Efficacy and safety of the third-generation chloroethylnitrosourea fotemustine for the treatment of chemorefractory T-cell lymphomas
Re-use of this article is permitted in accordance with the Terms and Conditions set out at http://wileyonlinelibrary.com/onlineopen#OnlineOpen_Terms
Abstract
Patients with recurring T-cell non-Hodgkin lymphoma (T-NHL) are incurable and candidate for investigational agents. Here, we report on five patients with T-NHL refractory to multiple chemotherapy lines, including in all cases alkylators and gemcitabine, who received the third-generation chloroethylnitrosourea fotemustine at a dose of 120 mg/m2 every 21 d, up to eight courses. Median actual dose intensity was 79%; toxicity was manageable and mainly hematological. One complete remission, one partial remission, two protracted disease stabilization, and one transient, minor response were achieved. Time to progression ranged from 48 to 240+ d. This is the first evidence ever reporting the activity of fotemustine in end-stage T-NHL. Formal studies with this agent are warranted in T-cell malignancies.
Non-Hodgkin lymphomas arising from mature post-thymic T cells (T-NHL) encompass biologically heterogeneous malignancies with a dismal prognosis (1, 2). Patients with histotypes such as peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), kinase-negative anaplastic large cell lymphoma (ALK-negative ALCL), angioimmunoblastic T-cell lymphoma, and Sezary syndrome (SS) experience early disease recurrence with 5-yr overall survival rates rarely exceeding 40%, also because of the lack of effective salvage strategies (1–3). While the biologic basis of T-NHL aggressiveness is under extensive research, investigation into novel active agents remains a critical need, especially in the setting of stem cell transplantation ineligibility/failure and end-stage disease (2, 4).
Fotemustine is a third-generation chloroethylnitrosourea displaying a significant clinical efficacy in highly chemoresistant cancers such as disseminated melanoma and primary or metastatic brain tumors (5–9). While second-generation nitrosoureas have been incorporated into standard and high-dose salvage regimens for NHL, including T-cell subtypes (10–13), the activity of fotemustine in lymphoma has been poorly explored, despite its lower hepatic and renal toxicity, as compared to congener agents, and several more favorable pharmacodynamic properties (14, 15). First, the presence of a phosphoalanine carrier group renders fotemustine more lipophilic because its octanol/water partition coefficient results within the optimal lipophilicity interval, differently from both carmustine and lomustine (16, 17). Second, preclinical studies on rat and human tissues have shown that intracellular penetration of fotemustine is superior to that of carmustine (18, 19). Third, studies have documented that fotemustine is cleared 2–5 times more slowly than carmustine from tumor tissues (17–19). The higher lipophilic properties, the more efficient tissue distribution, and slower elimination of fotemustine from tumor tissues, as compared to other nitrosoureas, might provide a preclinical rationale for testing this drug in chemorefractory lymphomas.
Because normal and malignant T cells express a low level of O6-alkylguanine-DNA alkyltransferase (AGT) and of O6-methylguanine-DNA methyltransferase (MGMT) (20, 21), two DNA repair enzymes implicated in cellular resistance to nitrosureas, and specifically to fotemustine (22–25), we speculated that alkylator-refractory T-NHL could represent a proper setting to explore the potential clinical activity of this newer nitrosourea derivative. Here, we report the first evidence ever that fotemustine is able to induce substantial clinical responses in patients with post-thymic T-cell malignancies.
Methods
Five consecutive patients (three men, two women; age range, 54–77 yr) with biopsy-proven recurrent T-NHL [ALK-negative ALCL (n = 2), PTCL-NOS (n = 1), and SS (n = 2)] were accrued into a named-patient program with single agent fotemustine. Treatment consisted of fotemustine 120 mg/m2, as a 1-h intravenous infusion, at day 1 every 3 wk until progression, unacceptable toxicity, or a maximum of eight administrations. The program was approved by the Internal Pharmaceutical Review Board, and after obtaining written informed consent from patients, fotemustine (Muphoran®, S10036; Servier, Neuilly-sur-Seine, France) was purchased from Italfarmaco S.p.A. (Milan, Italy). Within 2 wk prior to treatment, patients underwent a complete disease staging including bone marrow (BM) biopsy, computer-assisted tomography (CT), 18F-fluoro-deoxy-glucose (18F-FDG) positron emission tomography (PET), and echotomographic assessment of superficial disease sites. Skin involvement was assessed through the modified Severity Weighted Assessment Tool (mSWAT) (26). Levels of soluble interleukin-2 receptor (sIL-2R) were determined on cryopreserved serum samples obtained prior to fotemustine treatment. Primary prophylaxis with trimethoprim-sulfamethoxazole and valacyclovir was mandatory, while granulocyte colony-stimulating factor recommended only in case of grade 3/4 neutropenia. Response evaluation was planned every two courses, interim restaging after four courses, and final assessment at the completion of treatment, according to the International Workshop (27) and to ISCL/EORTC criteria (28).
Case report
Baseline clinical features and treatment outcomes for all five patients are summarized in Tables 1 and 2, respectively. All were stage IV disease; most of them presented with a poor performance status and had received three prior lines of therapy [CHOP or CHOP-like chemotherapy (n = 3), gemcitabine-based regimens (n = 5), platinum and ifosfamide containing regimens (n = 4), newer agents including vorinostat (n = 1), bortezomib (n = 2), and alemtuzumab (n = 1)]. The treatment was given on an outpatient basis, and the related complications never required hospitalization. A total of 28 infusions of fotemustine were delivered. Overall, treatment duration was 8–28 wk, and the cumulative dose ranged from 280 to 880 mg/m2, with a median dose intensity [defined as actually delivered dose (mg/m2/wk) divided by the planned dose (mg/m2/wk)] of 79% for the first four courses. A dose reduction of fotemustine to 75% was needed, after 1-wk delay, in patients 2 (3rd course), in patient 3 (4th course), and in patient 4 (from course 3rd to 5th). All patients were evaluable for response, and none of them had to discontinue treatment owing to excessive toxicity: two had a major response, one complete (patient 1) and one partial (patient 4), and two others achieved disease stabilization (patients 3 and 5), while only one progressed following an early minor response (patient 2).
| N | Age/sex | Histology | Tumor cell phenotype1 | Bone marrow involvement | Cutaneous involvement | mSWAT | Stage at entry | PS | Baseline | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC × 109/L | Lymph × 109/L | Hgb, g/L | Plt × 109/L | LDH > UNL | sIL-2R IU/mL | |||||||||
| 1 | 54/M | Sezary syndrome | CD2+, CD3dim, CD4+, CD5+, CD7−, CD8− | Yes | Desquamating erythroderma, patch lesions, palmar fissuring | 54 | IVB2 | 1 | 6.18 | 4.3 | 12.3 | 215 | No | 722 |
| 2 | 78/M | Sezary syndrome | CD2+, CD3−, CD4+, CD5+, CD7−, CD8− | Yes | Multiple regressing nodules at posterior trunk, multiple plaques at left wrist, face, and scalp, erythematous patches at the left thigh | 84 | IVA2 | 2 | 5.95 | 1.7 | 10.4 | 243 | Yes | 3829 |
| 3 | 57/F | ALCL ALK-neg | CD3+, CD2+, CD30+, Alk−, CD20−, CD15− | Yes | No | – | IVB | 3 | 10.8 | 0.3 | 9.5 | 89 | Yes | 928 |
| 4 | 74/F | ALCL ALK-neg | CD3+, CD30+, CD2−, Alk−, CD15− | No | Multiple infiltrating skin nodules at scalp and face | 28 | IVA | 2 | 4.6 | 1.2 | 9.3 | 194 | Yes | 3127 |
| 5 | 77/M | PTCL-nos | CD2+, CD3+, CD4+, CD5+, CD7+ | Yes | No | – | IVA | 2 | 303 | 298 | 13.7 | 147 | No | 1678 |
- M, male; F, female; ALCL, anaplastic large T-cell lymphoma; PTCL-nos, peripheral T-cell lymphoma not otherwise specified; sIL-2R, levels of soluble interleukin-2 receptor measured on prefotemustine cryopreserved serum samples with a sandwich enzyme-linked immunosorbent assay based on two monoclonal antibodies raised against two different epitopes of the p55 alpha-chain of the IL-2R complex (reference value: 180–570 IU/mL; Bender Medsystem GMBH, eBioscience, Milan, Italy); mSWAT, modified Severity Weighted Assessment Tool for skin assessment; PS, ECOG performance status.
- 1As defined by immunohistochemistry and flow cytometry.
- 2According to ISCL/EORTC (28).
| N | Age/sex | Histology | Prior therapy n (type) | Response to prior therapy | Fotemustine n of courses | Best response | Time to best response (d)1 | Time to progression (d)2 | Status | CTCAE v3.0 toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54/M | Sezary syndrome | 3 (CHOP, gemcitabine, vorinostat) | Refractory | 8 | CR | 150 | 240+ | Alive in CR | G3 thrombocytopenia |
| 2 | 78/M | Sezary syndrome | 2 (GIFOX; VCG) | Refractory | 3 | PD | – | 48 | Died for PD | G4 anemia |
| G4 infection | ||||||||||
| G4 platelets | ||||||||||
| 3 | 57/F | ALCL ALK-neg | 3 (CHOP, DHAP, GIFOX) | Refractory | 4 | SD | 42 | 86 | Died for PD | G4 thrombocytopenia |
| G3 febrile neutropenia | ||||||||||
| G4 anemia | ||||||||||
| 4 | 74/F | ALCL ALK-neg | 3 (CHOEP, GIFOX, VCG) | Refractory | 6 | PR | 54 | 165 | Died for PD | G3 neutropenia |
| G3 infection | ||||||||||
| 5 | 77/M | PTCL-nos | 2 (GIFOX, alemtuzumab) | Relapse | 7 | SD | 68 | 144+ | Alive in PD | None |
- M, male; F, female; ALCL, anaplastic large T-cell lymphoma; PTCL-nos, peripheral T-cell lymphoma not otherwise specified; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; CHOEP, cyclophosphamide, adriamycin, vincristine, etoposide, prednisone; GIFOX, gemcitabine, ifosfamide, oxaliplatin; VCG, bortezomib, cyclophosphamide, gemcitabine; DHAP, dexamethasone, cytarabine, cisplatin; CHOEP, cyclophosphamide, adriamycin, vincristine, etoposide, prednisone; CR, complete response; PR, partial remission; SD, stable disease; PD, progression of disease; CTCAE v3.0, Common Terminology Criteria for Adverse Events version 3.0.
- 1From day of the 1st dose of treatment to documentation of best response.
- 2From day of the 1st dose of treatment to documentation of progression.
A complete response (CR) was achieved in patient 1. He had a stage IVB chemorefractory end-stage SS with high blood burden of CD4+/CD7 tumor cells, BM involvement, multicentric nodal disease (bilateral axillary and inguinal, intercavoaortic, lomboaortic) (Fig. 1A) and highly symptomatic disseminated desquamating erythroderma with patch-like skin lesions, palmar fissuring and intense, disabling itching. Skin disease completely reverted after the first two doses of fotemustine, while a residual PET-positive left inguinal adenopathy was still documented at interim restaging. A total regression of all 18F-FDG uptakes and a complete clearance of tumor T cells from blood and BM were documented by the end of treatment (eight courses) leading to a CR (Fig. 1A), which was maintained up to +240 d.
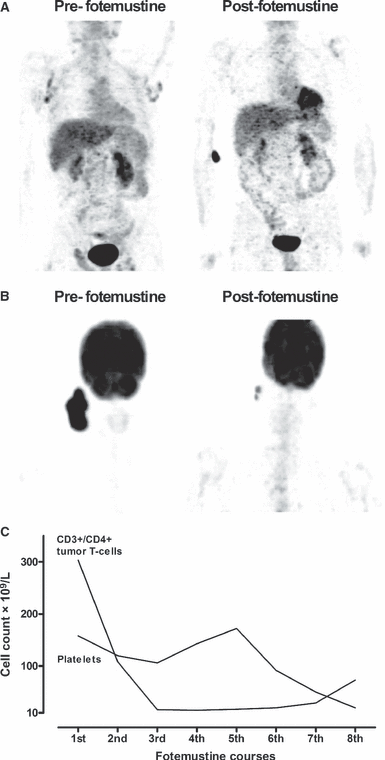
(A) Pretreatment nodal 18F-FDG uptakes (bilateral axillary and inguinal, intercavoaortic, lomboaortic) completely regressed after completion of fotemustine treatment in patient 1. (B) Changes 18F-FDG uptake of a cervical-parotid gross tumor lesion after three courses of fotemustine in patient 4. (C) The control of the leukemic (CD3+/CD4+) hypercytosis exerted by fotemustine in patient 5.
A partial response was achieved in patient 4. She suffered from a refractory ALK-negative ALCL with multiple skin-infiltrating nodules at the scalp and the right frontal-zygomatic area, together with a painful bulky swelling at right neck infiltrating cervical lymph nodes, parotid, and soft tissue planes. A complete regression of cutaneous involvement was achieved within 1 month from starting treatment; a maximum of 65% sharp reduction occurred, after three courses, in the sum of the products of the greatest diameters in the cervical/parotid gross lesion with an impressive decrease in the 18FDG uptake intensity and extension at PET scanning (Fig. 1B), as compared with baseline. Response persisted for 4 months, and thereafter, a progression was documented at day 165.
Disease stabilizations were observed in patients 3 and 5. Patient 3 had a chemorefractory ALK-negative ALCL with BM involvement and widespread nodal disease (laterocervical, retroclavear, subclavear, axillary, mediastinal, intercavoaortic, lomboaortic, splenic hilum, iliac, obturator, and inguinal) complicated by massive ascites. Treatment with fotemustine reduced nodal bulks and abrogated the need for paracentesis for about 3 months. Patient 5 had a multicentric nodal relapse of a PTCL-NOS together with a quickly onset leukemic (CD2+/CD3+/CD4+/CD5+/CD7+) hypercytosis (303 × 109/L). He achieved a long-lasting control of nodal and leukemic evolution, while receiving treatment, for an overall period of 144 d, as long as T cells continued to decline in the absence of any disease-related symptoms, cytopenia, and toxicity (Fig. 1C). Subsequently, a further rise in lymphoid neoplastic cells up to 73 × 109/L, paralleled by a drop in platelets count, down to below 20 × 109/L, impelled for a therapeutic diversion.
Only a minor response occurred in patient 2; he had a SS with large fungating skin lesions at posterior trunk, multiple plaques at left wrist, face, and scalp, and erythematous patches at the left thigh together with nodal and leukemic (CD2+/CD3−/CD4+, CD5+, CD7−, CD8−) disease. Three fotemustine courses were delivered to this patient. Following the early rapid fainting of skin lesions, accompanied by an impressive resolution of cutaneous symptoms, he developed a full-blown disease progression shortly afterward the third course. Differently from patient 1, also affected by SS, who achieved a CR, this patient had high-burden tumoral lesions, a higher mSWAT score, and a more aggressive disease behavior as also indicated by elevated LDH values and a high serum level of sIL-2R (29, 30).
Toxicity of treatment, as assessed according to the NCI Common Terminology Criteria for Adverse Events (version 3.0), was mainly hematological. Grades 3 and 4 thrombocytopenia occurred in three patients with a total of five courses requiring transfusion support. Grade 4 anemia required transfusions in two patients for overall four courses. Febrile neutropenia requiring intravenous antimicrobial therapy complicated three courses. A destructive infection of the deep skin and tissues at back chest wall, sustained by Gram-positive cocci and anaerobic clostridia, occurred in patient 2.
Discussion
Case reports presented herein provide the first evidence that fotemustine can achieve clinical responses in chemorefractory T-NHL. Two major responses (one complete) and two protracted disease stabilizations were recorded in four of five treated patients, all with highly unfavorable disease, recurring after alkylators and gemcitabine (i.e., two hallmarks of T-NHL therapy) and, in most cases, CHOP therapy or new agents such as bortezomib, vorinostat, and alemtuzumab. Several new agents are being actively explored in advanced T-NHL including, among others, folate antagonists such as pralatrexate, monoclonal antibodies, signaling inhibitors, and immunomodulatory agents (31). In the absence of a recognized gold standard for salvage and even upfront treatment for T-NHL (4, 31), we identified fotemustine, as a possible therapeutic option for our patients, owing to its pharmacodynamic advantages over other nitrosoureas (16–18), the low expression of AGT and MGMT in T-cell tumors (20, 21), and its favorable toxicity profile in pretreated patients (32).
Thus far, very few studies have addressed the role of fotemustine in hematological malignancies, with data published only for recurrent multiple myeloma (33, 34). More recently, we demonstrated that high doses of fotemustine (300 mg/m2) can be safely incorporated into an active new conditioning regimen (fotemustine, etoposide, cytarabine, and melphalan; FEAM), for autologous stem cell transplant (35). The FEAM regimen, as applied to 84 patients with lymphoma, favorably compared with the classical BEAM (carmustine, etoposide, cytarabine, and melphalan) conditioning in terms of activity and toxicity and was able to convert pretransplant PR into CR in 25 of 32 patients (78%) (35), including four of seven PTCL cases (Pinto A, unpublished observations). Interestingly, the FEAM regimen was devoid of pulmonary toxicity, as compared to the carmustine-containing BEAM, and displayed a lower rate of renal and hepatic toxicities (14, 35).
Noteworthy, fotemustine induced a fast and impressive activity on cutaneous lesions in our patients along with a rapid resolution of erythroderma and severe itching. In this respect, intravenous fotemustine may be of value in the palliative treatment for lymphoma-related refractory cutaneous symptoms. Whether this effect might be ascribed to a preferential accumulation/retention of the agent in the skin, as suggested by the highly lipophilic features (16) and significant activity of fotemustine in cutaneous melanoma (6), remains to be established.
Our patients with T-NHL were treated with a schedule differing from the treatment usually adopted for melanoma and brain tumors, i.e., 300 mg/m2 over an 8-wk period (100 mg/m2 on days 1, 8, 15 to be followed, after a 5-wk rest, by 100 mg/m2 at a 3-weekly interval) (6, 8). Owing to the rapid regrowth rate of tumors in our patients and the significant amount of previous myelotoxic therapy, we reasoned that fixing fotemustine dose to 120 mg/m2 at a 3-weekly interval could have realized a dose intensity able to control disease burden and kinetics, without a heavy load of early myelotoxicity. As a matter of fact, this allowed delivery of a sufficient number of courses at a median dose intensity approaching 80% with an acceptable hematological toxicity and without drug-related serious adverse events.
Overall, our report indicates that fotemustine is active in end-stage T-NHL and represents an agent worth of being further investigated in such disease setting. Beyond confirming our preliminary evidences, it would be of interest to study the impact of AGT expression and MGMT promoter methylation status on fotemustine activity in T-cell lymphomas. Expression of both these DNA repair enzymes was shown to predict sensitivity to fotemustine in glioma and melanoma (20–24). These studies should also be aimed at defining the optimal schedule of fotemustine for T-cell malignancies, which may differ from that used for melanoma and brain tumors.
Acknowledgement
The Authors are grateful to Dr. Alessandra Trocino, Librarian at the NCI 'G.Pascale' in Naples, for the excellent bibliographic assistance. Supported in part by the Ministry of Health (Ricerca Corrente and Ricerca Finalizzata, FSN), Rome, Italy.
Conflict of interest disclosure
All of the authors declare no competing financial interests.




