CRATER LAKE COLONIZATION BY NEOTROPICAL CICHLID FISHES
Abstract
Volcanic crater lakes are isolated habitats that are particularly well suited to investigating ecological and evolutionary divergence and modes of speciation. However, the mode, frequency, and timing of colonization of crater lakes have been difficult to determine. We used a statistical comparative phylogeographic approach, based on a mitochondrialDNA dataset, to infer the colonization history of two Nicaraguan crater lakes by populations of genetically and ecologically divergent cichlid lineages: Midas (Amphilophus cf. citrinellus complex) and moga (Hypsophrys nematopus). We compared estimates of diversity among populations within the two cichlid lineages and found that Midas were the most genetically diverse. From an approximate Bayesian computation analysis, we inferred that the crater lakes were each founded by both cichlid lineages in single waves of colonization: Masaya 5800 ± 300 years ago and Xiloá 5400 ± 750 years ago. We conclude that natural events are likely to have a dominant role in colonization of the crater lakes. Further, our findings suggest that the higher species richness and more rapid evolution of the Midas species complex, relative to other lineages of fishes in the same crater lakes, cannot be explained by earlier or more numerous colonization events.
When pristine habitats are colonized, the combination of genetic founder effects and novel environmental conditions can enhance the evolution of new species (Mayr 1954). How, when, and by how many individuals such environments were colonized influences both the tempo of evolution and the ecological conditions experienced by the founding populations (Grant 1998). In this regard, comparative phylogeographic studies, which include different species with shared geographical distributions, are particularly useful for inferring evolutionary and historical events (Bermingham and Moritz 1998). However, phylogeographic approaches with young populations have struggled analytically to accommodate the differing genetic histories, incomplete lineage sorting, and genetic and demographic bottlenecks that accompany recent founding events (Hickerson et al. 2006).
Crater lakes are initially barren habitats generated by a volcanic eruption. These aquatically isolated, often young environments provide ideal settings for investigating colonization patterns and subsequent sympatric and allopatric divergence, as demonstrated, for example, by African and Neotropical cichlid fish lineages (e.g., Schliewen et al. 1994, 2001; Sato et al. 2003; Schliewen and Klee 2004; Barluenga et al. 2006a; Gavrilets et al. 2007; Elmer et al. 2010b,c; Geiger et al. 2010). The crater lakes of western Nicaragua are particularly well suited for comparative analyses of colonization because they vary in size, age, and fish community assemblages (reviewed in Elmer et al. 2010b). For example, some lineages, such as the Midas cichlids, have speciated considerably within and among crater lakes, forming small adaptive radiations of up to six endemic species in each crater lake (Elmer et al. 2010b). Meanwhile, other lineages of fishes, including other cichlids, have not diversified to the same extent (Waid et al. 1999; Elmer et al. 2010b). Hence, Nicaraguan crater lakes offer a replicated setting for examining the evolutionary history of colonization events that may, or may not, be independent across lakes and species.
How and when fishes colonized crater lakes has been difficult to determine with certainty, even with extensive population genetic sampling (e.g., Barluenga et al. 2006a,b; Schliewen et al. 2006). The following three modes of colonization are open for speculation: aquatic connection, human introduction, and natural aerial introduction. Most Nicaraguan crater lakes have high walls and have never been connected to any neighboring lakes by rivers or streams (Parello et al. 2008), with the exception of crater lake Xiloá, which has a low eastern rim and may have been connected to the great lake Managua at some time in the past (Villa 1976c). Therefore, aquatic connection is a reasonable mode for fishes to colonize crater lake Xiloá but not crater lakes in general or other Nicaraguan crater lakes in particular. Second, humans may have purposefully introduced fishes to crater lakes for aquaculture (Villa 1976a), either recently or quite long ago (e.g., Nicaragua's oldest human footprints are dated to ca. 6 kya, Lockley et al. 2008). However, not all cichlid fishes are large enough for humans to bother introducing for aquaculture, so this is not equally likely for all species. Third, natural events such as storms that cause “fish rains” (Thomson 1849; McAtee 1917; Hora 1950) are another possible means of colonization, and presumably affect multiple species at the same time. Other natural aerial introductions may result from live fishes being transported by predatory birds: this mode would be unlikely to involve introduction of multiple species simultaneously.
In practice, the timings of crater lake colonizations need to be estimated by genetic methods informed by the geological maximal age of a crater lake. However, contemporary crater lake fish populations may in some cases be much younger than the crater lakes they inhabit (e.g., Elmer et al. 2010c), if crater lakes lie uninhabited for long periods of time or earlier populations go extinct, for example, by off-gassing (Tassi et al. 2009) or remnant volcanic events (Kutterolf et al. 2007; Pérez et al. 2009). Further challenges to inferring the age of crater lake fish populations include incomplete lineage sorting and demographic fluctuations. Fortunately, recent methodological advances, such as hierarchical approximate Bayesian computation (HABC), accommodate the demographic stochasticity of different evolutionary histories, incomplete lineage sorting, and the demographic bottlenecks that accompany founding events (Hickerson et al. 2006; Bertorelle et al. 2010; Csilléry et al. 2010). HABC has been recently applied to quantify divergence in regions with controversial phylogeographic histories (Hickerson et al. 2006; Leaché et al. 2007; Carnaval et al. 2009; McCulloch et al. 2010), with the particular strength of inferring across different codistributed taxa.
In the present study, we use a statistical comparative phylogeographic approach to infer the colonization histories of two Nicaraguan crater lakes by members of two genetically and ecologically distinct cichlid lineages: the species rich Midas cichlid species complex (Amphilophus cf. citrinellus complex) and the species depauperate lineage, moga cichlid (Hypsophrys nematopus) (previously Neetroplus nematopus, see Schmitter-Soto 2007). It has been argued that Midas cichlids may be exceptionally prone to rapid speciation, including diversification in sympatry, because Midas species richness exceeds that of any other cichlid lineage in any Nicaraguan crater lake (Elmer et al. 2010b; Meyer 2011). To date, however, it is not known whether the high species richness is solely due to exceptionally fast evolutionary or adaptive potential of Midas cichlids or if this lineage has had more time than other cichlid species to speciate within the crater lakes.
We used several complementary approaches to study the colonization history of Nicaraguan crater lakes. First, we compared estimates of genetic diversity and genetic differentiation within the two cichlid lineages, Midas and moga, in the ancestral great lake habitat and in the two derived crater lake habitats (Barluenga and Meyer 2010). Second, to infer whether each crater lake was colonized by the two cichlid lineages simultaneously or sequentially, we used an approximate Bayesian computation approach that accommodates the changing and unequal population sizes inherent during a divergence. Third, we estimated how many years ago the crater lake colonizations occurred. We chose moga as a comparison lineage to Midas because of the considerable geographical overlap and similarly high abundance. Our overall aim was to determine the frequency (single or multiple colonizations) and timing (years since divergence from ancestral population), and thereby infer the mode (i.e., natural or anthropogenic), of Nicaraguan crater lake colonizations by cichlid fishes. This information may serve as a basis for understanding the way fishes colonize new habitats in general, and crater lakes in particular, and how often such events occur. It also helps to determine whether differential rates of evolution underlie the species richness disparity between various Neotropical cichlid lineages.
Methods
MODEL SYSTEM
We focused on two lineages of amphilophine cichlid fishes, Midas and moga (also known as “little lake cichlid,”“picaculo,” or “poor man's tropheus”), which differ in clade species richness, ecology, body size, and morphology (López-Fernández et al. 2010). Midas cichlids are large enough to be eaten by humans and indeed are commonly marketed in the region (Barlow 1976; McCrary et al. 2007), whereas moga cichlids are very small (adult standard length 3–11 cm, McKaye et al. 2010) and are not fished or raised for food. Midas are found in at least eight crater lakes and moga in two crater lakes (Waid et al. 1999), and both taxa are widely distributed in lakes and rivers of northern Costa Rica and Nicaragua (Bussing 1998; Schmitter-Soto 2007). We sampled each of the Nicaraguan crater and great lakes in which these lineages are known to co-occur (Waid et al. 1999): crater lake Xiloá (maximally 6100 years old, Kutterolf et al. 2007), crater lake Masaya (age unknown, Kutterolf et al. 2007; Pérez et al. 2009), and great lake Nicaragua (Schmitter-Soto 2007) (Fig. 1). Moga's type locality is great lake Managua but the population is thought to be extirpated (Schmitter-Soto 2007).
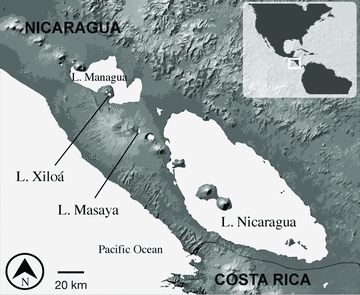
A map of Nicaragua, showing the location of the sampled crater lakes, Masaya and Xiloá, and the great lakes Managua and Nicaragua.
DNA SEQUENCE DATA
Samples of moga were collected in 2005 and 2007 using gill nets. Genomic DNA was extracted by standard high-salt methods. Mitochondrial DNA control regions were amplified using primers LProF (Meyer et al. 1994) and CIC3 (5′ TCT AGG GTC CGT CTT AAC ATC TTC A; designed for this study) using a standard PCR recipe and conditions. Products were purified using a standard Exonuclease/SAP protocol and then sequenced bidirectionally with BigDye using the same primers as for the PCR, before being electrophoresed on an ABI3130xl. Sequences were assembled into contigs in Sequencher version 4.2.2. Sequences are deposited in GenBank: JX003703-JX003839 (Masaya n= 33, Nicaragua n= 73, Xiloán= 31).
Control region sequences for Midas were downloaded from GenBank on October 30, 2009 (AY567132-AY567192, AY567194-AY567206, EF157332-EF157346-EF157395-EF157403, EF157347-EF157373, F157448-EF157476, EF157539-EF157547, EF219245; EF157327-EF157331, EF157374-EF157378, EF157485-EF157494, EF157573, GU017062-GU017090; Masaya n= 66, Nicaragua n= 98, Xiloán= 50). When multiple Midas species exist within lakes (Nicaragua: A. citrinellus, A. labiatus, Barlow and Munsey 1976; Xiloá: A. amarillo, A. sagittae, A. xiloaensis, Stauffer Jr. and McKaye 2002), they are genetically very similar (Elmer et al. 2009; Barluenga and Meyer 2010; Geiger et al. 2010) and exemplars of each species were combined for all analyses to be representative of intralacustrine diversity.
GENETIC DIVERSITY ANALYSES
Sequences were aligned in ClustalX (Thompson et al. 1997) and trimmed to a common length of 746 bp for Midas and 737 bp for moga. Genetic diversity statistics, haplotype sharing, and genetic differentiation were calculated in DnaSP version 5 (Librado and Rozas 2009). Gaps were not considered to be informative. Haplotype richness rarefied to a population size of 30 was calculated in Contrib version 1.02 (Petit et al. 1998) for each species. Median joining haplotype networks were calculated in Network version 4 (Fluxus Engineering). For Midas cichlids, haplotype loops were broken by eye based on clade inference from a maximum likelihood tree calculated in PAUP* version 4.0b10 (Swofford 2003) with likelihood setting approximating a K81 model of evolution, as inferred by AIC in Modeltest version 3.7 (Posada and Crandall 1998).
DIVERGENCE INFERENCE
We implemented the msBayes pipeline version 20081106 (Hickerson et al. 2007). The hyperparameters estimated include the possible number of colonization/divergence times (Ψ) per lake, the mean divergence (E(τ)) across Y taxon-pairs per lake, and the ratio of the variance and mean of the divergence time (Ω), which quantifies the variability in the possible Y divergence times (Hickerson et al. 2007). We estimated the transition-transversion rate ratio in Modeltest version 3.7 (Posada and Crandall 1998) as prior information for msBayes (Table S1). The upper and lower boundaries of the population mutation parameter θ were set to 0.5 and 40 as per the software recommendation (Hickerson et al. 2007). The number of possible colonization events (Ψ) for each taxon pair was estimated by 1000 random draws out of 1,000,000 HABC a priori simulations.
We estimated the timing of the colonization (t) by the equation t= T ×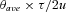 modified from (Hickerson et al. 2007) for mtDNA, where T is the generation time (one year per generation [McKaye et al. 2002, 2010]), θave is the average of the ancestral population mutation parameter, τ is the mode of the mean divergence time across taxa (i.e., E(τ)), and u is the mutation rate per gene (7.08% per site per million years Barluenga and Meyer 2004) multiplied by sequence length (737 bp). The per generation per gene DNA mutation rate (μ) is uniform across taxa. When we assume the mutation rate is universal between taxa for the simulations, we also assume the generation time is uniform. This mutation rate has been confirmed for Midas cichlids by a transcriptome-wide molecular clock (Elmer et al. 2010a) of approximately 1.25 × 10−6 per site per year, which was calibrated to the A. zaliosus mtDNA divergence time within Apoyo (Barluenga et al. 2006a) based on the Barluenga and Meyer (2004) rate. We then applied this transcriptome-wide mutation rate to the genetic divergence between two lake Xiloá Midas cichlids, which resulted in an interspecific divergence less than the age of the crater lake (K. R. Elmer et al., unpubl. ms.; Fan et al. 2012) and suggests that the Barluenga and Meyer (2004) rate is approximately correct and applicable to these recent divergences (despite the challenges of estimating mutation rates for recently diverged taxa, for example, see Ho et al. 2005).
modified from (Hickerson et al. 2007) for mtDNA, where T is the generation time (one year per generation [McKaye et al. 2002, 2010]), θave is the average of the ancestral population mutation parameter, τ is the mode of the mean divergence time across taxa (i.e., E(τ)), and u is the mutation rate per gene (7.08% per site per million years Barluenga and Meyer 2004) multiplied by sequence length (737 bp). The per generation per gene DNA mutation rate (μ) is uniform across taxa. When we assume the mutation rate is universal between taxa for the simulations, we also assume the generation time is uniform. This mutation rate has been confirmed for Midas cichlids by a transcriptome-wide molecular clock (Elmer et al. 2010a) of approximately 1.25 × 10−6 per site per year, which was calibrated to the A. zaliosus mtDNA divergence time within Apoyo (Barluenga et al. 2006a) based on the Barluenga and Meyer (2004) rate. We then applied this transcriptome-wide mutation rate to the genetic divergence between two lake Xiloá Midas cichlids, which resulted in an interspecific divergence less than the age of the crater lake (K. R. Elmer et al., unpubl. ms.; Fan et al. 2012) and suggests that the Barluenga and Meyer (2004) rate is approximately correct and applicable to these recent divergences (despite the challenges of estimating mutation rates for recently diverged taxa, for example, see Ho et al. 2005).
Results
GENETIC DIVERSITY AND DIFFERENTIATION
For Midas cichlids, haplotype richness and nucleotide diversity are all notably higher in Lake Nicaragua compared to the crater lakes (Fig. 2). Nucleotide diversity is higher in Masaya than Xiloá but haplotype richness is slightly higher in Xiloá. From a total of 72 haplotypes, eight haplotypes are private to Masaya, nine haplotypes are private to Xiloá, and three haplotypes are shared between the two crater lakes. FST between Nicaragua and Masaya is 0.0568 (χ2= 94.9 df = 61, P= 0.0035). FST between Nicaragua and Xiloá is 0.0408 (χ2= 78.7 df = 63, P= 0.087).
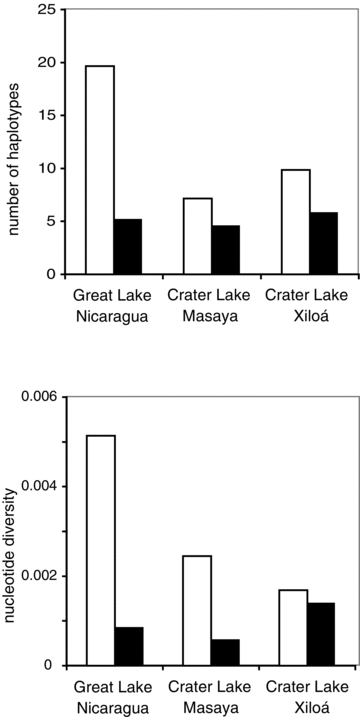
Rarefied haplotype richness (above) and nucleotide diversity (below) are higher for Midas cichlids (white bars) than for moga cichlids (black bars) in all lakes.
For moga cichlids, haplotype richness is highest in Xiloá and approximately equal in Masaya and Nicaragua (Fig. 2). Nucleotide diversity is higher in Xiloá than any other lake (Fig. 2). From a total of 20 haplotypes, four are private to Masaya, four are private to Xiloá, and two haplotypes are shared between crater lakes. FST between Nicaragua and Masaya is 0.0218 (χ2= 20.6 df = 15, P= 0.150). FST between Nicaragua and Xiloá is 0.0403 (χ2= 29.9 df = 16, P= 0.071).
Rarified haplotype richness and nucleotide diversity are always higher in Midas than in moga cichlids (Fig. 2), as are the number of variable sites and the transition-transversion rate ratio (Table S1). Haplotype networks also indicate the higher diversity (more haplotypes and more mutations) of Midas compared to moga cichlids (Fig. S1).
DIVERGENCE INFERENCE
Coalescence analyses in msBayes suggested a single, simultaneous colonization event (Ψ= 1) for both cichlid lineages from Lake Nicaragua into Masaya and Xiloá (Fig. 3). The ratio of the variance and mean of the divergence time (Ω) was approximately 0 for both crater lakes, which indicates simultaneous divergence of the two taxa (Hickerson et al. 2007). The mean divergence (E(τ)) for both species between Nicaragua and Masaya is 0.030 ± 0.0015, which corresponds to 5800 ± 300 years. For fishes in Xiloá, the mean divergence from Nicaragua is 0.028 ± 0.0039, which is equivalent to 5400 ± 750 years.
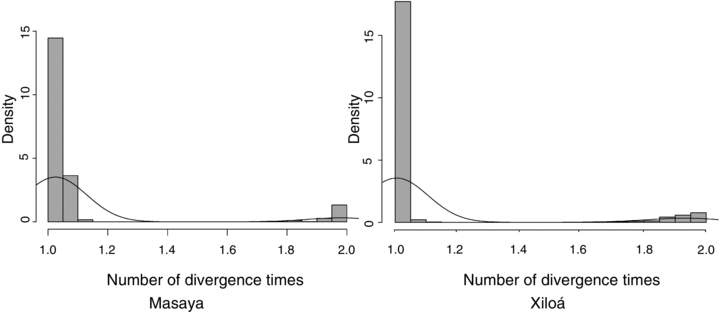
Posterior probability densities from msBayes analyses strongly favor a single (i.e., simultaneous) divergence for both taxa from great lake Nicaragua into each crater lake, Masaya and Xiloá. Model prior information gave equal weight to one (i.e., both lineages concurrently) versus two (i.e., Midas cichlids and moga cichlids separately) divergence events.
Discussion
We identified a high likelihood of single and simultaneous divergence of Midas cichlids and moga cichlids from ancestral lake Nicaragua into each of the crater lakes Masaya and Xiloá (Fig. 3). A single founding and subsequent expansion of Midas populations in these two lakes is supported by the results of previous distance-based mismatch analyses (Bunje et al. 2007; Barluenga and Meyer 2010). Thus, crater lake colonization by cichlid fishes seems to be a rare event (i.e., it has happened only once in a few thousand years). Alternatively, the first founding population may have become so well established that future colonizers (if they existed) left no discernable genetic contribution to the contemporary mtDNA signature.
Based on the combined coalescence analysis of both lineages, Midas and moga cichlids in crater lake Masaya diverged together from the ancestral great lake Nicaragua population 5800 ± 300 years ago and those in Xiloá diverged together from ancestral great lake Nicaragua's population 5400 ± 750 years ago. These divergence estimates can be considered to be approximately equal to crater lake colonization times, because both represent a discrete split of gene flow from the ancestral population. Because the colonizations for Masaya and Xiloá overlap considerably, from ca. 5500 to 6100 years ago, both lakes could have been colonized at the same time, although our analysis was not suitable to test such concurrence (msBayes currently only accommodates pairwise comparisons). For Midas cichlids, mismatch analyses have also identified colonization times that should be similar for Xiloá and Masaya (∼1 or 2 mutations ago) (Barluenga and Meyer 2010).
The concurrent colonization time estimates for the two cichlid lineages are likely explained by a single, nondiscerning natural event that affected both lineages of cichlids similarly, such as an exceptionally strong storm (or a wave of storms). Alternatively, it may be that Midas and moga cichlids colonized the crater lakes in different ways, although at approximately the same time.
How the fishes got in the crater lakes may also differ across localities. For Xiloá, it has been proposed that there was a partial or complete aquatic connection with great lake Managua, located only 700–1500 m away and lying at 11–15 m lower elevation (Villa 1976c) (Fig. 1). Managua's water level during the Pleistocene was 15–20 m higher than presently (Villa 1976b), but it is now known that this greatly predates the age of Xiloá (ca. 6.1 ky old; Kutterolf et al. 2007). In the era of Xiloá's formation, Managua's water level was only 9 m above present and it has been falling since (Cowan et al. 2002). Thus, it is possible that there was never a permanent aquatic connection between the two lakes but that storms or tsunamis (Freundt et al. 2006, 2007) carried great lake biota into Xiloá. Crater lake Masaya, in contrast, is more than 17 km from and 120 m above the nearest great lake (MacNeil et al. 2007) and has without doubt never had a connection with other bodies of water (Parello et al. 2008). We therefore suggest that a natural aerial introduction was most likely. Yet, ultimately any molecular or ecological analysis will only be able to determine when— not how—fishes colonized crater lakes.
Our finding suggests that crater lake Xiloá did not lie inhabited for long before it was colonized by cichlid fishes, because the colonization time (5400 ± 750 years ago) was only some hundreds of years less than, or even equal to, its geological age. Therefore, our molecular coalescent estimates concur with our present understanding of the Midas cichlid species flock in crater lake Xiloá being genetically isolated, endemic, and having likely speciated in situ (Elmer et al. 2009; Barluenga and Meyer 2010; Geiger et al. 2010). Crater lake Masaya is ca. 6 ky old, so again our divergence estimates for the cichlid populations therein (5800 ± 300 years ago) do not predate the age of the lake. However, Masaya has experienced dramatic volcanic activity even within the last 2 ky (Kutterolf et al. 2007; Pérez et al. 2009), and all young crater lakes are subject to boiling and off-gassing and remnant volcanic activity (MacNeil et al. 2007), making it debatable how hospitable an environment it has been for fish. It was proposed that this is why the Midas cichlid population in crater lake Apoyeque is only some hundreds of years old whereas the crater lake is ca. 1.8 ky old (Elmer et al. 2010c). Thus, the lag time between crater lake formation and its colonization by fish, as well as the occurrence of extinction cycles after lake formation, are likely to be strongly influenced by the physical properties of each crater lake. Limnological coring of crater lake floors could detect the first physical presence of fish and other biota and would be a direct method to address these questions in each specific case.
Genetic diversity differed notably between the two cichlid lineages in each crater lake and in the great lake. Haplotype richness and genetic diversity were always found to be lower in moga than Midas (Fig. 2). The difference is most striking in Lake Nicaragua, but this is unlikely to be attributed to cryptic Midas species; morphological (Barlow 1976; Klingenberg et al. 2003; Elmer et al. 2010b) and genetic analyses (Barluenga and Meyer 2004, 2010) have to date only identified two morphologically distinct, although genetically almost homogeneous, species. Crater lakes Masaya and Xiloá are thought to harbor undescribed Midas cichlid species, which may or may not be included in our study. Barluenga and Meyer (2010) also found haplotype diversity to be slightly higher in Xiloá, nucleotide diversity slightly higher in Masaya, and both estimates considerably higher in great Lake Nicaragua than the crater lakes. Nonetheless, our analyses found both crater lakes to be colonized by Midas and moga at the same time, which suggests that waves of admixture from multiple colonizations do not underlie the higher endemic species richness or the higher genetic diversity of Midas cichlids.
Intriguingly, the sophisticated HABC analysis clearly indicated that the two crater lakes were colonized around the same time period and that each crater lake was colonized simultaneously by the two cichlid lineages. In contrast, distance-based population genetic analyses could have led us to believe that moga colonized Masaya more recently than did Midas cichlids. Specifically, population differentiation between Nicaragua and Masaya is lower and nonsignificant for moga, suggestive of moga having a more recent cessation of gene flow with the ancestral population (Avise 2000). However, the inherent pitfall of distance methods is that they do not accommodate demographic and genetic variability across taxa. We are not aware of any difference between Midas and moga sexual dimorphism or dispersal ecology that would suggest a sexbias (reviewed in Avise 2000) to the mtDNA patterns observed.
Two issues with molecular evolution and demographics could affect our HABC-derived estimate of colonization time. For one, mitochondrial DNA can be subject to selective sweeps that distort contemporary signatures; thus we encourage future studies to test our current results using multiple, rapidly evolving nuclear DNA markers. However, such analyses are not currently possible within the comparative phylogeographic framework of the msBayes analysis. In support of our current dating, a study based on coalescence analysis of 14 microsatellite loci and mtDNA using a single lineage (i.e., noncomparative) statistical methodology inferred a divergence time for Midas cichlids in Xiloá that overlaps with our estimates here (5700 years ago; minimum and maximum 95% highest posterior density interval 3400–8500 from three independent runs, A. Kautt et al., unpubl. ms.). Second, we have applied a uniform generation time for Midas and moga of one year. We think this is justified because Midas and moga cichlids have a unimodal annual breeding peak (McKaye et al. 2002, 2010) and, although the age of first reproduction in the wild is not documented, in laboratory conditions both these species begin breeding before their first year (A. Meyer, pers. obs.). Increasing the average generation time across both species would commensurately increase the divergence time estimation; further empirical research on life history for both species is needed. However, the results of the coalescent analysis will not change: mean divergence across taxa (i.e., E(τ)) will remain the same and, most importantly, the divergence events remain simultaneous (Ω still ∼0).
It is unlikely that the disparity in extant genetic diversity between Midas and moga cichlids is due to a considerable difference in abundance. Moga is one of the most abundant cichlids in Xiloá (McKaye et al. 2010; T. Lehtonen, pers. obs.). Its abundance in Masaya has not been formally documented but our net samples suggest that the species is moderately, if not very, abundant also in that lake (T. Lehtonen, A. Meyer, pers. obs.). Midas cichlids are abundant in both crater lakes (Barlow and Munsey 1976; K. Elmer, T. Lehtonen, A. Meyer, pers. obs.). In great lake Nicaragua, in turn, the Midas cichlid lineage seems to be more common of the two: it represents 12.5% of the biomass at its most abundant habitat depth, whereas moga represents 0.6% at its most abundant depth (Koenig et al. 1976). Regardless, the coalescent analyses we employed implicitly accommodate unequal demographic histories (Hickerson et al. 2006, 2007) for our divergence estimates.
Based on patterns of species richness within crater lakes, it has been argued in the past that there is something “special” about Midas cichlids that makes them exceptionally prone to diversification (Elmer et al. 2010b). Our current findings confirm that Midas cichlids are particularly genetically diverse in the ancestral and derived populations relative to other sympatric cichlids. Furthermore, the greater species richness and propensity to sympatric speciation (Barluenga et al. 2006a; Elmer et al. 2009) of Midas cichlids compared to more depauperate cichlid lineages is not due to either a greater number of colonization events nor a longer time to speciate within crater lakes. This suggests that future studies can rightly focus on searching for fundamental ecological or genetic characteristics of Midas cichlids that underlie their tendency for rapid ecological speciation in sympatry.
Associate Editor: G. Mayer
ACKNOWLEDGMENTS
We thank H. Recknagel, E. Hespeler, J. Brasseit, and M. Barluenga for help in collecting sequence data, and M. Barluenga and W. Salzburger for moga samples from 2003 and 2005. Thanks to M. Hickerson and W. Huang for help with msBayes. This research was funded by Alexander von Humboldt (TKL, KRE) and NSERC (KRE) fellowships, the Department of Biology at the University of Turku (TKL), a University of Konstanz Young Scholar's Award (KRE), and various grants of the DFG (AM). We thank the Associate Editor and two anonymous reviewers for comments that improved the manuscript.




