Quantitative analysis of the synthesis and secretion of type VII collagen in cultured human dermal fibroblasts with a sensitive sandwich enzyme-linked immunoassay
Abstract
Abstract: Type VII collagen is the major component of anchoring fibrils in the epidermal basement membrane. Its expression has been analyzed by immunostaining or Northern blotting, but rarely at the protein level. In this study, we have quantitatively examined the effects of ascorbic acid and various cytokines/growth factors on the protein synthesis and secretion of type VII collagen by human dermal fibroblasts in culture, using a developed, highly sensitive sandwich enzyme-linked immunoassay with two kinds of specific monoclonal antibodies against the non-collagenous domain-1. Ascorbic acid and its derivative induced a twofold increase in type VII collagen synthesis, and markedly increased the secretion of type VII collagen into the medium when compared with the control culture. This effect was not influenced by the presence of transforming growth factor-β1 (TGF-β1). The synthesis of type VII collagen was elevated by TGF-β1, platelet-derived growth factor, tumor necrosis factor-α, and interleukin-1β, but not by TGF-α. Thus, our data indicate that the synthesis and secretion of type VII collagen in human dermal fibroblasts are regulated by ascorbate and the enhancement of type VII collagen gene expression by cytokines/growth factors is accompanied with elevated production of type VII collagen at the protein level.
Introduction
The dermal-epidermal junction of the skin contains unique structure and highly specialized functions (1,2). One of its main functions, which requires strong cohesion of the skin layers, is to provide the resistance of the skin against shearing forces (1,2). This is achieved by unique macromolecular networks (the anchoring complex), which attach the epidermis to the basement membrane and to the underlying papillary dermis (1). One of the major structures mediating the attachment is the anchoring fibrils, which originate within the basement membrane and project into the upper regions of the papillary dermis (1). The presence of anchoring fibrils is apparently critical for the stability of the epidermal basement membrane zone, as abnormalities of the anchoring fibrils lead to separation of the epidermis from the dermis and to clinical blistering of the skin (3–5).
Type VII collagen is the main component of anchoring fibrils, stabilizing the association of epidermal basement membrane with the underlying dermis (6–8). Previous studies have indicated that both fibroblasts and keratinocytes, the principal cell types in the skin, synthesize type VII collagen (9–12). Type VII collagen expression has been shown to be transcriptionally regulated by several cytokines, including transforming growth factor-β1 (TGF-β1) and various pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) (9,13–18). Recently, it was also demonstrated that TGF-β1 and TNF-α response elements are distinct entities within the COL7A1 promoter (13). These factors strongly elevate type VII collagen mRNA levels, as measured by Northern blot hybridization (9). These effects were also observed at the protein level in a semi-quantitative manner by indirect immunofluorescence (9) or by an enzyme-linked immunoassay (ELISA) based on immunoblotting (11).
For quantitative analysis at the protein level, we have established a sandwich ELISA for epidermal basement membrane protein, laminin 5, by using monoclonal antibodies (19). To measure the total synthesized laminin 5, the intracellular and deposited protein was solubilized by treatment with a mixture of several kinds of detergents that did not interfere with the sandwich ELISA, and the laminin 5 in both the culture medium and the solubilized cell layer was quantified (19). By using similar methods, we have also developed a sandwich ELISA for type IV collagen and type VII collagen (20).
In this study we examined quantitatively the protein synthesis of type VII collagen by skin fibroblasts using a specific and sensitive sandwich ELISA. We found that ascorbic acid or its derivative stimulated type VII collagen synthesis and was essential for the extracellular secretion of type VII collagen. In all fibroblasts examined, type VII collagen synthesis was more responsive to TGF-β1 than to TNF-α, while there was no correlation between the age of the cell donors and the level of synthesis of type VII collagen.
Materials and methods
Cell cultures
Human dermal fibroblast cultures, established by explanting tissue specimens obtained from foreskins and skins from subjects of different ages (newborn to 91 years old), were utilized in passages 3–15. The cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum. Dissociated fibroblasts, 4 × 104 cells/cm2, were plated on a 24-well culture dish incubated at 37°C in a moist atmosphere containing 5% CO2. The cultures were treated for 48 h with ascorbate at a concentration of 250 μM. l-Ascorbic acid (AsA) and l-ascorbic acid 2-phosphate magnesium salt (Asc 2-P) were purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan).
Antibodies
Antibodies used in this study were NP-32 and NP-185, which recognize non-collagenous domain 1 of type VII collagen (6,8).
Cytokines/growth factors
Transforming growth factor-β1 (R&D Systems, Hercules, MN, USA), transforming growth factor-α (TGF-α; Upstate Biotechnology, Lake Placid, NY, USA), platelet-derived growth factor (PDGF; Becton Dickinson, Bedford, MA, USA), interleukin-1β (IL-1β; Genzyme Techne, MPLS, MN, USA), and tumor necrosis factor-α (TNF-α; Sigma Chemical Co., Saint Louis, MO, USA) were used.
Sample preparation for sandwich ELISA
Conditioned medium of human fibroblast culture was clarified at 20000 g for 5 min and stored at −20°C. To solubilize type VII collagen in the cells and on the culture dish, 1 ml of detergent mixture (10 mM Tris-HCL, pH 7.4, 150 mM NaCl, 2 mM EDTA, 250 mM PMSF, 0.1 mM N-ethylmaleimide, 0.3% NP-40, 0.05% Triton X-100, 0.3% sodium deoxycholate, 0.1% SDS, and 0.1% BSA) was added to each well and frozen at −20°C. The next day, each well was sonicated for 20 s after thawing and then the culture dishes were frozen again at −20°C. On the third day, the thawed samples were spun at 14 000 rpm for 5 min and stored at −20°C. Type VII collagen in the medium and the detergent mixture was stable at least 3 months when frozen at −20°C. Each sample was diluted with the blocking buffer before starting the assay. In the following sandwich ELISA, the detergent mixture did not affect the standard curve of type VII collagen, at least when it was diluted more than 10 times.
Sandwich ELISA for type VII collagen
The sandwich ELISA was developed using two monoclonal antibodies, NP-32 and NP-185 (6,8). Each well of 96-well ELISA microtiter plates (Corning Incorporated, Corning, NY, USA) was coated overnight with 100 μl of protein G purified NP-185 (5 μg/ml) in phosphate-buffered saline (PBS) at room temperature, followed by treatment at room temperature for 1 h with blocking buffer containing Block Ace, which is skimmed milk purchased from Dainihon Seiyaku Co. Ltd (Osaka, Japan), diluted 1:4 with PBS-Tween. Dried plates were stored at −20°C until use. Samples were diluted with the blocking buffer and added to each well, and the plates were incubated at 37°C for 2 h. The plates were washed thrice with PBS-Tween, and then 100 ml of a mixture of biotinylated NP-32 antibody (0.2 μg/ml in blocking buffer) and horseradish peroxidase-avidin D (0.5 μg/ml in blocking buffer) (Vector Laboratories, Inc., Burlingame, CA, USA) was added to each well. The plates were incubated for 1 h at 37°C and then washed thrice with PBS-Tween. The substrate solution, 3,3′,5,5′-tetramethylbenzidine (Bio-Rad Laboratories, Hercules, CA, USA) was added (100 μl/well), and the absorbance was recorded at 650 nm after incubation for 0.5 h and then at 450 nm after the addition of 100 μl/well of 2 N H2SO4 solution to stop the enzyme reaction.
Type VII collagen was purified from the spent media from WISH cells using NP-32 antibody-Sepharose (21). The purified type VII collagen was used as a standard reference in the concentration range of 156.25 pg–2.5 ng for the sandwich ELISA.
Results
Sandwich ELISA for type VII collagen
A sensitive and reproducible sandwich ELISA for type VII collagen was developed using two monoclonal antibodies, NP-32 and NP-185, against non-collagenous domain-1 of type VII collagen. This sandwich ELISA showed a linear standard curve in the range of 156.25–2.5 ng of type VII collagen (Fig. 1), and was sensitive enough to determine the amount of type VII collagen produced by cultured human foreskin and skin fibroblasts.
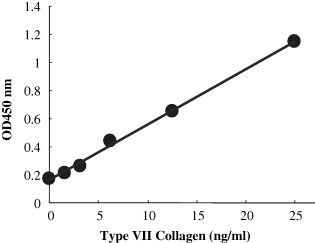
A typical ELISA standard curve for type VII collagen.
Effect of ascorbic acid on synthesis and secretion of type VII collagen
In order to analyze the effects of ascorbic acid or its derivative on the synthesis and secretion of type VII collagen at the protein level, the amounts of type VII collagen in conditioned medium or in the cell layer were separately determined in human foreskin fibroblasts cultured for 48 h. Ascorbic acid and its derivative stimulated twofold the total production of type VII collagen, and markedly increased its secretion into the medium when compared with the control cultured without ascorbic acid (Fig. 2). TGF-β1, which enhances the synthesis of extracellular matrix proteins (22,23), stimulated the total production of type VII collagen independently of ascorbic acid, while secretion into the medium was <10% of the total production of type VII collagen in the absence of ascorbic acid (Fig. 2a). The extracellular secretion of type VII collagen stimulated by TGF-β was also enhanced by the presence of ascorbic acid and its derivative (Fig. 2b,c). These results indicate that ascorbic acid or its derivative is essential for the extracellular secretion of type VII collagen by fibroblasts.
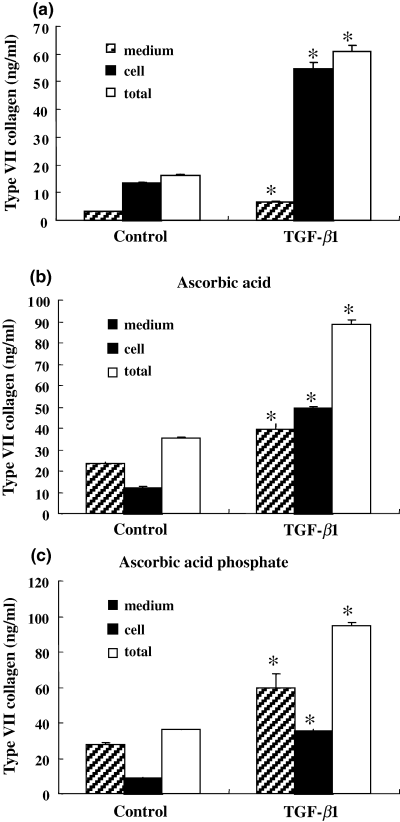
Effect of ascorbic acid on synthesis and secretion of type VII collagen. Human foreskin fibroblasts were cultured with or without TGF-β1 (1 ng/ml) for 48 h in the absence (a) or presence of ascorbic acid (b) or ascorbic acid phosphate (c) to examine the effects of ascorbate on the synthesis and secretion of type VII collagen at the protein level. The amounts of type VII collagen in conditioned medium or in the cell layer were separately determined by specific sandwich ELISA as described in Materials and methods. Ascorbic acid and its derivative increased twofold the total production of type VII collagen and markedly accelerated its secretion into the medium compared with the control cultured without ascorbic acid. Data are representative of three independent experiments, and are presented as mean ± SD. *P < 0.001 compared with control.
Effects of cytokines on the synthesis of type VII collagen
The effects of the growth factors, TGF-β1, PDGF, and TGF-α, and the pro-inflammatory cytokines, IL-1β and TNF-α, on the production of type VII collagen at the protein level were examined in human foreskin fibroblast culture. TGF-β1, IL-1β, and TNF-α have been reported to activate type VII collagen gene expression in fibroblasts (9,13–18). As shown in Fig. 3, these factors stimulated type VII collagen synthesis at the protein level. PDGF also enhanced the synthesis, but TGF-α had little effect. The production of type VII collagen at the protein level was also increased by TGF-β1 or TNF-α in all human skin fibroblasts derived from 21 persons (newborn to 91 years old) although there was no correlation between the age of fibroblast donors and the synthesis of type VII collagen (data not shown).
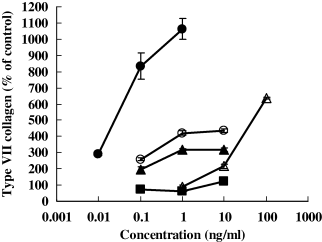
Effects of TNF-α, IL-1β, TGF-α, TGF-β1, and PDGF on the synthesis of type VII collagen. Human foreskin fibroblasts were cultured for 48 h in the presence or absence of TNF-α, IL-1β, TGF-α, TGF-β1, and PDGF to examine their effects on the total amounts of the synthesized and secreted type VII collagens at the protein level. The amounts of type VII collagen in conditioned medium or on cell layer were separately determined by specific sandwich ELISA as described in Materials and methods. TGF-β1 (closed triangles), PDGF (open triangles), IL-1β (closed circles), and TNF-α (open circles) but not TGF-α (closed squares), increased the production and secretion of type VII collagen in a concentration-dependent manner at the protein level.
Discussion
Previous studies have demonstrated that TGF-β1 and pro-inflammatory cytokines such as TNF-α or IL-1β are capable of upregulating type VII collagen gene expression at the mRNA level in cultured dermal fibroblasts (9,13–18). The synthesis of type VII collagen at the protein level has been analyzed in a semi-quantitative manner by indirect immunostaining assay (9) or by an enzyme-linked solid-phase immunoassay, which was based on immunoblotting (11). In this study we assessed the production of type VII collagen in a quantitative manner by using a sensitive sandwich ELISA specific to type VII collagen, and we determined the effects of ascorbic acid and cytokines/growth factors on the synthesis of type VII collagen at the protein level.
Ascorbic acid is an essential factor for the biologic expression and deposition of collagen in the extracellular matrix (24,25). Ascorbate is thought to enhance the transcription of procollagen mRNA in fibroblasts (25). One of the essential functions of ascorbic acid is its role as a cofactor for the hydroxylation of proline and lysine residues in procollagen (26,27). This hydroxylation is essential for the subsequent assembly of procollagen monomers into a triple helix, which in turn is an essential requirement for procollagen stability (28) and subsequent secretion (29–31). The effects of ascorbate on type VII collagen expression were observed both at the mRNA level and at the semi-quantitative protein level in previous studies (12), though there has been no report on the secretion of type VII collagen. We demonstrated that the synthesis of type VII collagen was enhanced and its secretion was markedly stimulated by ascorbic acid and its derivative with or without TGF-β1, as shown in Fig. 2. These results indicate that the synthesis and secretion process of type VII collagen in fibroblasts are similar to those of fibril collagens such as type I collagen and type III collagen, which have been well analyzed.
About 30–35% of type VII collagen was associated with the cell layer even in the presence of ascorbate (Fig. 2), while over 95% of type IV collagen, as determined by using monoclonal antibody against a collagenous region near the 7S domain and polyclonal antibodies (20), was secreted from the same fibroblasts (data not shown), as was the case for type I collagen. In the absence of ascorbate, type VII collagen was rarely detected extracellularly even in the presence of TGF-β1 (data not shown), whereas intracellular type VII collagen was increased by TGF-β1, as shown in Fig. 2a. Therefore, the hydroxylation of proline in the collagen domain of type VII collagen in the presence of ascorbic acid (26,27) appears to be necessary for extracellular secretion. A possible reason for the prolonged intracellular residence of type VII collagen is that its maturation might be slower than that of other collagens because of its large molecular size. It is also possible that additional mechanisms are involved in the secretion of type VII collagen when compared with other collagens, although further investigation will be necessary to examine this.
The effects of various cytokines/growth factors on the expression of type VII collagen at the mRNA level and at the semi-quantitative protein level by indirect immunostaining and immunoblotting in dermal fibroblasts were examined in previous studies (9,11). It has been demonstrated that TGF-β1, IL-1β, and TNF-α response elements are distinct entities within the COL7A1 promoter (13). The effect of TGF-β1 and IL-1β or TNF-α on the expression of type VII collagen gene (COL7A1) is mediated by the immediate-early transcription factor SMAD 3/4 or NF-kappaB, respectively (13). In this study, we confirmed that TGF-β1, TNF-α, and IL-1β elevated type VII collagen synthesis at the protein level, as determined by sandwich ELISA. These results indicate that the enhancement of type VII collagen gene expression by these factors is accompanied with elevated production of type VII collagen at the protein level.
A previous study has indicated that the basal expression of the type VII collagen gene in fibroblasts decreases in an age-dependent manner (9). Therefore, we examined the effects of the age of fibroblast donors (from newborn to 91 years old) on the basal level of protein synthesis of type VII collagen. However, there was little correlation between donor age and basal protein production. Almost all fibroblasts showed similar relative increases of type VII collagen production in response toTGF-β1 and TNF-α. These results suggest that type VII collagen protein synthesis may not decline with aging.
In conclusion, our data indicate that the synthesis and secretion of type VII collagen in human dermal fibroblasts are regulated by ascorbic acid or its derivatives, as is the case for type I and III collagens, and the enhancement of type VII collagen gene expression by cytokines/growth factors is accompanied with elevated production of type VII collagen at the protein level.
Acknowledgements
We thank Ms Tomoko Yoshida for her skillful technical assistance. We also thank Dr Marie-France Champliaud for her gift of purified type VII collagen. This study was supported in part by a Grant-in-Aid (No. 16380227) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to TN).




