Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche
Abstract
Abstract: Since the discovery of epithelial hair follicle stem cells (eHFSCs) in the bulge of human hair follicles (HFs) an important quest has started: to define useful markers. In the current study, we contribute to this by critically evaluating corresponding published immunoreactivity (IR) patterns, and by attempting to identify markers for the in situ identification of human eHFSCs and their niche. For this, human scalp skin cryosections of at least five different individuals were examined, employing standard immunohistology as well as increased sensitivity methods. Defined reference areas were compared by quantitative immunohistochemistry for the relative intensity of their specific IR.
According to our experience, the most useful positive markers for human bulge cells turned out to be cytokeratin 15, cytokeratin 19 and CD200, but were not exclusive, while β1 integrin and Lhx2 IR were not upregulated by human bulge keratinocytes. Absent IR for CD34, connexin43 and nestin on human bulge cells may be exploited as negative markers. α6 integrin, fibronectin, nidogen, fibrillin-1 and latent transforming growth factor (TGF)-beta-binding protein-1 were expressed throughout the connective tissue sheath of human HFs. On the other hand, tenascin-C was upregulated in the bulge and may thus constitute a component of the bulge stem cell niche of human HFs.
These immunophenotyping results shed further light on the in situ expression patterns of claimed follicular ‘stem cell markers’ and suggest that not a single marker alone but only the use of a limited corresponding panel of positive and negative markers may offer a reasonable and pragmatic compromise for identifying human bulge stem cells in situ.
Introduction
The correct identification of a cell type is essential for accurate phenotype analyses. The previously published results of the immunoreactivity (IR) pattern of human epithelial hair follicle stem cells (heHFSCs) include and confirm that cytokeratin 15 (CK15) IR is upregulated in the human bulge, compared with other regions of the outer root sheath (ORS) and appears to identify HF bulge located clonogenic heHFSCs, which was shown by immunostainings and in microarray analyses, which demonstrated CK15 upregulation in mice and human HF bulge cells (1–4). However, it has also been documented that CK15 IR is not restricted to the human bulge, as initially claimed (3). Our IR results, which were obtained by employing methods that provide enhanced immunodetection sensitivity compared with standard ones, define a set of useful markers for heHFSCs and their local niche – that has become a major ongoing challenge being both biologically and clinically important (5–7). Since the discovery of epithelial hair follicle stem cells (eHFSCs) in the bulge region of mouse hair follicles (HFs) (8) and the identification of equivalent cells in human HFs (3,9), a controversial debate on which markers are best suited to identify heHFSCs and their niche began (10). In the current study, we attempt to contribute to this debate by critically evaluating the selected immunohistological markers that have been reported/proposed as being useful for the identification of heHFSCs in situ and/or the bulge stem cell niche (5–7).
Recently, we have identified a prominent ‘follicular trochanter’-like epithelial structure in the bulge region of the ORS of human scalp anagen VI HFs, which could perhaps serve as morphological marker structure for a bulge epithelial stem cell-rich compartment (6,7,11). The bulge of both mouse and human HFs describes the region of the HF epithelium below the entry of the sebaceous gland duct where the arrector pili muscle (APM) inserts into the upper ORS. This region is now firmly established as at least one major site of eHFSCs (2,3,5,7,8,11,12). While the human HF bulge area clearly contains keratinocyte stem cells, the hair matrix represents a compartment of proliferating and differentiating transient amplifying cells (2,5,7,9). In contrast to mouse and fetal human HFs, the adult human anagen bulge is usually morphologically undistinctive (5) – unless one could specifically screen for the above-mentioned ‘follicular trochanter’ (11).
For this particular study, normal human scalp skin cryosections of at least five different individuals per antigen were examined, employing standard immunohistology and immunofluorescence as well as sensitivity-enhanced methods [EnVision®, DAKO, Glostrup, Denmark; tyramide signal amplification (TSA), Perkin-Elmer, Boston, USA] (13–17). Care was exercised to obtain as many longitudinal scalp HF sections as possible. When accidentally visible, ‘follicular trochanter’ structures were included in the analyses, for which purposely only terminal scalp HFs in the anagen VI stage of HF cycling (18,19) were used.
Stem cell populations exist in specific protective anatomical locations (niches), where they interact dynamically with their environment (12,20–23). The well-recognized fact that laser epilation techniques do not easily stop a HF from producing new hair shafts and routinely have to be applied several times until the HF regeneration potential has finally been exhausted, suggests that the eHFSCs reside in a relatively protected, damage-resistant niche (bulge region) (23). Since epithelial stem cells usually reside on the basement membrane (BM), a high level of expression of certain integrins such as β1 and α6 integrins, which mediate the attachment to the extracellular matrix, is required to maintain their position in the niche (12,21,24,25). Together with even more obscure neural and endocrine inputs, an as yet ill-defined number of secreted factors produced by both the epithelial and the mesenchymal niche components are likely to impact on the niche biology in the bulge region of human HFs (7,20,21,26–29).
From the many potential heHFSCs and/or bulge niche markers previously discussed in the literature (5–7,11,12,30) Table 1, we have selected the antigens as listed in Table 2, for which we had access to appropriate primary antibodies, and for most of which published reports on human HFs were available for comparison. In addition, the transcription factor LIM (limatin) homeobox 2 (Lhx2) was selected as a potential marker, since it has previously been reported to be a regulatory key factor in murine bulge stem cell maintenance, responsible for keeping the stem cells in an undifferentiated state (7,31,32).
| Antigen | Primary antibody | Previously reported IR pattern of human HF in situ | Comments | References |
|---|---|---|---|---|
| Cytokeratin 15 (CK15) | CD8 (Clone C8/144b) | Bulge in anagen, catagen, telogen and early anagen HFsIsolated cells in the suprabulbar ORS as well | Lyle was the first describing CK15+ bulge cellsBulge shown as: proximal part of the isthmus and distal part of the lower follicle | (3) |
| LHK15 | Absent in hair bulb, present in cells of the ORS located above the hair bulb | Waseem has produced the LHK15 clone | (84) | |
| Mouse anti-human CK15 (clone LHK15, Chemicon, Billerica, USA) | FT | (7,11) | ||
| Mouse anti-human CK15 (clone LHK15, Chemicon) | Outermost layer of the ORS in the bulge and in the lower HF | (7,11) | ||
| CK15 (clone C8/144B, DAKO, Glostrup, Denmark) | No co-expression with CK19+ cells in upper ORS and lower ORS in anagen HF | (44) | ||
| No information about antibody | Bulge cells are detected preferentially | Bulge marks the lower end of the permanent follicle | (1,5) | |
| MAb C8/144 | Predominantly detected in the outermost layer of the ORS between the insertion point of the APM and the SG | Bulge shown as: ORS from the region, upper border SG and lower border APM insertion siteUsing laser capture microdissection and microarray analysis | (2) | |
| LHK15 (Chemicon, Temecula, CA, USA) | Variable staining of the infundibulumORS just below the entrance of the SG involving the bulge regionNo staining of the hair bulb | Laser-mediated hair removalCD34 IR of suprabulbar ORS does not colocalize with CK15 | (45) | |
| LHK15 and C8/144B | Outermost layer of the ORS in the bulge in anagen, catagen and telogen HFsA few positive cells were also detected in the ORS just above the bulb | At the level of the isthmus, between the SG duct and down to the insertion of the APM (bulge zone)Does not overlap with CD34LHK15 and C8/144B show the same IR pattern | (85) | |
| C8/144B (DAKO, Copenhagen, Denmark) | Basal cells of the ORS of the HF bulge show cytoplasmic labelling | (86) | ||
| C8/144B (DAKO) | Basal cells of the ORS show cytoplasmic labelling, restricted to the bulge | (87) | ||
| LHK15 Neomarkers (Freemont, CA, USA) | Anagen HF: 1, outermost layer of the ORS of the isthmus in the upper segment; 2, ORS above the hair bulbCatagen HF: upper part of the epithelial strand and the germ capsuleTelogen HF: outermost layer of the ORS and weak IR in the secondary hair germ and matrix | Used normal human scalp skinIsthmus is including the bulge area | (88) | |
| C8/144B (DAKO) | In anagen, catagen and telogen HFs CK15 highlights the bulge area | A few Oct4+ cells are present within the CK15-highlighted areas | (89) | |
| LHK15 (Novocastra, Newcastle, UK)C8/144B (DAKO, Kyoto, Japan) | IR in the basal cells of the bulge in vellus HF and terminal HF during all hair cycle stages | Both antibodies hat no basic difference in IR patterns | (90) | |
| CK15 (clone LHK15 Novocastra) | Outermost layer of the upper and middle portions of the ORS and in a group of the cells in the bulge area | (91) | ||
| LHK15 (84) | CK15 is only expressed from the mid-region of the isthmus (middle of the upper ORS) to the lower ORS of the hair follicle bulbCK15 was not expressed in either the upper ORS or the lower follicle bulbSecondary hair germ of telogen HF is positive for CK15 | Region of attachment of the APM is defined as bulge | (56) | |
| Cytokeratin 19 (CK19) | Mouse anti-human CK19 (Ks 19.1, IBL, Cambridge) | Bulge cells in anagen and telogen HFs | Bulge: below the SG, the deepest region of the permanent portionUsed hairy and glabrous skinCK19 cells are also α3β1-bright cells | (48) |
| Mouse anti-human CK19, LPK2 | Cells in the bulge and the outermost layer of the ORS along the whole HF | Used lanugo HFs | (58) | |
| Mouse anti-CK19 (clone 19.1, Progen, Heidelberg, Germany) | FT | (7,11) | ||
| CK19 (Am. Res. Products, clone Ks19.1) | Throughout the ORS; bulge and bulb areas | Bulge shown as: proximal part of the isthmus and distal part of the lower follicle | (3) | |
| CK19 (clone Ks19.1, Progen, Heidelberg, Germany) | Cryosections: Upper ORS and lower ORS in anagen HFsCatagen HFs: epithelial column and in the ORS at the club hair level and aboveEarly anagen HFs: around the club hair of the previous HF and in the lower part of the growing epitheliumTelogen HFs: throughout the telogen capsule | Stained whole HFs (dispase/microdissected) | (44) | |
| Anti-human CK19 | ORS in the bulge | Bulge is a protrusionUsed human scalp skin from the nape of the neck | (54) | |
| Mouse anti-human CK19 (Santa Cruz, California, USA) | IR restricted to the bulge ORS | (55) | ||
| CK19 (clone Ks.19.1, Sigma, St Louis, MO, USA) | ORS: 1, below the entrance of the SG; 2, lower portion of the HF | Laser-mediated hair removalCK15 and CD34 colocalize with CK19 | (45) | |
| Mab Ks19.1 | IR in the bulge and in the infundibulum | (27,49) | ||
| Mouse monoclonal antibody anti-cytokeratin 19 cod. NCLCK19, Novocastra | Positive cells in the ORS | CK19+ and TUNEL+ cells should demonstrate stem cell apoptosis in HIV patient with diffuse alopeciaHorizontal cut at the bulge level (the proximal APM insertion indicates the bulge level) | (92) | |
| LHP2K against CK19 | Bulge and lower ORS of the HFSometimes IR was continuous in lower and upper ORSSecondary hair germ of telogen HF | (56) | ||
| Mouse anti-CK19 (Sigma) | CK19-expressing bulge region | (53) | ||
| CK19 (clones RCK108 and LP2K) | Two distinct areas from skin biopsies are positive (upper ORS and lower ORS)Most peripheral layer of the ORS is positive | (57) | ||
| CK19 (clone RCK108, DAKO, Glostrup, Denmark) | Basal layer between the lower part of the isthmus and the upper transient portions of the ORS in early anagen phase and the telogen bulbOutermost layer of the middle to lower ORS in some HFs | (91) | ||
| mAb CK19 (clone RPN1165) | Constant expression in the ORS and bulge region of vellus HFsShowed variable expression in basal cell carcinoma ORS | (59) | ||
| CK19 (Santa Cruz Biotechnology, CA, USA) | Anagen HF: 1. outermost layer of the ORS of the isthmus in the upper segment, 2. ORS above the hair bulbCatagen HF: upper part of the epithelial strand and the germ capsuleTelogen HF: outermost layer of the ORS and weak IR in the secondary hair germ and matrix | Used normal human scalp skin | (88) | |
| CD200 | CD200 (Serotec, clone MRC OX104) | Expressed in the bulge ORS and in the companion layer of anagen HFs | Bulge: ORS from the region, upper border SG and lower border APM insertion site (per given definition: bulge = isthmus)Used anti-desmin (D33) to show the APMUsing laser capture microdissection and microarray analysis | (2) |
| Mouse anti-human CD200 (clone MRC OX104, Serotec) | FT | (7,11) | ||
| CD200 is identified on human bulge cells using laser capture microdissection and microarray analysis by Ohyama et al. (2) | Bulge marks the lower end of the permanent follicle (bulge is: proximal part of the isthmus and distal part of the lower follicle) | (1,5) | ||
| Tenascin-C | Anti-human tenascin-C monoclonal antibody (RCB 1) | Intense perifollicular staining in the lower part of the HF and hair the hair bulb | (64) | |
| Monoclonal anti-human tenascin-C antibody (clone T2H5, Progen BiotechnikGmbH, Heidelberg, Germany) | Perifollicular mesenchyme around the hair peg especially in the upper part of the hair pegDP cells in the lanugo HF | Skin samples from human fetal developing skin | (93) | |
| Mouse anti-tenascin-C (clone 143DB7, Biohit, Helsinki, Finland) | Fibrous sheaths of HFs in some specimens | Punch biopsies taken | (65) | |
| Mouse anti-human tenascin-C (clone DB7, Biomol) | FT | (7,11) | ||
| Antiserum against tenascin-C purified from rat embryo fibroblasts cultures raised in rabbits | Perifollicular CTS, connective tissue papilla, DP, APM | Normal human scalp used | (66) | |
| Antiserum against tenascin-C purified from rat embryo fibroblasts cultures raised in rabbits | Continuous and intense IR around HFs | Used normal human skin | (67) | |
| Clone F9A5 (W. Carter, Fred Hutchinson Cancer Research Center, Seatlle, WA, USA) | Tenascin-C IR remained prominent in the BM zone and extracellular matrix of the HF sheath during subsequent morphogenetic stages | HF morphogenesis in fetal human skinTwo forms of tenascin were revealed by Western blots | (94) | |
| α6 integrin | α6 integrin (MoAb 1972, Chemicon) | Basal pole of the matrix cells surrounding the DP, vitreous membrane above the Auber’s lineBasal pole of the basal lower ORS cells | Unfixed cryosections | (68) |
| Mouse anti-human α6 integrin (clone 4F10, Chemicon) | FT | (7,11) | ||
| α6 integrin (gift from Dr Sonnenberg) | Strong IR in the upper part of the BM zone outside the HF, intensity became weaker towards the lower part | (70) | ||
| GoH3 (rat) from Dr Sonnenberg (the Netherlands) | BM zone: upper and middle part of the HF, not detectable around the hair bulb, weak around DP | (69) | ||
| 4F10 against α6 integrin (95) | Bulge and lower ORS of the HFSometimes IR was continuous in lower and upper ORSSecondary hair germ of telogen HFs | (56) | ||
| β1 integrin | Mouse anti-human β1 integrin, Clone DE9 Upstate Biotech | Entire hair germ and outer cells of the hair pegOutermost layer of the ORS and mid-portion of the lanugo HF | Human fetus skin, lanugo HFs | (58) |
| FITC-conj. anti-β1 integrin (DAKO) | Basal layer of the ORS in the upper portion of the anagen HF | Double exposure of β1 integrin and C8/144BBulge shown as: proximal part of the isthmus + distal part of the lower follicle | (3) | |
| Anti-human β1 integrin | ORS in the bulge | Bulge is a protrusionUsed human scalp skin from the nape of the neck | (54) | |
| P5D2 against β1 integrin (96) | Expressed throughout the basal layer of the ORS of the whole HF, not restricted to the bulge | Eccrine sweat glands were as well positive | (56) | |
| Fibronectin | Anti-human plasma fibronectin (Sigma) | APM | (97) | |
| Human plasma fibronectin raised in rabbits | Especially abundant in BM | Studied different human tissues (skin, spleen, lymph node, artery, lung, muscle, liver, etc.) | (98) | |
| Anti- plasma fibronectin (clone F7387, Sigma) | Perifollicular CTS, DP, APM | Normal human scalp | (66) | |
| Nidogen | Rabbit anti-human Nidogen (Calbiochem, San Diego, CA, USA) | FT | (7,11) | |
| LTBP-1 | Rabbit anti-human LTBP-1 (BD Pharmingen, New Jersey, USA) | CTS of the HF | (36) | |
| CD34 | CD34 | Expression is low or absent in bulge ORS cells (two out of three)Expressed in the suprabulbar ORS | RNA-expression as well | (2) |
| Not expressed by bulge cells which; do express CK15 (telogen HF)Lower ORS cells express CD34 (anagen HF) | Bulge: proximal part of the isthmus and distal part of the lower follicle (double pos cells: CD34+ cells may be immediate descendents of CK15+ stem cells of the bulge)Using laser capture microdissection and microarray analysis | (1,5) | ||
| Mouse anti-human CD34 (clone QBEND 10, Acris) | High expression in all the HF bulge regions | Used stripped HFs from the occipital scalp regionSay but do not show that CK15 and CK19 are found in the ORSWe used the same antibody clone | (99) | |
| CD34 (clone QBEnd/10, Biogenex, San Ramon, CA, USA) | ORS above the hair bulb and often about half of the length of the HF | Laser-mediated hair removalCD34 IR does not colocalize with CK15Bulge: proximal part of the isthmus + distal part of the lower follicle | (45) | |
| CD34 (clone QBEND 10, DAKO) | Most peripheral layer of the ORS in the transient portion of the HF, below the isthmus and above the matrix cellsNot detected in catagen or telogen HFs | At the level of the isthmus, between the SG duct and down to the insertion of the APM (bulge zone)Does not overlap with CK15Same clone as we have used | (85) | |
| CD34 (clone QBEND 10, DAKO) | Membranous staining of the ORS cellsLimited to cells located below the attachment of the APM and above matrix cells | First who describe CD34 in human HF keratinocytesUsed normal human skin | (71) | |
| Connexin43(Cx43) | Rat Cx43 (Chemicon Int., Temecula, CA, USA) | ORS and IRS express Cx43ORS bulge shows no Cx43 IR | Bulge: position below the sebaceous gland, position correlates with the insertion of the APMNeonatal foreskin: Cx43 in suprabasal layers but not in the basal layers | (53) |
| Mouse anti-human Cx43 (clone 4E6.2, Chemicon) | 88d EGA: inner part of the hair peg135d EGA: IRS, HM, weak IR in the ORS163d EGA: inner part of the ORS, subset of cells in the bulge, IRSAdult HFs: inner part of the ORS, subset of cells in the bulge, IRS | Looked at developing human HFs (fetal HFs)At day 163 EGA lanugo HFsUsed also adult HFs | (75) | |
| Nestin | Mouse anti-human Nestin (Chemicon) | CTS, DP | (83) | |
| Mouse anti-human nestin (Santa Cruz) | Bulge, strong IRS IR, SG cells with little IRNo IR in DP, MKs and HS | (55) |
- APM, arrector pili muscle; BM, basement membrane; CTS, connective tissue sheath; DP, dermal papilla; EGA, estimated gestational age; FT, follicular trochanter; HF, hair follicle; HM, hair matrix; HS, hair shaft; IR, immunoreactivity; IRS, inner root sheath; MK, matrix keratinocyte; ORS, outer root sheath; SG, sebaceous gland.
| Primary antibody | Clone | References | Ig class | Dilution | Origin/vendor | Secondary detection system |
|---|---|---|---|---|---|---|
| α6 integrin | 4F10 | (11,100) | IgG2b | 1:400 | Chemicon, Billerica, MA, USA | ABC-AP, EnVision® |
| β1 integrin | mAb 13 | (101–103) | IgG2a | 1:500 | K. Yamada, Washington, D.C., USA | ABC-AP, IF-FITC/Rh |
| CD34 | QBEND 10 | (71,72,85) | IgG1 | 1:500 | Acris, Hiddenhausen, Germany | EnVision®IF-FITC/Rh |
| CD200 | MRC OX 104 | (2,11) | IgG1 | 1:250 | Serotec, Oxford, UK | TSA |
| Connexin43 | 4E6.2 | (75) | IgG1 | 1:100 | Chemicon, Billerica, MA, USA | IF-FITC/Rh |
| Cytokeratin 15 | LHK15 | (45,56,84) | IgG2a | 1:400 | Chemicon, Billerica, MA, USA | TSA, EnVision® |
| Cytokeratin 19 | Ks 19.1 | (3,11,44) | IgG2a | 1:10 | Progen, Heidelberg, Germany | IF-FITC/Rh |
| Fibrillin-1 | Polyclonal | (104) | NA | 1:500 | D. P. Reinhardt, McGill University, Canada | IF-FITC/Rh |
| Fibronectin | P1H11 | (105) | IgG1 | 1:600 | Chemicon, Billerica, MA, USA | IF-FITC/Rh |
| Lhx2 | Polyclonal | NA | NA | 1:200 | Chemicon, Billerica, MA, USA | IF-FITC/Rh |
| LTBP-1 | 35409 | (106) | IgG1 | 1:500 | R & D Systems, Minneapolis, MN, USA | IF-FITC/Rh |
| Nestin | 10C2 | (83) | IgG1 | 1:1000 | Chemicon, Billerica, MA, USA | TSA |
| Nidogen | Polyclonal | (107) | NA | 1:800 | Calbiochem, San Diego, CA, USA | IF-FITC/Rh |
| Tenascin-C | DB7 | (11,108) | IgG1 | 1:200 | Biomol, Plymouth Meeting, OA, USA | IF-FITC/Rh |
- ABC-AP, avidin–biotin complex, alkaline phosphatase; FITC, fluorescein isothiocyanate; IF, immunofluorescence; NA, not applicable; Rh, rhodamine; TSA, tyramide signal amplification conjugated.
Fibrillin-1, a component of elastic microfibrils, which may attach to BMs in many tissues (33), has also been incorporated in these analyses, since this protein has been shown to bind latent transforming growth factor (TGF)-beta-binding protein-1 (LTBP-1), a recognized murine bulge marker, which plays a role in epithelial and mesenchymal stem cell differentiation (34,35). LTBP-1 targets latent TGFβ complexes to the extracellular matrix whose expression has already been reported in human HFs (36). Finally, nidogen (entactin 1), which operates as a linker molecule joining laminin and collagen IV networks in BMs (37) was included, as nidogen transcription has been reported to be a functionally important component of other stem cell niches (38) and plays a crucial role in BM assembly by connecting the collagen IV and laminin networks (39). Recently published, nidogen could promote prosurvival and promigratory effects of neural progenitors (40), suggesting that it regulates, among other extracellular matrices (41), neurogenesis of HF bulge located neural precursors (42).
Material and methods
Tissue collection
Human temporal and occipital uninflamed scalp skin was obtained from a total of 13 patients with informed consent and following Helsinki guidelines during routine face-lift surgery. Per antigen, scalp skin cryosections derived from at least five different individuals were examined (3–10 cryosections per patient). The specimens were embedded in Shandon Cryomatrix (Pittsburgh, PA, USA), snap-frozen in liquid nitrogen and stored at −80°C until use. The samples were cut into 6–8 μm thick cryosections and then processed for immunohistochemistry.
Immunohistochemistry (ABC-AP)
The cryosections were first air dried for 10 min and then fixed in acetone at −20°C for another 10 min. After air drying for 10 min the slides were washed three times for 5 min in Tris-buffered saline (TBS) and were then preincubated with 10% goat normal serum in TBS. The application of the different primary antibodies (Table 2) followed in their respective dilution in TBS overnight at 4°C. After washing three times for 5 min in TBS, sections were stained either with goat anti-mouse, goat anti-rabbit (1:200 in TBS, Jackson ImmunoResearch, Cambridgeshire, UK) or goat anti-rat (1:200 in TBS, Beckman Coulter, Marseille, France) biotinylated antibodies for 45 min. After washing three times for 5 min in TBS, a 30 min application of avidin–biotin complex, alkaline phosphatase conjugated solution (ABC-AP, Vector Laboratories, Burlingame, CA, USA; 30 min) followed, both at room temperature (RT). Finally, the slides were labelled with Fast Red (DAKO, Glostrup, Denmark) and counterstained with haematoxylin with washing steps in between.
EnVision®-AP
The fixation, the preincubation and the incubation of the primary antibody (Table 2) were performed as described above using standard immunohistochemistry. Then the cryosections were incubated with EnVision® solution (alkaline phosphatase, DAKO, rabbit/mouse) for 30 min. After that, Fast Red AP-chromogen was employed for visualizing the immunosignals and finally, sections were counterstained with haematoxylin.
Immunofluorescence (FITC, rhodamine)
To identify the IR in the bulge region of the human HFs in human skin via immunofluorescence staining we used primary antibodies (Table 2) and the secondary antibodies goat anti-mouse, anti-rat or anti-rabbit IgG conjugated with fluorescein isothiocyanate (FITC) or rhodamine (1:200 in TBS, Jackson ImmunoResearch, Cambridgeshire, UK) for 45 min at RT. The cryosections were counterstained with DAPI (4′,6-diamidin-2′-phenylindol-dihydrochlorid, Boehringer Mannheim, Mannheim, Germany) for 1 min and mounted with Fluoromount-G (Southern Biotechnologies, Birmingham, AL, USA).
Tyramide signal amplification
After standard fixation the cryosections were washed three times for 5 min using TNT (Tris-HCL NaCl Tween) buffer (0.1 mol/l Tris–HCl, pH 7.5; containing 0.15 mol/l NaCl and 0.05% Tween 20). Then horseradish peroxidase was blocked by washing with 3% H2O2 in phosphate-buffered saline (PBS) for 15 min. Preincubation was performed with the incubation of avidin and biotin for 15 min and 5% goat normal serum in TNT for 30 min with washing steps in between. Primary antibodies (Table 2) were diluted in TNT and incubated overnight at 4°C followed by a biotinylated secondary antibody goat anti-mouse (1:200 in TNT) for 45 min at RT. Next, streptavidin horseradish peroxidase (TSA kit; Perkin-Elmer, Boston, MA, USA) was administrated (1:100 in TNT) for 30 min at RT. The reaction was amplified by tetramethylrhodamine- or FITC-tyramide amplification reagent at RT for 5 min (1:50 in amplification diluent provided with the kit). The cryosections were counterstained with DAPI for 1 min and mounted with Fluoromount-G.
For all immunostaining assays, primary antibodies were omitted as a negative control. As routine internal positive controls, reproduction of published follicular IR patterns was chosen. Only those specific IR patterns that were well reproducible between at least five different individuals and were clearly above background were photodocumented and are reported here (Table 3).
| Antigen | Primary antibody | Specific HF IR pattern (anagen VI) in situ | Differences to published IR patterns |
|---|---|---|---|
| IR upregulated in the human bulge region | |||
| Cytokeratin 15 (CK15) | Mouse anti-human CK15 (clone LHK15, Chemicon, Billerica, USA) | Outermost layer of the ORS in the bulge and the proximal part of the isthmus (homogeneous IR intensity)Often found also in the outermost layer of the ORS in the proximal HF (homogeneous IR intensity)FT (for photodocumentation see (11)) | Commo et al. (44): Upper ORS and lower ORS in anagen HF are negative for CK15Orringer et al. (45): Variable staining of the infundibulum |
| Cytokeratin 19 (CK19) | Mouse anti-CK19 (clone 19.1, Progen, Heidelberg, Germany) | Outermost layer of the ORS in the bulge and the proximal part of the isthmus (heterogeneous IR pattern, some cells brighter than others, sometimes isolated cells)Sometimes single cells in the outermost layer of the ORS of the lower HFFT (for photodocumentation see (11)) | Akiyama et al. (58): In the lanugo HF cells in the bulge and the outermost layer of the ORS along the whole HFCommo et al. (44): Upper ORS and lower ORS in anagen HF are positive for CK19Ghali et al. (56): Bulge and lower ORS of the HF, sometimes IR was continuous in lower and upper ORSGho et al. (57): Two distinct areas from skin biopsies are positive (upper ORS and lower ORS)Kruger et al. (59): Constant expression in the ORS and bulge region of vellus HFLyle et al. (3): Throughout the ORS, bulge and bulb areasMatic et al. (53): CK19-expressing bulge regionMichel et al. (48): Bulge cells in anagen and telogen HFOzawa et al. (88): 1, Outermost layer of the ORS of the isthmus in the upper segment; 2, ORS above the hair bulb – Wang et al. (55): IR restricted to the bulge ORSZhang et al. (54): ORS in the bulge |
| CD200 | Mouse anti-human CD200 (clone MRC OX104, Serotec, Oxford, UK) | Outermost layer of the ORS in the bulge and the proximal part of the isthmus (homogeneous IR intensity)DP (including blood vessel), CLFT (for photodocumentation see (11))APM intensive IR and SW mesenchyme/ epithelium most prominent IR | Ohyama et al. (2): Expressed in the bulge ORS and in the companion layer of anagen HFs |
| Lhx2 | Rabbit anti-human Lhx2 (Chemicon) | Isolated cells in the inner layers of the ORS in the bulge and the proximal part of the isthmus (heterogeneous IR intensity), CLSometimes single cells in the ORS of the lower HF, in the mesenchyme of the SG and in the infundibulum (heterogeneous IR intensity) negative FT | First report in human HF |
| Tenascin-C | Mouse anti-human Tenascin-C (clone DB7, Biomol, Hamburg, Germany) | CTS from the proximal part of the isthmus, the bulge and the lower HF including the hair bulb (most intensive IR in the bulge)CTS of the FT (for photodocumentation see (11)) weak IR of the APM | Dang et al. (65): Fibrous sheaths of HFs in some specimensSchalkwijk et al. (67): Continuous and intense IR around HFsShikata et al. (64): Intense perifollicular staining in the lower part of the HF and hair the hair bulbVan Baar (66): Perifollicular connective tissue, APM, homogeneous IR in the DP |
| IR unchanged in the human bulge region | |||
| α6 integrin | Mouse anti-human α6 integrin (clone 4F10, Chemicon) | Outermost layer of the ORS and the BM of the whole HF (homogeneous IR intensity)DP-IR, FT, CTSAPM shows weak IR | Chuang et al. (69): BM zone: Upper and middle part of the HF, not detectable around the hair bulbCommo et al. (68): Basal pole of the basal lower ORS cellsGhali et al. (56): Bulge and lower ORS of the HF, sometimes IR was continuous in lower and upper ORSJoubeh et al. (70): Strong IR in the upper part of the BM zone outside the HF, intensity became weaker towards the lower part |
| β1 integrin | Rat anti-human mAb13(K. Yamada, Washington, D.C., USA) | Outermost layer of the ORS and the BM of the whole HF (homogeneous IR intensity)FTCTS, DP | Lyle et al. (3): Basal layer of the ORS in the bulge and the upper portion of the anagen HF |
| Fibronectin | Mouse anti-human Fibronectin (clone P1H11, Chemicon) | CTS and BM along the whole HF (homogeneous IR intensity)Weak IR in the DP(APM most intensive IR) | |
| Nidogen | Rabbit anti-human Nidogen (Calbiochem, San Diego, USA) | CTS and BM along the whole HF (homogeneous IR intensity)IR in the DP (including blood vessel), FT(APM most intensive IR) | First report in human HF |
| LTBP-1 | Mouse anti-human LTBP-1 (clone 35409, RD Systems, Minneapolis, USA) | CTS of the HFWeak DP, APM | |
| Fibrillin-1 | Rabbit anti-human Fibrillin-1 (D. P. Reinhardt, McGill University, Canada) | CTS of the HFWeak DP, APM | First report in human HF |
| IR absent in the human bulge region | |||
| CD34 | Mouse anti-human CD34 (clone QBEND 10, Acris, Hiddenhausen, Germany) | Outermost layers of the ORS proximal to the bulge region shows homogeneous IR, the bulge and the isthmus and the infundibulum show no IRHeterogeneous IR in the CTS | Ohyama et al. (2): Sometimes low expression in bulge ORS cells (2 out of 3)Raposio et al. (72): High expression in all the HF bulge regions |
| Connexin43 | Mouse anti-human Connexin43 (clone 4E6.2, Chemicon) | Entire ORS proximal and distal to the bulge show a homogeneous IR, the bulge shows no IRFT is negative | Arita et al. (75): Adult HFs: Inner part of the ORS, subset of cells in the bulge, IRSMatic et al. (53): ORS and IRS express Connexin43, ORS bulge show no Connexin43 IR |
| Nestin | Mouse anti-human Nestin (Chemicon) | Isolated cells in CTS and the DP (including blood vessel)No specific IR in the HF epitheliumSW epithelium | Wang et al. (55): Bulge, strong IRS-IR; no IR in DP, MKs and HS |
- APM, arrector pili muscle; BM, basement membrane; DP, dermal papilla; CL, companion layer; CTS, connective tissue sheath; FT, follicular trochanter; HF, hair follicle; HS, hair shaft; IR, immunoreactivity; IRS, inner root sheath; ORS, outer root sheath; SW, sweat gland.
Microscopy
For fluorescence microscopy we used a Zeiss Axiovert 200 M microscope using a green fluorescent protein (GFP) filter set (AHF Analysentechnik AG, Tübingen, Germany). The slides were photographed using a Zeiss AxioCam MRm Rev.3 Fire Wire (D) and the Zeiss AxioVision Rel. 4.5 software.
For light microscopy we used an Olympus BH-2 microscope (Olympus Optical Co., Hamburg, Germany). Photos were taken using a ColorView12 camera from Olympus and the analySIS® software (Soft Imaging System GmbH, Münster, Germany).
Quantitative immunohistochemistry
The immunostaining intensity levels for the selected examined antigens were compared by quantitative immunohistochemistry as previously described (13,17,43), using NIH image software (NIH, Bethesda, MD, USA). Two reference areas were defined (1, insertion point of the APM; 2, middle of the isthmus) and then analysis was performed on sections deriving from three to seven different individuals.
Statistical analysis
For evaluating statistical significance, the measurements were pooled and the mean and the SEM were calculated. With the statistical analysis software SPSS (SPSS Inc., Chicago, IL, USA) P-values (*P < 0.05) were assessed using the Mann–Whitney U-test for unpaired samples.
Results and discussion
Cytokeratin 15, cytokeratin 19 and CD200 are upregulated in the bulge region of human HFs, but their expression is not restricted to it
CK15 is upregulated in human and murine bulge cells which are able to reconstitute all components of the cutaneous epithelium (2,30). Staining for CK15, by either EnVision®- or TSA-immunohistochemistry showed homogeneous IR in the outermost layer of the ORS (1, 5 ) – not only in the bulge region, but also in the proximal part of the isthmus of human HFs – and is significantly upregulated (Fig. 4). As a morphological marker for the bulge, the ‘follicular trochanter’ also showed positive IR [not shown, for documentation see (11)]. Importantly, CK15 IR was also found in the outermost layer of the proximal ORS (1, 5) and in human sweat glands (not shown).
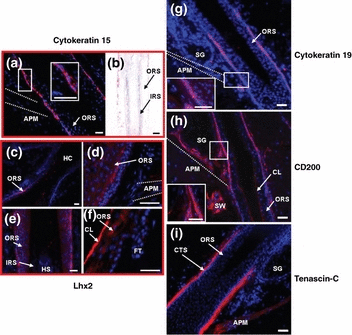
Immunoreactivity is upregulated in the human bulge region for cytokeratin 15, cytokeratin 19, CD200 and tenascin-C. Lhx2 is not a useful marker of the bulge region. (a, b) Cytokeratin 15 shows homogeneous immunoreactivity in the outermost layer of the outer root sheath not only in the bulge region, but also in the proximal part of the isthmus of human hair follicles (a) and in the outermost layer of the proximal outer root sheath (b) (red). (c–f) Cells positive for Lhx2 with different immunoreactivity intensity are found in the suprainfundibular outer root sheath (c), in the inner layers of the outer root sheath of the bulge and the proximal isthmus (d) as well as in the proximal outer root sheath of the human hair follicle (e). The follicular trochanter is negative for Lhx2 (f) (red). (g) Cytokeratin 19 showing heterogeneous immunoreactivity intensity is detected in the bulge region and distal to it (red). (h) CD200 immunoreactivity is found in the bulge region and in the proximal part of the isthmus. The companion layer, the arrector pili muscle and the sweat gland mesenchyme and epithelium and the sebaceous gland mesenchyme are positive for CD200, too (red). (i) Tenascin-C (red) shows immunoreactivity in the connective tissue sheath of the proximal part of the isthmus, the bulge and the hair bulb. The arrector pili muscle is stained positive. APM, arrector pili muscle; CL, companion layer; CTS, connective tissue sheath; HC, hair channel; HS, hair shaft; IRS, inner root sheath; ORS, outer root sheath; SG, sebaceous gland; SW, sweat gland, dotted white line indicates the APM. Bars (a–i) 50 μm.
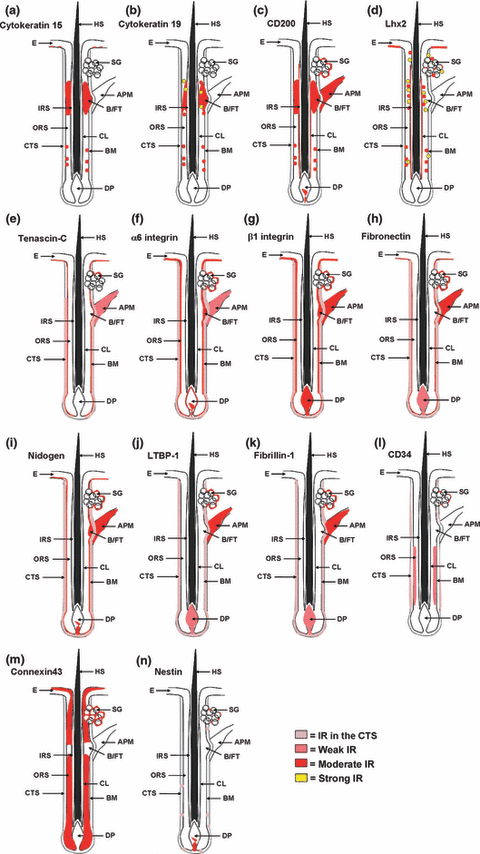
Schematic drawings of the immunoreactivity patterns of the employed antibodies in the pilosebaceous unit and in the epidermis. (a–n) Tested antigens show an immunoreactivity pattern as indicated above. APM, arrector pili muscle; B/FT, bulge/follicular trochanter; BM, basement membrane; CL, companion layer; CTS, connective tissue sheath; DP, dermal papilla; E, epidermis; HS, hair shaft; IRS, inner root sheath; ORS, outer root sheath; SG, sebaceous gland; IR, immunoreactivity.
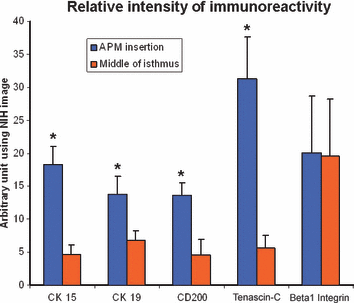
The immunostaining intensity for cytokeratin 15, cytokeratin 19, CD200, tenascin-C and β1 integrin was compared by quantitative immunohistochemistry using NIH image software. The first reference area is the insertion point of the arrector pili muscle and the second was situated in the middle of the isthmus. n = 3–7, *P < 0.05, mean + SEM, P-values were calculated by Mann–Whitney U-test for unpaired samples.
Immunohistochemistry and -fluorescence methods contrast with the CK15 negativity of the upper and lower ORS in human anagen HFs (44). Although a previous report of infundibular CK15 IR (45) supports our finding that CK15 IR in human HFs is not restricted to the bulge, we did not observe CK15 expression in the peri-infundibular ORS (see Table 3 for summary of our own IR results, which highlights differences of our findings to previously published IR patterns of human HFs). Given its expression in the mitotically active basal cell layers of the HF, CK15 expression appears to regulate an early stage in the pathway of keratinocyte differentiation that precedes the decision of a cell to become epidermal or hair-like, which is provided by the bulge region as a reservoir for eHFSCs.
CK19, which should commit stem cells to an epidermal cell fate and differentiation in vivo and in vitro (46,47), shows upregulated IR in the outermost layer of the ORS in the bulge region of normal human anagen VI scalp HFs, including the ‘follicular trochanter’ (11), but was again not restricted to it (1, 4, 5). The detected IR pattern for CK19 in the bulge and distal to it (i.e. in the proximal part of the isthmus) was heterogeneous, with some cells displaying stronger CK19 IR than their neighbours, and sometimes only isolated cells in the ORS being highly CK19 positive (1, 5). This observation goes in line with previously reported murine CK19 positive cells, which were identified as [3H]thymidine-label-retaining cells (48). Occasionally, isolated CK19 positive cells were also identified in the outermost layer of the proximal ORS, at a large distance of the bulge. These isolated CK19 positive HF cells may represent Merkel cells (48–52).
Our findings confirm an upregulation of CK19 expression (Fig. 4) in the human bulge, but contrast with the previously reported restriction of CK19 expression to this ORS region (48,53–55) and with several other published IR patterns (3,44,56,57) [(58) cave: lanugo HF examined!] [(59) cave: vellus follicles examined!] (see Table 3).
As previously reported by Ohyama et al. (2), CD200-positive cells obtained from a human HF suspensions demonstrated high colony-forming efficiency in clonogenic assays, indicating successful enrichment of living human bulge stem cells. By using different immunostaining techniques, we show that prominent CD200 IR can be found in the outermost layer of the ORS between the insertion of the APM and that of the sebaceous gland duct [note that this region is classically defined as isthmus (19), while only its proximal end includes the bulge region]. In addition, by extrasensitive TSA immunofluorescence, we found that the dermal papilla (DP) including its blood vessels, the APM and the sweat gland mesenchyme and epithelium of normal human scalp skin show very prominent CD200 positive IR, and stains the companion layer of the human HF homogeneously (1, 5). The ‘follicular trochanter’ was also prominently CD200 positive [not shown, see (11)]. Therefore, even though CD200 is indeed upregulated in the human bulge, on the protein and gene expression level (2) as confirmed by quantitative immunohistochemistry (Fig. 4), once again is not exclusively expressed in the region and therefore CD200 alone does not suffice being a specific heHFSC marker.
Lhx2 is not a useful bulge marker in human anagen scalp HFs
Here, we also report the first IR pattern for Lhx2 in human HFs. Lhx2 has a role for maintaining the growth and undifferentiated properties of murine HF progenitors (31). Unexpectedly, the ‘follicular trochanter’, a proposed morphological bulge marker structure (11), was predominantly negative for Lhx2 IR (1, 5). Only relatively weak Lhx2 IR was found in the innermost cell layer of the ORS in the proximal isthmus and bulge region (1, 5).
Instead, the companion layer located between the ORS and the IRS, which may be an integral component of the latter (60) and is not considered to harbour heHFSCs (5,6), was most prominently positive for Lhx2 (1, 5). In addition, isolated Lhx2 positive cells were also seen in the proximal ORS (1, 5) and in the suprainfundibular ORS (1, 5), i.e. distant from the bulge in both distal and proximal directions.
These immunostaining results render it unlikely that Lhx2 can be a useful bulge marker in the HF, but raise intriguing new questions about the functions of this transcription factor in human hair biology. Based on the previously reported expression patterns, including our staining results of the differentiated epithelial compartments, Lhx2 maintains epithelial stem cells in an undifferentiated state in murine skin/hair epithelium (31), but it is unlikely that Lhx2 exerts direct effects on human eHFSCs and more likely represents, among others, a regulator of the stem cell niche quiescence (61).
Tenascin-C is upregulated in the bulge connective tissue sheath of human HFs
Next, tenascin-C was examined, since it is a key extracellular matrix protein that has been discussed as a functionally important component of stem cell niches (30,62) and is co-expressed with β1 integrin and fibronectin in human HFs (63). Interestingly, tenascin-C is significantly upregulated in the bulge region of human scalp HFs (Fig. 4), even though there is also extensive homogeneous IR for this antigen along the connective tissue sheath (CTS) of human scalp HFs (1, 5).
Tenascin-C IR is present both throughout the CTS and in the BM at the levels of the proximal part of the isthmus, the bulge and the hair bulb, with the strongest IR signal seen in the bulge. In addition, the APM is also positive (1, 5). This confirms our previous report that the CTS around the ‘follicular trochanter’ and the APM show tenascin-C IR (11) and is in line with the less detailed expression analyses of Shikata et al. (64–66) and Schalkwijk et al. (67). However, these authors had not noted an upregulation of tenascin-C IR in the bulge mesenchyme, which is clearly documented here (Fig. 4).
α6 integrin, β1 integrin, fibronectin, nidogen, LTBP-1 and fibrillin-1 are not upregulated in the bulge epithelium and/or mesenchyme
α6 integrin is expressed throughout the outermost layer of the ORS and the BM of the whole HF and demonstrates gene upregulation in murine bulge cells compared with basal keratinocytes (12). This IR intensity can also be seen in the DP and in the ‘follicular trochanter’, whereas only weak IR was shown in the APM (2, 5). Previously reported differential expression patterns in human HFs (56,68–70) (see Table 3 for details) could not be exactly reproduced by our group. Quantitative immunohistochemistry did not reveal significant differences in the IR intensity between the bulge region and other parts of the ORS (data not shown).
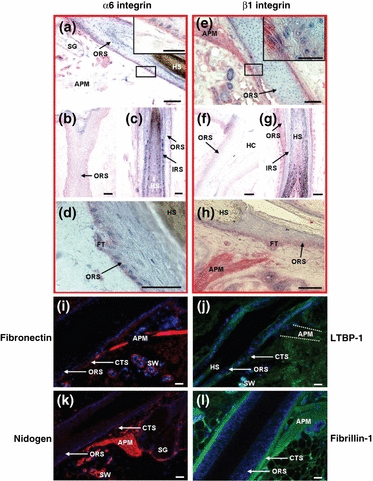
Immunoreactivity in the human bulge region for α6 integrin, β1 integrin, fibronectin, LTBP-1, nidogen and fibrillin-1. (a–d) α6 integrin immunoreactivity can be found throughout the whole outermost layer of the outer root sheath, the basement membrane, the connective tissue sheath and the ‘follicular trochanter’ (a–d) (red). The arrector pili muscle is weakly stained for α6 integrin (a) (red). (e–h) β1 integrin is expressed in the outermost layer of the outer root sheath and in the basement membrane throughout the entire length of the hair follicle (e–h) (red). The connective tissue sheath, the arrector pili muscle and the ‘follicular trochanter’ are also positive for β1 integrin (e, h) (red). (i–l) Fibronectin, LTBP-1, nidogen and fibrillin-1 show positive immunoreactivity in the complete connective tissue sheath (i–l) (red/green). The basement membrane is positive for fibronectin and nidogen (i, k) (red). APM, arrector pili muscle; CTS, connective tissue sheath; FT, follicular trochanter; HC, hair channel; HS, hair shaft; IRS, inner root sheath; ORS, outer root sheath; SG, sebaceous gland; SW, sweat gland; dotted white line indicates the APM. Bars (a–l) 50 μm.
Essentially, this was also the case for β1 integrin IR. As documented in 2, 4, 5, there is homogeneous IR in the outermost layer of the ORS and the BM throughout the entire length of human HFs. In addition, the CTS, the DP and the ‘follicular trochanter’ region are positive for β1 integrin. This contrasts with the reported relative upregulation of β1 integrin expression in the bulge (3). However, closer examination of this paper shows that the authors did report prominent β1 integrin IR also well above and below the bulge area of the ORS, with no conclusive evidence provided that β1 integrin IR really is more intense in the bulge zone than in adjacent ORS regions. Therefore, our study discourages the use of exploiting β1 integrin antigen expression as a marker for human bulge-associated eHFSCs.
Fibronectin, nidogen, LTBP-1 and fibrillin-1 were all prominently expressed throughout the HF CTS and in the DP, with fibronectin and nidogen also expressed in the BM (2, 5), without noticeably higher IR in the bulge mesenchyme, thus rendering them unsuitable as immunohistological markers for this region.
We hereby present the first report of fibrillin-1 antigen expression in the human pilosebaceous unit – fibrillin-1 was expressed along the entire follicular CTS uniformly, with no upregulation in the bulge mesenchyme (2, 5).
CD34, connexin43 and nestin IR are useful negative markers for human bulge epithelial HF stem cells
Confronted by this paucity of convincing positive human bulge and heHFSC markers in situ, we advise the incorporation of postulated negative bulge stem cell markers to the positive markers (5–7,11). Since, in contrast to murine eHFSCs, where CD34 is a viable upregulated marker, together with CK15, to isolate bulge cells which possess stem cell characteristics, including multipotency and high proliferative potential (5), CD34 is not detectable in the human bulge (2,5,71). We have re-examined the CD34 IR pattern, using also more sensitive immunohistology methods (Envision®). Indeed, we found that the isthmus, bulge and infundibulum regions of the human ORS are negative for CD34 (3, 5), while the outermost layers of the ORS proximal to the bulge (i.e. lower ORS) exhibits slight, homogeneous IR (3, 5). Heterogeneous IR is also seen in scattered cells of the human CTS (3, 5). Our findings are largely in line with the report by Ohyama et al. (2) that human CD34 in the bulge is downregulated on both the gene and protein level, but contrast with an isolated report that claims CD34 expression is ‘in all human bulge regions’ (72).
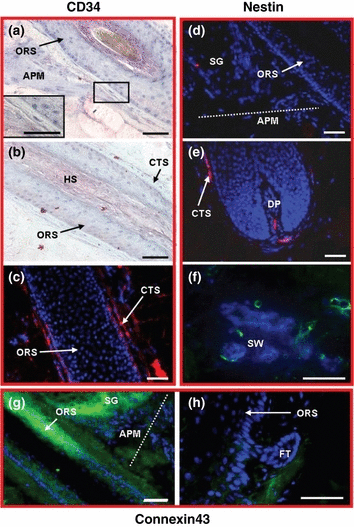
Immunoreactivity is absent in the human bulge region for CD34, connexin43 and nestin. (a–c) CD34 is absent in the isthmus and in the bulge of the hair follicle (a) but outermost layers of the outer root sheath proximal to the bulge are positive for CD34 (b) (red). Immunoreactivity is also seen in scattered cells of the connective tissue sheath using immunofluorescence staining (red). (d–f) Nestin immunoreactivity was not found within the entire HF epithelium including the bulge (d). Isolated nestin positive cells were detected in the connective tissue sheath, the dermal papilla (e) and in the sweat gland epithelium (f) (red/green). (g, h) Connexin43 is absent in the bulge but expressed in the whole outer root sheath proximal and distal to the bulge (g). The follicular trochanter is also negative for connexin43 (h) (green). APM, arrector pili muscle; CTS, connective tissue sheath; DP, dermal papilla; FT, follicular trochanter; HS, hair shaft; ORS, outer root sheath; SG, sebaceous gland; SW, sweat gland; dotted white line indicates the APM. Bars (a–h) 50 μm.
A second potential negative heHFSC marker is connexin43, a gap junction protein (73) which is downregulated in epithelial stem cells. This demonstrates the incompetence of heHFSCs for gap junction-mediated cell-to-cell communication (74). Using a different immunohistology protocol, our studies essentially confirm a report (which examined human newborn foreskin) by showing that the entire ORS of adult human scalp HFs proximal and distal to the bulge shows homogeneous IR for connexin43 (53) (3, 5), while it is negative in the bulge itself. Importantly, the ‘follicular trochanter’ (3, 5) is also negative for connexin43. Contrary to a report by Arita et al. (75), no connexin43 IR was detected in any subset of cells in the human bulge or the IRS.
The intermediate filament protein nestin has long been used as a marker for neural stem cells, but is now widely accepted to be a general progenitor cell marker (76–78). While in nestin promoter-driven GFP transgenic mice, cells that are highly GFP positive have been reported in the murine epithelium (79–82). This GFP positive cells produce several cell types, including cells with neuronal, astrocytic, oligodendrocytic, smooth muscle, adipocytic and other phenotypes (42). We had previously not been able to detect nestin IR cells in the human bulge epithelium (83). However, Wang et al. (55) have reported nestin IR in the human bulge epithelium. Therefore, we systematically re-examined nestin IR, using an antibody specifically directed against human nestin as well as highly sensitive TSA immunofluorescence. In all human scalp HFs examined, contrary to what has been reported in mice, we did not detect any prominent nestin IR within the HF epithelium (including the bulge and the rest of the ORS) (3, 5). However, isolated nestin-positive cells were routinely noted in the human HF CTS and DP (3, 5), and in the human sweat gland epithelium (while non-specific nestin IR was prominent in the IRS, data not shown) (3, 5).
Conclusions
Taken together, this suggests that the absence of CD34, connexin43 and nestin expressions in the human bulge are indeed useful negative markers for heHFSCs in situ, while a relative upregulation of CK15, CK19 and CD200 IR in the bulge region can serve as positive markers. However, the latter three markers are by no means exclusively expressed in the bulge so that the highest ‘hit rate’ for heHFSC identification in situ can be expected when combining them with the above negative markers. A relative increase in their IR here marks tenascin-C potentially as a useful marker for the elusive human bulge stem cell niche.
Acknowledgements
The study was supported by a grant from the Federal Ministry of Education and Research, Germany, to RP and RF (BMBF #01GNO517-19) and by a grant from the Research Focus Programme ‘Regenerative Medicine’, Medical Faculty, University of Luebeck, to RP. The authors thank Katherine Lau for language editing.




