MALE-SPECIFIC GENOTYPE BY ENVIRONMENT INTERACTIONS INFLUENCE VIABILITY SELECTION ACTING ON A SEXUALLY SELECTED INVERSION SYSTEM IN THE SEAWEED FLY, COELOPA FRIGIDA
Abstract
In the seaweed fly, Coelopa frigida, a large chromosomal inversion system is affected by sexual selection and viability selection. However, our understanding of the interaction between these two selective forces is currently limited as research has focused upon a limited range of environments. We allowed C. frigida larvae to develop in two different algae, Fucus and Laminaria, and then measured viability and body size for each inversion genotype. Significant male-specific genotype-by-environment interactions influenced viability and body size. For males developing in Laminaria, the direction of viability selection acts similarly on the inversion system as the direction of sexual selection. In contrast, for males developing in Fucus, viability selection opposes sexual selection. These results demonstrate that through considering viability selection in different environments, the costs and benefits associated with sexual selection can be found to vary.
Sexual selection and viability selection can often be found to act in opposing directions. Classically, the exaggeration of secondary sexual ornaments by sexual selection is held in check when viability becomes sufficiently compromised (Andersson 1994). In contrast, it is also proposed that sexual selection can act in concert with viability selection (Darwin 1859), for example, to promote local adaptation (van Doorn et al. 2009), to increase the spread of beneficial alleles within a population (Whitlock 2000; Proulx 2001, 2002; Lorch et al. 2003), or to help purge deleterious alleles (Whitlock and Agrawal 2009).
A key challenge for evolutionary biologists is to understand the interaction between sexual selection and viability selection. For example, sexual selection was not found to increase the rate of adaptation of the fruit fly, Drosophila melanogaster, to either thermal stress (Holland 2002) or a novel food source (Rundle et al. 2006). In contrast, more recent work using the same species demonstrated rapid elimination of a deleterious allele under sexual selection (Hollis et al. 2009). Such diverse results highlight that the interaction between sexual selection and viability selection is not fixed, either within or between species. Apparently, conflicting results in fact prompt a more interesting question—under what conditions will sexual selection either assist or oppose viability selection? In this study, we show that, within a single species, sexual selection can assist viability selection in one environment yet oppose viability selection in another.
Variation in the fitness of sexually selected genotypes in different environments, known as genotype by environment interactions (G×Es), can contribute to our understanding of the relationship between sexual selection and viability selection (Ingleby et al. 2010). For example, in the lesser wax moth, Achroia grisella, female choice leads to the production of higher quality offspring under favorable conditions, but lower quality offspring under unfavorable conditions (Jia and Greenfield 1997). The seaweed fly, Coelopa frigida, is an ideal species to study G×Es as both viability and sexually selected fitness can be attributed to a large chromosomal inversion system (Butlin et al. 1982a,b).
Coelopa frigida are dependent, as adults and larvae, upon decomposing marine algae washed up on beaches (Dobson 1974). The C. frigida mating system is characterized by a sexual conflict over mating frequency as males harass females intensely to coerce them into mating (Blyth and Gilburn 2006). Male ability to overcome female resistance increases with body size, hence sexual selection favors larger males (Butlin et al. 1982b; Crean and Gilburn 1998). A large inversion system is a major determinant of male body size and occurs in two forms, α and β (Butlin et al. 1982b; Day et al. 1982). αα-homokaryotypes are the largest, ββ-homokaryotypes are smallest, and heterokaryotypes are of intermediate size (Day et al. 1980; Butlin et al. 1982b). Sexual selection for large male size therefore exerts significant selection for male inversion genotype (αα > αβ > ββ; Butlin et al. 1982b).
The inversion system also influences egg to adult viability, which is characterized by strong heterokaryotype advantage, or “heterosis” (Butlin et al. 1982a). Consequently, sexual selection and viability selection can act in different directions, thus maintaining multiple forms of the inversion (Butlin et al. 1982a). αα females are predicted to experience greater costs of this mating bias, as they are less likely to produce heterokaryotype offspring when mating with larger males. However, these conclusions are limited as previous studies have not considered variation in viability selection among different environments.
A principal source of environmental variation is the algae present in wrack beds. Two genera, Fucus and Laminaria, are commonly found in varying proportions, yet the majority of studies have used only Fucus in experiments and for laboratory culture. However, it has been found that females will oviposit more readily (Phillips et al. 1995) and males increase mating effort (Edward and Gilburn 2007) when exposed to Laminaria. The objective of this study is to measure viability selection on the genetic inversion system when C. frigida larvae develop in either Fucus or Laminaria. We also compare the size of adults as a plastic response to developing on either Fucus or Laminaria.
Methods
LABORATORY CULTURES
All aspects of the study were carried out in a controlled temperature room set at 25°C on a 12:12 light:dark cycle. Five hundred male and 500 female C. frigida were collected as larvae from Roome Bay, Crail, Fife, UK and reared in the laboratory in large culture boxes (27 × 19 × 36 cm) with an excess of minced Laminaria and Fucus in equal proportion. As it is currently not possible to identify the inversion genotype of C. frigida before eclosion as an adult, cultures were established that had the same, but unknown, initial proportions of each inversion genotype at the same larval density. To achieve this, the following procedure was adapted from Leggett et al. (1996).
Male flies were placed into a large culture box that contained a thin layer, approx 1-cm deep, of coarsely minced Laminaria. Twenty-four hours later, the female flies were introduced to the box containing males and left for 16 h to mate and oviposit. A 16-h oviposition period was chosen to maximize egg yield while minimizing the number of eggs that hatched before they could be collected. Laminaria was used as this alga is known to stimulate both male mating behavior (Edward and Gilburn 2007) and female oviposition rate (Phillips et al., 1995) more than Fucus. After the 16-h period of mating and oviposition, all flies were removed. Eggs were collected from the algae by shaking in 3% saline solution and straining through a mesh. Further saline solution was then added to reach a total volume of 1500 mL.
To estimate the concentration of viable eggs in this solution, 150 mL was filtered onto pieces of black cotton cloth so that eggs were evenly distributed. This cloth was then placed inside a culture box on a layer of damp tissue paper to allow eggs to hatch. After 48 h, 1138 hatched eggs were counted, thus estimating the concentration of viable eggs to be 7.59 eggs per mL. Concurrent with estimating the concentration of eggs, further 75-mL aliquots of the solution (each containing approximately 569 eggs) were filtered onto pieces of the same cloth and placed into small culture boxes (13 × 13 × 14 cm) that each contained 200 g of coarsely minced algae; either Laminaria (N= 4) or Fucus (N= 4). This generated a starting density of approximately 2.85 eggs per gram of algae in each box. After 48 h, the pieces of cloth were removed. This procedure ensured that each culture box was seeded with an equal and unbiased number of larvae. Furthermore, this procedure minimized any bias between cultures in the initial starting frequencies of each inversion genotype. Cultures were checked daily to ensure sufficient algal resources for development. Additional algae were added to all culture boxes in equal measure if larvae were observed crawling up the side of boxes. Consequently, a further 150 g of algae was added to each culture during development, giving a final density of 1.63 larvae per gram of algae.
All newly eclosed flies were removed from cultures twice daily at intervals of no more than 16 h to ensure virginity and prevent a second generation. This continued until no further adult flies emerged for 48 h. All flies were placed at –20°C and body size was estimated by measuring wing length and body mass.
DETERMINING INVERSION GENOTYPE
A subset of five male and five female flies was selected at random from each culture box on each day that flies eclosed. This resulted in a sampling bias because a higher proportion of flies was genotyped on days when fewer flies eclosed. We therefore applied a transformation to scale the genotype frequencies for each sex and on each alga according to the number of flies that eclosed each day. For each day, the proportion of each genotype found in the sample was multiplied by the total number of individuals collected that day. This estimated the total number of individuals belonging to each genotype on that day. We then summed these genotype frequencies across all days to determine the frequency of each genotype in the entire population. Finally, we multiplied these values by the proportion of all flies that had been successfully genotyped. This final step ensured that the sample size following this transformation was equal to the number of flies that were genotyped, hence we did not artificially increase the sample size.
Inversion genotype was inferred through linkage disequilibrium with allozymes of alcohol dehydrogenase (Adh) that are visible using starch gel electrophoresis (Day et al. 1980, 1982). Starch gels were prepared as 12% w/v starch in a 1:1 mix of TEB buffer (0.17M tris (hydroxymethyl) aminomethane, 0.002M ethylenediaminetetraacetic acid (EDTA), 0.05M boric acid) and water. Flies were prepared by homogenizing in the well of a spotting tile with a drop of water and a few grains of carborundum powder. The homogenate was soaked into circles of filter paper (6 mm diameter), which were then inserted into wells cut in the gel surface. An electrical current (300 V/70 mA) was applied to the gel for 90 min at 4°C. Enzyme activity was visualized by slicing gels in half along the horizontal axis and applying an agar overlay stain (10 mL 0.1 M Tris-HCl buffer (pH 8.6), 6 mL propanol, 1.5 mL 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (10 mg mL−1), 1 mL phenazine methosulphate (2 mg mL−1), 10 mL nicotinamide-adenine dinucleotide (1.5 mg mL−1), 20 mL bacteriological agar (2% w/v)). Stained gels were incubated in the dark at 37°C for a minimum of 30 min or until bands could be scored.
STATISTICAL ANALYSIS
The day that females were introduced to males in the large culture box used for oviposition was defined as day 0 so that development times include the period from mating to eclosion. Larval to adult viability, defined as the number of adult flies that eclosed from each culture box, was compared between algae in a Wilcoxon rank sum test. Development times were compared using a Cox's regression with sex, culture alga, and culture box (nested within culture alga) as fixed effects.
The frequencies of each inversion genotype were compared in multinomial logistic regression models with sex, culture alga, and culture box (nested in culture alga) as fixed effects. To illustrate the direction of significant sex by environment interactions in this model, additional multinomial logistic regression models were compared for each sex with culture alga and culture box (nested in culture alga) as fixed effects.
Differences in body size, estimated through wing length and body mass, were compared in general linear models with sex, culture alga, inversion genotype, and culture box (nested within culture alga) as fixed effects. Because of a significant three-way interaction between sex, alga, and genotype, we also compared differences in body size in separate models for each sex with culture alga, inversion genotype, and culture box (nested within culture alga) as fixed effects.
All statistical models were initially constructed to include each main effect and all possible interactions. Models were subsequently simplified by stepwise removal of the highest order nonsignificant factor until the minimum adequate model was reached (Crawley 2005). At each step, the current and reduced models were compared using a likelihood-ratio test. Factors were removed only where the change did not significantly influence the fit of the model at P= 0.05. The significance of individual factors was determined as the effect of removal upon fit of the minimal adequate model using a likelihood-ratio test. As model simplification may increase the chance of committing Type 1 error (Forstmeier and Schielzeth 2011), full models, prior to simplification, are included in the Supporting information (Table S1). All analyses were conducted in R version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria). Multinomial logistic regression models were fitted using the nnet package (Venables and Ripley 2002).
Results
SUMMARY OF CULTURES
A total of 1904 adult flies were collected from all the culture boxes, hence larval to adult survival rates averaged 41.8%. Of these, 422 were electrophoresed to determine inversion genotype. Fifty two of these flies possessed an allozyme that is found on both forms of the inversion system, hence inversion genotype cannot be determined (Day and Buckley 1980), and the bands of a further 34 flies could not be accurately scored. Flies whose genotypes could not be determined were not significantly biased according to size (t408= 0.396, P= 0.692), culture alga (χ21= 0.058, P= 0.810), or sex (χ21= 2.442, P= 0.118). These flies were excluded from further analyses.
PRODUCTIVITY OF LAMINARIA AND FUCUS CULTURES
The total number of flies that eclosed from each of the Laminaria culture boxes was higher than the total number that eclosed from any of the Fucus culture boxes (NLam= 323, 262, 257, and 254, NFuc= 244, 213, 209, and 142). Consequently, larval to adult viability was greater in Laminaria than Fucus (Proportion surviving on Laminaria= 0.48; Proportion surviving on Fucus= 0.36; Risk Ratio = 1.36; U= 0, P= 0.029). Once all flies had eclosed, sex ratios did not significantly differ from 50:50 (Binomial test: N= 1904, P= 0.347) and this was unaffected by culture alga (χ21= 0.031, P= 0.859) or culture box (χ27= 10.569, P= 0.159). Female flies and flies cultured on Fucus had the shortest development times (Sex: χ21= 477.16, P < 0.001, HR = 2.96; Algae: χ21= 546.11, P < 0.001, HR = 3.48). The influence of culture alga on development time was the same for both sexes (χ21= 0.181, P= 0.671, HR = 0.96). The effect of culture alga on development time was consistent across all culture boxes as the median development times of flies from Laminaria cultures were all longer than the median development times of flies from any of the Fucus cultures (Lam: 11,11,12, and 12 days; Fucus: 9, 10, 10, and 10 days).
INVERSION GENOTYPE
There was a highly significant sex-specific effect of culture alga that influenced the proportion of each inversion genotype that survived as adults. Although there was no difference in the effect of each culture alga on the viability success of each female inversion genotype, this factor did significantly affect the success of male inversion genotypes (Table 1, Fig. 1). A greater number of heterokaryotype females, than either of the homokaryotypes, were found irrespective of the culture alga. Likewise, when males were cultured on Fucus, there was a greater number of heterokaryotypes than either homokaryotype. In contrast, when males were cultured on Laminaria, there was a greater number of αα homokaryotypes (Fig. 1). This sex-specific effect of culture alga was consistent across all culture boxes. In all four Laminaria cultures, the proportions of each inversion genotype were in the order αα > αβ > ββ. In all four Fucus culture boxes, the proportions of each inversion genotype were in the order αβ > αα > ββ. Furthermore, the proportion of αα males that eclosed from each of the Laminaria culture boxes always exceeded the proportion of αα males that eclosed from any of the Fucus boxes.
| Model | Model term | Significance of term | Males | ||
|---|---|---|---|---|---|
| Males and females | Sex | χ22=11.53, P=0.003 | Fucus | Laminaria | |
| Culture alga | χ22=13.00, P=0.001 | 0.51 | 2.03 | ||
| Sex×alga | χ22=15.55, P<0.001 | αα/ββ | 1.50 | 2.74 | |
| Culture box | χ212=25.96, P=0.011 | 2.93 | 1.35 | ||
| Sex×box | χ212=10.16, P=0.602 | Females | |||
| Males | Culture alga | χ22=28.18, P<0.001 | Fucus | Laminaria | |
| Culture box | χ212=9.738, P=0.639 | αα/αβ | 0.52 | 0.50 | |
| Females | Culture alga | χ22=0.269, P=0.874 | 0.75 | 0.85 | |
| Culture box | χ212=26.38, P=0.009 | αβ/ββ | 1.44 | 1.69 | |
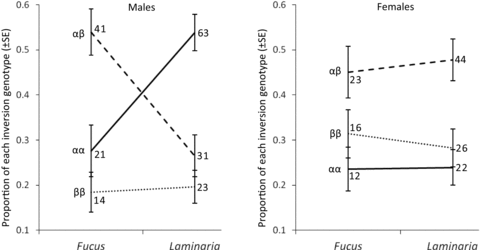
Reaction norm plots showing the proportion of each inversion genotype that eclosed from cultures of Laminaria and Fucus for males (left panel) and females (right panel). Data shown are the proportion and SE of a proportion and are derived from pooling all individuals across replicate boxes. Values to the right of each datapoint are the total number of individuals in each group.
BODY SIZE
Body size was determined by numerous main effects and interactions that included a significant sex by genotype by algal environment interaction for both wing length and body mass. The complexity of these effects is more easily interpreted in the sex-specific models (Table 2). Female body size was influenced by culture alga and genotype, but this influence was small and for culture alga only significant when body size was estimated through body mass and not wing length (Fig. 2). There was no significant interaction between genotype and culture alga that influenced female body size when estimated through either wing length or body mass. In contrast, inversion genotype and culture algae were much more influential in determining male body size (Fig. 2; Table 2). An interaction between genotype and culture algae was found to influence male body size. This male specific G×E did not influence the rank order of male body sizes according to genotype, instead in a Laminaria environment the effect of the inversion system to influence male size was increased.
| Model | Term | Wing length | Body mass | ||
|---|---|---|---|---|---|
| Significance of term | ω2 | Significance of term | ω2 | ||
| Males and females | Sex | χ21=178.2, P<0.001 | 0.367 | χ21=128.1, P<0.001 | 0.287 |
| Alga | χ21=23.69, P<0.001 | 0.035 | χ21=34.21, P<0.001 | 0.061 | |
| Genotype | χ22=175.9, P<0.001 | 0.246 | χ22=156.7, P<0.001 | 0.240 | |
| Sex×alga | χ21=14.29, P<0.001 | 0.011 | χ21=12.27, P<0.001 | 0.015 | |
| Sex×genotype | χ22=156.7, P<0.001 | 0.126 | χ22=123.0, P<0.001 | 0.117 | |
| Algae×genotype | χ22=3.828, P=0.148 | 0.001 | χ22=6.376, P=0.041 | 0.003 | |
| Sex×alga×genotype | χ22=6.056, P=0.048 | 0.002 | χ22=13.80, P=0.001 | 0.009 | |
| Culture box | χ26=11.36, P=0.078 | 0.004 | χ26=11.38, P=0.077 | 0.005 | |
| Culture box×sex | χ26=1.229, P=0.975 | 0.000 | χ26=4.733, P=0.579 | 0.000 | |
| Culture box×genotype | χ212=16.20, P=0.182 | 0.002 | χ212=19.26, P=0.082 | 0.005 | |
| Males | Alga | χ21=28.67, P<0.001 | 0.096 | χ21=30.65, P<0.001 | 0.128 |
| Genotype | χ22=227.6, P<0.001 | 0.629 | χ22=175.0, P<0.001 | 0.513 | |
| Alga×genotype | χ22=7.527, P=0.023 | 0.008 | χ22=12.60, P=0.002 | 0.018 | |
| Culture box | χ26=9.750, P=0.136 | 0.005 | χ26=9.171, P=0.164 | 0.005 | |
| Culture box×genotype | χ212=17.52, P=0.131 | 0.005 | χ212=14.31, P=0.281 | 0.002 | |
| Females | Alga | χ21=0.379, P=0.538 | 0.000 | χ21=4.789, P=0.029 | 0.027 |
| Genotype | χ22=7.533, P=0.023 | 0.039 | χ22=9.340, P=0.009 | 0.048 | |
| Alga×genotype | χ22=0.217, P=0.897 | 0.000 | χ22=3.806, P=0.149 | 0.011 | |
| Culture box | χ26=3.640, P=0.725 | 0.000 | χ26=10.41, P=0.108 | 0.024 | |
| Culture box×genotype | χ212=16.66, P=0.163 | 0.018 | χ212=18.09, P=0.113 | 0.024 | |
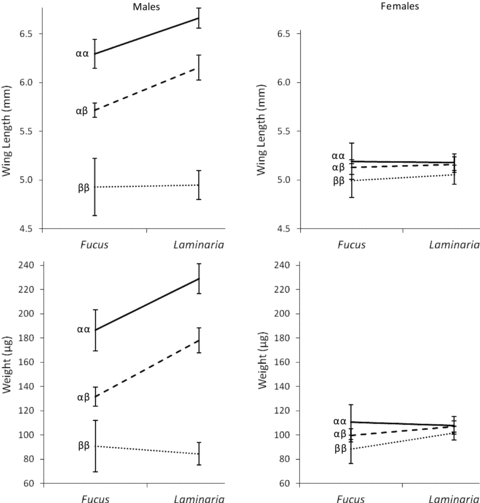
Reaction norm plots showing the wing length and body mass of males (left panel) and females (right panel) of each inversion genotype following development in Laminaria or Fucus. Data shown are the mean and 95% confidence intervals and are derived from pooling all individuals across replicate boxes.
Discussion
In this study, we found significant fitness benefits for C. frigida of development in Laminaria compared to Fucus. Larval to adult viability was greater and body size was greater when larvae developed in Laminaria. However, these benefits appear to be at the expense of a longer development time. The potential benefits of larval development in Laminaria help to explain previous reports of increased female oviposition and male reproductive effort within this environment (Phillips et al. 1995; Edward and Gilburn 2007). Results also demonstrate two significant male-specific G×Es. The first G×E is a difference in male viability among inversion genotypes during development in each alga (Fig. 1). The second G×E is a difference in the influence that the inversion system has in determining male body size during development in each alga (Fig. 2). Both of these G×Es are of interest because both inversion genotype and male body size are already known to be targets of sexual selection.
The proportions of each female genotype were approximately the same following development in either alga. Heterokaryotype females were most abundant, followed by ββ, and then αα genotypes (Fig. 1). This indicates that viability selection for female genotype was the same on each alga. However, a male-specific G×E is identified because the proportion of male genotypes differed following development in either alga. Following development in Fucus, heterokaryotype males were most abundant, followed by αα, and then ββ genotypes (Fig. 1). In contrast, following development in Laminaria, αα-homokaryotype males were most abundant, followed by heterokaryotype, and then ββ genotypes (Fig. 1). Because the starting proportion of each genotype was the same on both algae, this shows that viability selection for male genotype differed in each environment. This G×E is important because it influences the relationship between viability selection and sexual selection. Relative to a Fucus environment, αα-homokaryotypes are favored by viability selection in a Laminaria environment. Thus, the direction of viability selection and sexual selection, which is also known to favor larger αα-homokaryotype males, is more similar in a Laminaria than a Fucus environment. In contrast, greater selection for heterokaryotypes in a Fucus environment, relative to Laminaria, is more likely to conflict with the direction of sexual selection.
The different relationship that is predicted between viability selection and sexual selection in each of these environments is central to considering how this species will adapt to each environment. Sexual selection is more likely to aid adaptation to a Laminaria environment as a mating bias toward larger males that increases the likelihood of producing αα offspring will be less costly to females. This is because αα males are more likely to survive in this environment when compared to a Fucus environment. In contrast, sexual selection is more likely to hinder adaptation to a Fucus environment as heterokaryotypes of both sexes are known to prosper, when compared to development in Laminaria. A further consideration is that in a Laminaria environment, as both sexual and viability selections are more likely to favor αα males, the β form of the inversion could be lost completely. Nevertheless, heterokaryotype females are still the most abundant genotype in both environments. This means that the optimal inversion genotype, according to the combined effects of viability selection and sexual selection, will differ for each sex in each environment. There is currently no evidence for sex linkage of the inversion system (Day et al. 1982), however we would predict that sex linkage is more likely to evolve in environments that are dominated by Laminaria, than Fucus.
This G×E has further implications for predicting the sexual conflict load of male harassment for females with different inversion genotypes. It was previously thought that αα females experience the greatest conflict load as they are less likely to produce heterokaryotype offspring when mating with larger males that are likely to share the same genotype. This is in contrast to ββ females that were predicted to indirectly benefit from mating with larger males through the greater probability of producing more viable heterokaryotype offspring. However, these predictions were based upon an assumption of viability selection favoring heterokaryotypes, which we now demonstrate as being environmentally dependent. Instead, the conflict load for αα females is expected to be lower in a Laminaria environment, than a Fucus environment, as αα male offspring are more likely to survive. This environmental variation in conflict load could explain why the resistance of αα females can vary between populations from a mating bias toward large males (Gilburn et al. 1992, 1993) to a mating bias toward small males (Gilburn et al. 1993, 1996; Gilburn and Day 1994; Day and Gilburn 1997; Blyth and Gilburn 2011).
The second G×E to be discussed is variation in the degree of influence the inversion system has on male body size during development in each alga (Fig. 2). Even though the reaction norms do not cross in this instance, that is, the rank order of male size across genotypes is unaltered, this still constitutes a G×E because the reaction norms are nonparallel (Ingleby et al. 2010). The effect of this G×E is that the range of male body size across the three inversion genotypes is greater when males develop in Laminaria than when they develop in Fucus. This will influence the likelihood that female resistance will bias matings in favor of a particular male genotype, because male size is a better predictor of genotype in Laminaria. In essence, whenever a genotype has greater influence upon a phenotype, any selection acting upon that phenotype is more likely to influence those genes. In many respects, this is analogous to the finding that G×Es can influence the reliability of sexually selected traits to signal potential fitness benefits of mating (Greenfield and Rodriguez 2004; Higginson and Reader 2009). In C. frigida, this is important because, following development in Laminaria, αα males are more likely to be larger than other males, hence more likely to succeed at overcoming female resistance and will be more likely to mate. The sexual selection differential is therefore predicted to be greater following development in Laminaria.
In this study, differences in male viability selection and adult size have been identified that predict alternate relationships between viability selection and sexual selection following development in different environments. This example is not expected to be unique and reiterates the need for further investigation of G×Es in relation to sexual selection (Ingleby et al. 2010). It is evident from many studies that the relationship between sexual selection and viability selection is not a fixed property. Future work would therefore benefit our understanding of sexual selection most by trying to understand the conditions and circumstances that can influence this relationship.
Associate Editor: E. Morrow
ACKNOWLEDGMENTS
This work was funded by Natural Environment Research Council Ph.D. studentship to DAE. We are grateful to L. Bussiere, P. Edelaar, and two anonymous reviewers whose comments greatly improved the manuscript.




