in the hyperplastic epidermis of eczematous samples (Fig. 1a). Expression was also detected in infiltrating lymphocytes in the dermis as CTIP2 is known to be expressed by T cells as well (Fig. 1a, AD and ACD, shown by arrowheads). Germline disruption of CTIP2 results in an arrest of T cell development at an immature CD4-CD8- stage(s), with the complete absence of αβ T cells (20). CTIP2-positive cells were found to comprise only 29 ± 1% of normal, healthy epidermis (Fig. 1b). In contrast, in both AD and ACD samples, numerous CTIP2-positive cells were found throughout the epidermis and their numbers were 60 ± 2 and 52 ± 1% respectively, of the total epidermal cell population. Thus, our results show that CTIP2 is expressed in basal layer of the NHE, extending all the way up to the suprabasal layer of hyperplastic epidermis from AD and ACD. The number of CTIP2+ cells in AD and ACD was significantly higher as compared with normal epidermis (P < 0.05), and this correlated well with the hyperplastic nature of diseased skin.
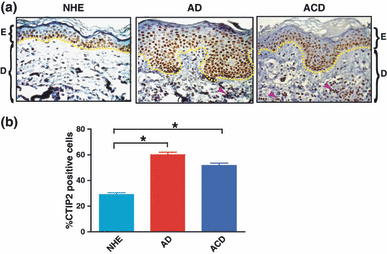
Chicken ovalbumin upstream promoter-transcription factor-interacting protein 2 (CTIP2) expression in human AD and ACD. (a) Analyses of CTIP2 expression by IHC in NHE, AD and ACD skin samples. Brown staining indicates CTIP2-positive cells. Arrowheads indicate dermal CTIP2-positive cells (magnification, 40×). The yellow dotted line defines the epidermal-dermal boundary. E, Epidermis; D, Dermis. (b) The mean frequency of CTIP2-positive cells in NHE, AD and ACD is expressed as percentages. Plotted are mean measurements (± SEM). *P < 0.05.
Hyperproliferative skin generally exhibits a high level of expression of the proliferation marker Ki-67 (21–23). To evaluate the proliferation status of CTIP2-expressing cells, we performed co-labelling studies using our anti-CTIP2 monoclonal antibody and an antibody against the proliferation marker Ki-67. All Ki-67 positive cells co-labelled with CTIP2, confirming that all proliferative cells expressed CTIP2 (Fig. 2). A significantly higher number of double-positive cells were present in both basal and suprabasal cell layers of the AD (26 ± 1%) and ACD (23 ± 1) samples compared to normal (9 ± 1%) skin (Fig. 2b; P < 0.05). Altogether, our results suggest that elevated CTIP2 expression might be linked to increased epidermal proliferation in the skin of AD and/or ACD patients.
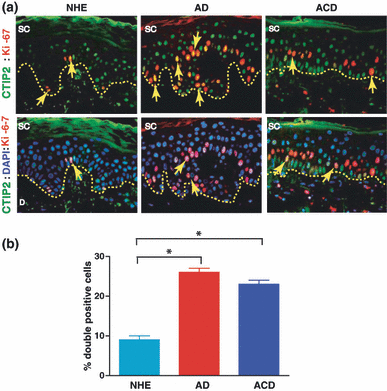
Ki-67 expression in human AD and ACD. (a) Co-labelling of CTIP2 (green), Ki-67 (red) and DAPI (blue) in NHE, AD and ACD samples (original magnification, 40×). Arrows indicate double-positive cells. SC, stratum corneum (b) The mean frequency of Ki-67+CTIP2+ double-positive cells in NHE, AD and ACD is expressed as percentages. The mean measurements (± SEM) are shown. *P < 0.05.
Conclusions
In this study, we have demonstrated by IHC that CTIP2 is expressed in NHE. Enhanced expression of CTIP2 was observed in hyperproliferative skin of AD and ACD patients. Co-labelling experiments confirmed CTIP2 expression in all proliferating cells, as well as in some suprabasal cells of the normal and diseased skin. In summary, our results suggest that CTIP2 may play a role in initiation and/or maintenance of proliferative/differentiation state, and possibly in the barrier defects seen in AD, by regulation of genes such as Id2, GATA-3, Dgat2 and eLox-3 (18).
Barrier dysfunction and impaired keratinocyte proliferation and differentiation are crucially involved in the pathogenesis of AD (10,11). Our studies in a mouse model have confirmed that CTIP2 is crucial for the skin barrier formation and for terminal differentiation of keratinocytes during development (18). It is likely that an optimum level of CTIP2 expression is required for the maintenance of skin homeostasis. An increase in CTIP2 expression might lead to an increase in epidermal proliferation and subsequent altered differentiation in AD and/or ACD skin. Additional studies are required to better understand the role of CTIP2 in AD pathogenesis.
Acknowledgements
We thank Adam Campbell and Sophie Truck for help in cell counting during early phase of the project. These studies were supported by Award Number R01AR056008 from the National Institute of Arthritis And Musculoskeletal And Skin Diseases to AI, OSU startup fund (AI) and a NIEHS Center grant (ES00210) to OSU Environmental Health Sciences Center. We also thank Dr Wayne Kradjan and Dr Gary DeLander of the OSU College of Pharmacy for continuous support and encouragement. [Correction added after online publication 29 March 2009: NIH grant number added.]




