DEVELOPMENTAL BASIS OF TOOTHLESSNESS IN TURTLES: INSIGHT INTO CONVERGENT EVOLUTION OF VERTEBRATE MORPHOLOGY
Abstract
The tooth is a major component of the vertebrate feeding apparatus and plays a crucial role in species survival, thus subjecting tooth developmental programs to strong selective constraints. However, irrespective of their functional importance, teeth have been lost in multiple lineages of tetrapod vertebrates independently. To understand both the generality and the diversity of developmental mechanisms that cause tooth agenesis in tetrapods, we investigated expression patterns of a series of tooth developmental genes in the lower jaw of toothless turtles and compared them to that of toothed crocodiles and the chicken as a representative of toothless modern birds. In turtle embryos, we found impairment of Shh signaling in the oral epithelium and early-stage arrest of odontoblast development caused by termination of Msx2 expression in the dental mesenchyme. Our data indicate that such changes underlie tooth agenesis in turtles and suggest that the mechanism that leads to early-stage odontogenic arrest differs between birds and turtles. Our results demonstrate that the cellular and molecular mechanisms that regulate early-stage arrest of tooth development are diverse in tetrapod lineages, and odontogenic developmental programs may respond to changes in upstream molecules similarly thereby evolving convergently with feeding morphology.
As a major component of the vertebrate feeding apparatus, teeth play a crucial role in species survival. The developmental programs for teeth have been subjected to strong selective constraints since their appearance in the oral cavity over 400 million years ago (Ma). Although the basic structure, that is, a pulp cavity surrounded by a dentine crown covered by enamel or enameloid, is generally conserved, teeth diversified in their location, size, shape, ornamentation, mode of attachment, and number of generations in the course of vertebrate evolution, thereby permitting vertebrate lineages to adapt to a variety of diets (Davit-Béal et al. 2009). However, irrespective of their importance for animal survival, teeth have been lost in multiple lineages of the tetrapods independently (Davit-Béal et al. 2009). The most familiar example is modern birds (Neorthines) whose common ancestor lost their teeth and acquired a horny beak (rhamphotheca) approximately 100 Ma (Louchart and Viriot 2011) (Fig. 1). Embryonic data from the chicken (Gallus gallus) suggest three types of developmental events that lead to the reduction and/or loss of teeth in birds: inactivation of odontogenic genes, a shift in epithelial-mesenchymal interactions, and diversion of gene function (Louchart and Viriot 2011). However, the cellular and molecular mechanisms underlying the loss of teeth in nonavian tetrapod lineages remain unknown.
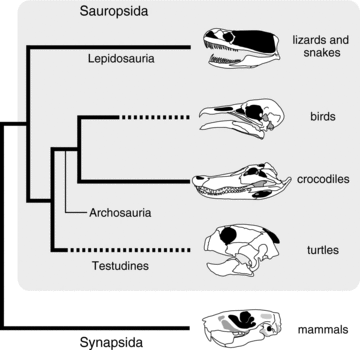
Simplified amniote phylogeny that highlights independent tooth loss in birds and turtles (dashed line). This phylogeny represents recent molecular phylogenetics studies that support an archosaurian affinity of turtles.
Turtles (Testudines), which have acquired a unique body plan represented by their shell (carapace and plastron) (Nagashima et al. 2009; Kuratani et al. 2011), and which have succeeded to exploit a variety of ecological niches from marine to terrestrial (Bonin et al. 2006), also lack teeth (Davit-Béal et al. 2009) (Fig. 1). Although the phylogenetic position of turtles within “reptiles” is still debated (see Werneburg and Sánchez-Villagra 2009; Lyson et al. 2010; Kuratani et al. 2011; Lyson et al. 2012), there is no doubt on that they were derived from an ancestor with teeth. Supporting this view, the fossil of the oldest turtle species (Odontochelys semitestacea), which was recently discovered from the Late Triassic (220 Ma) in China, had small and peg-like, pointed teeth on both upper and lower jaws (Li et al. 2008). Interestingly, the second oldest known turtle, Proganochelys quenstedti, from a Late Triassic formation (220 Ma) in Germany possessed no marginal teeth, possessing only conical teeth on the vomers, palatines, and pterygoids (Gaffney 1990). Therefore, it is possible that the oral teeth of turtles were lost in a relatively short timescale, and this is in stark contrast to the process of dentition loss in birds where various intermediate conditions of tooth reduction are observed in fossil taxa (Louchart and Viriot 2011).
In this paper, we reveal potential cellular and molecular mechanisms that underlie toothlessness in turtles through the comparative analysis of gene expression patterns in the developing lower jaw. We then compare these data to two archosaurian lineages with or without teeth: crocodiles and birds that are considered closely related to turtles (Hedges and Poling 1999; Kumazawa and Nishida 1999; Iwabe et al. 2005; Shedlock et al. 2007; Shen et al. 2011; Tzika et al. 2011; Crawford et al. 2012), to understand both the generality and the diversity of developmental mechanisms that cause tooth loss (edentulism) in vertebrates, and ultimately test whether edentulism in turtles was brought about by changes of developmental programs different from those seen in birds. Our results demonstrate that tooth agenesis in turtles is the result of impairment of Shh signaling in oral epithelium and early-stage arrest of odontoblast development caused by termination of Msx2 expression in the dental mesenchyme, implying that the developmental program underlying tooth loss is diverse in tetrapods. Our study provides an opportunity to address alteration of gene expression patterns that regulates cellular differentiation and generates convergent evolution of similar cranial morphology in vertebrates.
Methods
SAMPLE COLLECTION AND STAGING OF EMBRYOS
Fertilized eggs of the Chinese softshelled turtle, Pelodiscus sinensis, were purchased commercially from a local breeder in Japan. Fertilized eggs of the Siamese crocodile, Crocodylus siamensis, were provided by a local breeder in Thailand. Embryonic stages of P. sinensis were determined according to Tokita and Kuratani (2001). Because there is currently no embryonic staging system for C. siamensis, we instead used the staging table for Alligator mississippiensis (Ferguson 1985). All animal experiments were approved by the University of Tsukuba Committee for Animal Care.
MOLECULAR CLONING
Total RNA was extracted from embryos using ISOGEN reagent (Nippon Gene Co., Ltd., Tokyo, Japan). We used RT-PCR to amplify fragments of Pelodiscus Col1a1, Dlx2, Dlx5, Patched1, Pitx2 and Crocodylus Bmp4, Col1a1, Fgf8, Msx2, Patched1, Pax9, Pitx2, Shh messenger RNA (primer sequences used for isolation of the fragments of these genes are available upon request). Because Bmp4, Fgf8, Msx1, Msx2, Pax9, and Shh of P. sinensis were already sequenced and sequence data were deposited in the database, we isolated the orthologous fragments by RT–PCR with primers constructed by referring to the reported sequence data. The fragments were isolated using the pGEM T-easy vector systems (Promega, Madison, WI) or TOPO® TA cloning kit (Invitrogen) and sequenced using an ABI 3130 sequencer (Applied Biosystems). To identify the orthologous genes of the isolated fragments, comparable sequence data were surveyed using a BLAST search, and phylogenetic trees with neighbor joining method were constructed after sequence alignment using the CLUSTALX software.
GENE EXPRESSION ANALYSIS
Embryos were fixed in 4% PFA, dehydrated using a graded methanol series, placed in xylene, embedded in paraffin, and sectioned on a microtome. Serial sections were hybridized with digoxigenin-labeled riboprobes as described in Neubüser et al. (1995) with slight modifications. To identify the expression domain of Msx1 in crocodile tissues, we hybridized tissues with a chicken Msx1 antisense riboprobe. To identify expression domain of Dlx2 and Dlx5 in crocodile tissues, we hybridized tissues with antisense riboprobes for Pelodiscus orthologues. Generally, heterospecific RNA probes easily hybridize among reptilian (including birds) lineages (Harris et al. 2006). To confirm expression pattern of each gene in the tissue of the lower jaw, at least three individuals representing each embryonic stage were sampled for analysis. For interspecific comparison of general histology of the lower jaw, hematoxylin and eosin (H & E) or Milligan's Trichrome staining was performed following standard protocols.
Results
We first describe the process of teeth development and expression pattern of tooth developmental genes in the lower jaw of crocodile embryos. We then present data on the development of the lower jaw in turtle embryos without teeth to compare the pattern of odontogenesis between toothed and toothless tetrapods.
GENE EXPRESSION IN CROCODILIAN TOOTH DEVELOPMENT
In Alligator embryos, the development of the first-generation tooth is initiated in the lower jaw of embryos around stage 14, forming a small epithelial evagination at the anteromedial part of the mandible (Westergaard and Ferguson 1986). We could not find any epithelial evaginations on the oral surface of the upper and lower jaws of crocodile embryos at stage 13 in histological sections (Fig. 2A). In crocodile embryos at the same stage, Pitx2, a gene known as a marker for dental epithelium (Mucchielli et al. 1997; Keränen et al. 1999; Smith et al. 2009a) and that regulates tooth formation (Lin et al. 1999), was expressed along the entire oral epithelium of both the upper and lower jaws (Fig. 2B). Another marker for early odontogenic epithelium, Shh (Hardcastle et al. 1998; Cobourne et al. 2004; Fraser et al. 2006; Buchtová et al. 2008; Vonk et al. 2008; Smith et al. 2009a), was not expressed in the lower jaw, but only expressed at the medial region of the oral epithelium of the upper jaw (Fig. 2C). The gene encoding the receptor for Shh, Patched-1 (Ptc1), was also not expressed in the lower jaw (Fig. 2D). Instead, its expression was detected along the oral epithelium of the upper jaw where Shh was expressed and in the adjacent mesenchyme dorsal to the oral epithelium. We next examined the expression patterns of a series of tooth developmental genes that are known to regulate the positioning of the sites of tooth formation as well as odontogenesis in mammalian embryos: Fgf8, Pax9, Bmp4, Msx1, and Msx2 (Vainio et al. 1993; Neubüser et al. 1997; Peters et al. 1998; Tucker et al. 1998; Chen et al. 2000; Stock 2001; Tucker and Sharpe 2004; Matalova et al. 2008; Zhou et al. 2011). Consistent with the expression pattern observed in mouse embryos, Fgf8 was expressed in the lateral part of the oral epithelium close to the jaw joint (Fig. 2E) and Pax9 was expressed in the mandibular mesenchyme that was distributed in the lateral to intermediate domain of the lower jaw and just ventral to the oral epithelium (Fig. 2F). We found the expression domains of Bmp4, Msx1, and Msx2 to be almost identical in crocodile embryos at stage 13 (Fig. 2G-I). All three genes were expressed in the mesenchyme of the lower jaw just ventral to the oral epithelium and the distribution of the cells that express these genes was biased to the medial portion of the mandible.
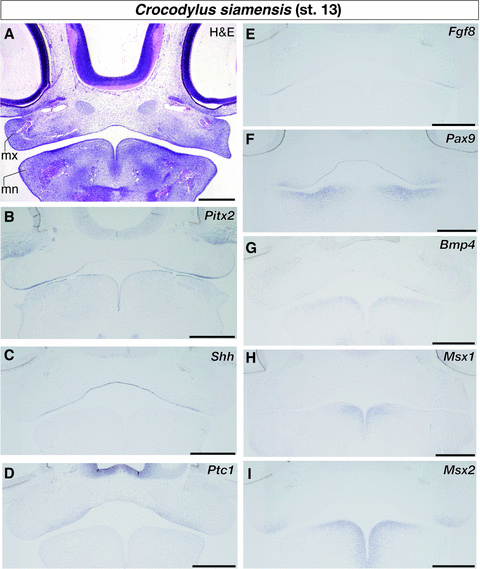
Expression of genes that play key roles in vertebrate odontogenesis in the jaw region of crocodile embryos at stage 13. (A) Hematoxylin and eosin (H & E) staining of the frontal section of the embryo to show general histology. (B) Pitx2 is expressed along the entire oral epithelium of both upper and lower jaws. (C) Shh is expressed at the medial region of the oral epithelium of the upper jaw. (D) Ptc1 is expressed along the oral epithelium of the upper jaw and in the adjacent mesenchyme dorsal to the oral epithelium. (E) Fgf8 is expressed in the lateral part of the oral epithelium close to the jaw joint. (F) Pax9 is expressed in the lateral population of the mandibular mesenchyme. (G) Bmp4, (H) Msx1, and (I) Msx2 are expressed in the medial population of the mandibular mesenchyme. mn, mandibular process (primordium of the lower jaw); mx, maxillary process (primordium of the upper jaw). Scale bars are 0.5 mm.
In crocodile embryos at stage 15, the primordium of the first-generation teeth (tooth germ) was clearly recognized as the pointed epithelial evagination at the anteromedial part of the mandible (Fig. 3A). Internal domains of the tooth germs were filled by condensed mesenchyme. Although Pitx2 was still strongly expressed in the entire oral epithelium, including the epithelium of the nascent tooth germ (Fig. 3B), expression of Shh was initiated at the apical domain of the tooth germs in addition to the medial part of oral epithelium of the upper jaw (Fig. 3C). We detected its expression only in the inner layer of the dental epithelium. Expression of Ptc1 was detected at both the inner and outer layers of the dental epithelium and the mesenchyme beneath the dental epithelium (Fig. 3D). We still observed Fgf8 expression at the lateral domain of the oral epithelium that was close to the jaw joint (Fig. 3E). Although the expression domain of Pax9 was expanded in the mandibular mesenchyme medially, no cells that expressed Pax9 were observed in the mesenchyme that fills the tip of the tooth germ (Fig. 3F). Like at the former stage of embryogenesis, Bmp4 and Msx1 were expressed in the mesenchyme of the lower jaw with medially biased expression (Fig. 3G and H). The internal side of the tooth germs was filled with the cells that express Bmp4 and Msx1. We also observed Bmp4 expression at the limited sites of the mandibular oral epithelium that were located somewhat lateral to the tooth germs (Fig. 3G). The sites may correspond to the place where new tooth germs were formed in subsequent embryonic stages. Although Msx2 showed similar expression pattern with that of Bmp4 and Msx1 in former embryonic stages (Fig. 2G-I), its expression pattern was different at this stage (Fig. 3I). We observed Msx2 expression at both the apical epithelium of tooth germs and the mesenchyme that underlies the epithelium. Msx2 was also expressed at the sites where new tooth germs appear subsequently. Additionally, we examined expression of two genes that belong to the distal-less family of homeobox genes: Dlx2 and Dlx5. They are expressed in dental tissues of mouse, teleost, and shark embryos and regulate tooth development (Thomas et al. 1997, 2000; Davideau et al. 1999; Zhao et al. 2000; Jackman and Stock 2006; Stock et al. 2006; Lézot et al. 2008; Debiais-Thibaud et al. 2011; Venugopalan et al. 2011). In crocodile embryos at stage 15, Dlx2 was broadly expressed in the mesenchyme in proximity to the oral epithelium of both the upper and lower jaws (Fig. 3J). Expression of Dlx5 was detected in both the dental epithelium and the mesenchyme that fills the internal domain of the tooth germs in the lower jaw (Fig. 3K).
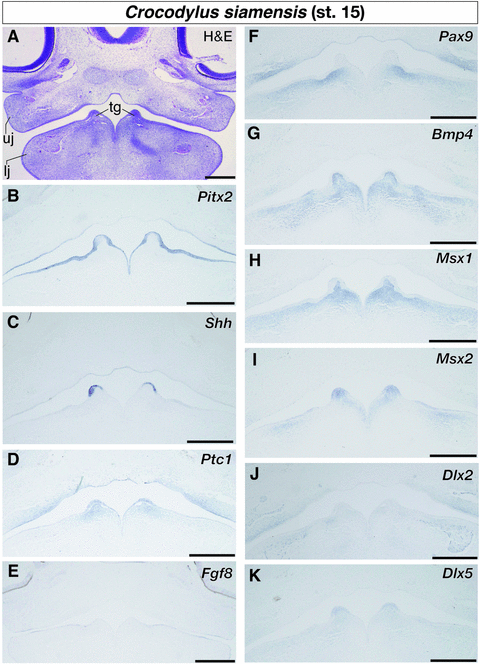
Expression of tooth developmental genes in the lower jaw of crocodile embryos at stage 15. (A) Frontal section of the embryo stained by hematoxylin and eosin (H & E). (B) Pitx2 is expressed in the entire oral epithelium, including the epithelium of the nascent tooth germ. (C) Shh is expressed in the inner layer of the dental epithelium, as well as in the medial part of oral epithelium of the upper jaw. (D) Ptc1 is expressed at both the inner and outer layers of the dental epithelium and the mesenchyme beneath the dental epithelium. (E) Fgf8 is expressed at the lateral domain of the oral epithelium. (F) Pax9 is expressed in the mandibular mesenchyme, but not expressed in the mesenchyme that fills the tip of the tooth germ. (G) Bmp4 and (H) Msx1 are expressed in the mandibular mesenchyme with medially biased expression. Expression of these genes is also detected in the mesenchyme that fills internal side of the tooth germs. (I) Msx2 is expressed at both the apical epithelium of tooth germs and the intervening mesenchyme. (J) Dlx2 is broadly expressed in the mesenchyme in proximity to the oral epithelium of both upper and lower jaws. (K) Dlx5 is expressed in both the dental epithelium and the intervening mesenchyme in the lower jaw. lj, lower jaw; tg, tooth germ; uj, upper jaw. Scale bars are 0.5 mm.
Gene expression patterns in stage 17 crocodile embryos, where several rows of tooth germs were formed in the anterior part of the mandible (Fig. 4A), were almost identical to that observed in earlier embryonic stages. Pitx2 was expressed in the epithelial tissue that covers the entire part of the oral cavity, including dental epithelium (Fig. 4B). Expression of Shh was detected at the inner layer of the dental epithelium (Fig. 4C) and that of Ptc1 was observed at both the dental epithelium and the mesenchyme that underlies the dental epithelium (Fig. S1A). At this embryonic stage, expression of Fgf8 was no longer observed at the anterior part of the mandible where pairs of tooth germs were formed. We observed weak expression of Pax9 in the lower jaw mesenchyme medial to the tooth germs (Fig. 4D). As in the upper jaw, no cells that express Pax9 were observed within either dental epithelium or dental mesenchyme. Both Bmp4 and Msx1 were expressed in the mesenchyme of the lower jaw. We found cellular condensations that express Bmp4 and the odontoblast maker, Col1a1, simultaneously in the inner space of the tooth germs (Fig. 4E and H). The tissue corresponds to dental papilla histologically. On the other hand, the cells that express Msx1 were more broadly distributed in proximity to the tooth germs (Fig. 4F). We observed intensive expression of Msx2 at both the dental epithelium and dental papilla in addition to the oral epithelium medial to the tooth germs and ventral mesenchyme of the lower jaw (Fig. 4G).
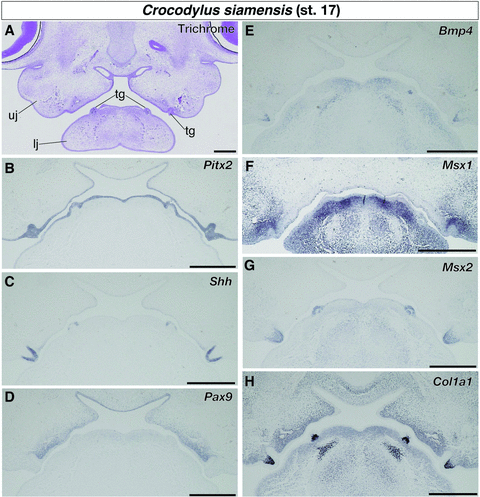
Expression of tooth developmental genes in the lower jaw of crocodile embryos at stage 17. (A) Trichrome staining of the frontal section of the embryo. (B) Pitx2 is expressed in the epithelial tissue that covers the entire part of the oral cavity, including dental epithelium. (C) Shh is expressed at the inner layer of the dental epithelium. (D) Pax9 is expressed in some mesenchymal cells medial to the tooth germs. Pax9-positive cells are not observed within either dental epithelium or dental mesenchyme. (E) Bmp4 and (F) Msx1 are expressed in the jaw mesenchyme. (G) Msx2 is expressed at both the dental epithelium and the dental papilla, as well as in the oral epithelium medial to the tooth germs and ventral mesenchyme of the lower jaw. (H) Col1a1 is broadly expressed in jaw mesenchyme, especially intensively expressed in cellular condensations that occupy the inner space of the tooth germs. lj, lower jaw; tg, tooth germ; uj, upper jaw. Scale bars are 0.5 mm.
GENE EXPRESSION IN TURTLE LOWER JAW DEVELOPMENT
We next described the pattern of lower jaw morphogenesis in turtles to compare it to the pattern of crocodile mandibular odontogenesis described above and that already reported for toothless modern birds. In turtle embryos at stage 13 that morphologically correspond to crocodile embryos at stage 13, the histological condition of the mandibular process is almost identical (Fig. 5A). As in crocodile embryos, Pitx2 was expressed in entire part of the oral epithelium (Fig. 5B). Although we could not find a morphologically distinct boundary between the oral and aboral epithelia in early-stage crocodile and turtle embryos we examined, Pitx2 would work as a good molecular marker to distinguish oral epithelium that includes future dental epithelium from aboral epithelium in early-stage embryos. Although Shh was only detected at the medial region of the oral epithelium of the upper jaw (Fig. 5C), its receptor gene Ptc1 was expressed in Shh-positive oral epithelium and the mesenchyme just dorsal to the epithelium (Fig. 5D). As in crocodile embryos, Fgf8 was expressed in the oral epithelium close to the jaw joint (Fig. 5E). Expression of Pax9 was not detected in the mesenchyme of the mandible, and instead was apparently expressed only in the medial oral epithelium and a limited population of the upper jaw mesenchyme (Fig. 5F). Although intensive expression of Msx1 was already detected in the mesenchyme that occupied the medial part of the mandible (Fig. 5H), Bmp4 was only expressed at the medial part of the lower jaw oral epithelium at this stage of turtle embryogenesis (Fig. 5G). We observed expression of Msx2 in the medial part of the oral epithelium of the lower jaw and the mesenchyme that underlies the epithelium (Fig. 5I).
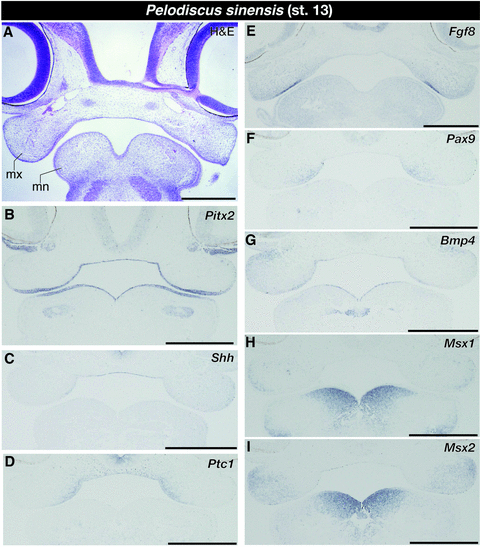
Expression of tooth developmental genes in the jaw region of turtle embryos at stage 13. (A) Frontal section of the embryo stained by hematoxylin and eosin (H & E). (B) Pitx2 is expressed in entire part of the oral epithelium. (C) Shh is only expressed at the medial region of the oral epithelium of the upper jaw. (D) Ptc1 is expressed in oral epithelium of the upper jaw and the mesenchyme just dorsal to the epithelium. (E) Fgf8 is expressed in the oral epithelium close to the jaw joint. (F) Pax9 is expressed only in the medial oral epithelium and a limited population of the upper jaw mesenchyme. (G) Bmp4 is expressed at the medial part of the mandibular oral epithelium. (H) Msx1 is expressed in the mesenchyme that occupies the medial part of the mandible. (I) Msx2 is expressed in the medial part of the oral epithelium of the lower jaw and the mesenchyme that underlies the epithelium. mn, mandibular process; mx, maxillary process. Scale bars are 0.5 mm.
In turtle embryos at stage 15 that are morphologically equivalent to crocodile embryos at stage 15 where peg-like tooth germs were visible, no tooth germs were observed in oral cavity (Fig. 6A). As in embryos at stage 13, Pitx2 continued to be expressed in entire part of the oral epithelium (Fig. 6B). Interestingly, expression of Shh was only detected in the epithelium at the roof of oral cavity and a limited domain of the upper jaw epithelium that occupies the intermediate region of the upper jaw in transverse plane (Fig. 6C). We could not detect Shh expression in the tissue of the lower jaw. Focal expression of Ptc1 was detected only in the upper jaw (Fig. 6D) and was expressed in limited parts of oral epithelium that were Shh-positive and the mesenchyme adjacent to the epithelium. We detected Fgf8 expression at the oral epithelium close to the jaw joint (Fig. 6E) and Pax9 expression at the jaw mesenchyme adjacent to Fgf8-positive oral epithelium (Fig. 6F). Intensive expression of Bmp4 and Msx1 was observed in the mesenchyme distributed at dorsomedial part of the mandible (Fig. 6G and H). We also observed intensive expression of Msx2 in the medial portion of the mandibular oral epithelium as well as in the ventral population of the mandibular mesenchyme (Fig. 6I). However, Msx2 was not expressed in the mandibular mesenchyme that occupies the space just ventral to Msx2-positive oral epithelium. Although Dlx2 was expressed in the mesenchymal cells that are distributed broadly in both the upper and lower jaws as in stage-matched crocodile embryos (Fig. 6J), Dlx5 was expressed at the medial part of the mandibular oral epithelium as well as in the mesenchyme that occupies almost entire domain of the mandible (Fig. 6K).
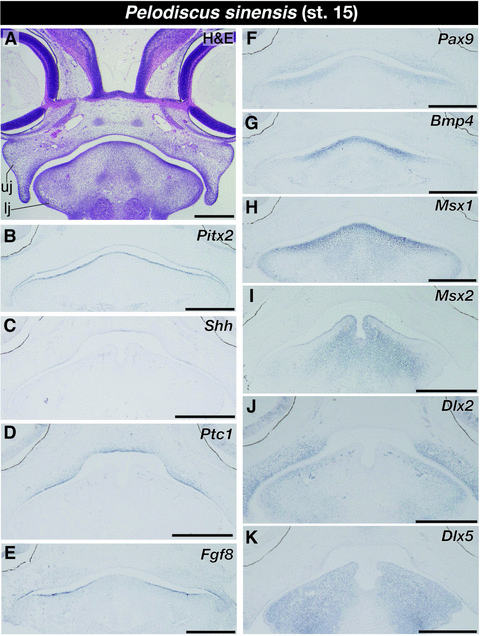
Expression of tooth developmental genes in the lower jaw of turtle embryos at stage 15. (A) Frontal section of the embryo stained by hematoxylin and eosin (H & E). (B) Pitx2 continues to be expressed in entire part of the oral epithelium. (C) Shh is only expressed in the epithelium at the roof of oral cavity and a limited domain of the upper jaw epithelium. (D) Ptc1 is expressed in the oral epithelium where the expression of Shh is observed as well as in the mesenchyme adjacent to these epithelial tissues. (E) Fgf8 is expressed in the oral epithelium close to the jaw joint. (F) Pax9 is expressed at the jaw mesenchyme adjacent to Fgf8-positive oral epithelium. (G) Bmp4 and (H) Msx1 are intensively expressed in the mesenchyme distributed at dorsomedial part of the mandible. (I) Msx2 is expressed in the medial portion of the mandibular oral epithelium as well as in the ventral population of the mandibular mesenchyme. (J) Dlx2 is expressed in the mesenchyme distributed broadly in both the upper and lower jaws. (K) Dlx5 is expressed at the medial part of the mandibular oral epithelium as well as in the mesenchyme that occupies almost entire domain of the mandible. lj, lower jaw; uj, upper jaw. Scale bars are 0.5 mm.
In stage 17 turtle embryos, which are morphologically equivalent to crocodile embryos at stage 17, we found a pair of tissue outgrowths that was covered with thickened epidermis on the oral side of both the upper and lower jaws (Fig. 7A). We observed that Pitx2 continued to be expressed in the oral epithelium and its expression domain terminated at the lateral margin of the tissue outgrowth (Fig. 7B). Although Shh was still weakly expressed at the roof of oral cavity, no Shh expression was detected in the tissues of the lower jaw (Fig. 7C). We found Ptc1 expression in the epithelium that covers the tongue primordium and the mesenchyme within it and in the mesenchyme that fills inside of the tissue outgrowth in both the upper and lower jaws. Expression of Ptc1 was also detected in the oral epithelium of the upper jaw, which also expresses Shh, and in the mesenchyme just dorsal to the epithelium (Fig. 7D). At this stage of turtle embryogenesis, we could not detect any Fgf8 expression in the tissues of the anterior part of the jaws as we observed in stage-matched crocodile embryos, but Pax9 was expressed in the mesenchymal cells that were distributed in the medial part of both the upper and lower jaws, and its expression domain laterally ended at the medial midpoint of a pair of tissue outgrowths on the jaws (Fig. 7E). Expression of Bmp4 and Msx1 was detected in the mesenchyme of both upper and lower jaws (Fig. 7F and G). The mesenchyme that expresses these genes was concentrated to the internal domain of the tissue outgrowths that appeared on the jaws. We observed intensive expression of Msx2 in the thickened epidermis that covers the tissue outgrowth in both upper and lower jaws, as well as in the ventral population of mandibular mesenchyme, which was located away from the oral epithelium (Fig. 7H). We found no Msx2 expression in the mesenchyme that occupied the internal space of the tissue outgrowths. Although Dlx2 was broadly expressed in the jaw mesenchyme adjacent to the oral epithelium in a mediolateral direction (Fig. S1B), Dlx5 was strongly expressed in the thickened epidermis that covers the tissue outgrowth in both the upper and lower jaws as well as in some mesenchymal cells that underlie these epidermal tissues (Fig. S1C). Unlike the situation in stage-matched crocodile embryos, we observed uniform expression of Col1a1 in the mesenchyme of both the upper and lower jaws, without concentration of Col1a1-positive cells in the mesenchyme within the tissue outgrowths (Fig. 7I). The results on expression domain of each gene analyzed in crocodile and turtle embryos, along with already reported data for chick and mouse embryos, are summarized in Table S1.
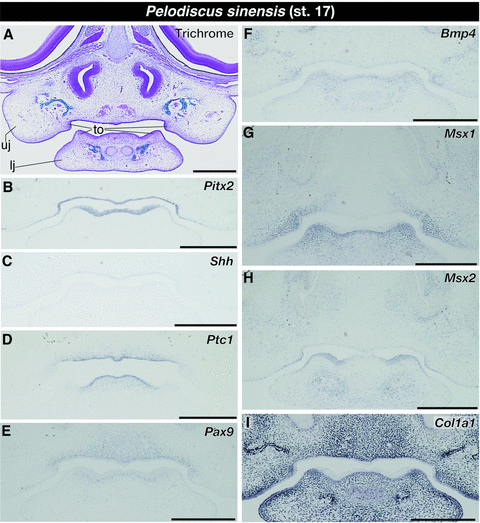
Expression of tooth developmental genes in the lower jaw of turtle embryos at stage 17. (A) Trichrome staining of the frontal section of the embryo. (B) Pitx2 continues to be expressed in the oral epithelium and its expression domain terminated at the lateral margin of the tissue outgrowth. (C) Shh is weakly expressed at the roof of oral cavity. (D) Ptc1 is expressed in some mesenchymal cells that fill inside of the tissue outgrowth in both upper and lower jaws, as well as in the epithelium and the mesenchyme of the tongue primordium and the oral epithelium of the upper jaw and the mesenchyme dorsal to it. (E) Pax9 is expressed in the mesenchymal cells distributed in the medial part of both upper and lower jaws. Its expression domain is laterally ended at the medial midpoint of a pair of tissue outgrowths on the jaws. (F) Bmp4 and (G) Msx1 are expressed in the mesenchyme of both upper and lower jaws and their intensive expression is observed in the internal domain of the tissue outgrowths on the jaws. (H) Msx2 is intensively expressed in the thickened epidermis covering the tissue outgrowth over both upper and lower jaws, as well as in the ventral population of mandibular mesenchyme, which is located away from the oral epithelium. (I) Col1a1 is uniformly expressed in the mesenchyme of both upper and lower jaws. lj, lower jaw; to, tissue outgrowth; uj, upper jaw. Scale bars are 0.5 mm.
Discussion
Through comparative analysis of tooth developmental genes, we found there is little difference in the patterns of gene expression between early crocodile embryos where tooth development has not been initiated and morphologically equivalent turtle embryos (Fig. 8A). In this embryonic stage, Pax9 is expressed in the lateral population of the mandibular mesenchyme only in crocodiles, and Msx2 is expressed in medial part of the mandibular oral epithelium only in turtles. However, these genes are expressed in spatially corresponding tissues in both reptilian lineages at subsequent ontogenetic stages. Judging from these observations, we speculate temporal differences of the onset of Pax9 and Msx2 expressions do not have a major influence on the odondogenesis of these animals. However, we observe striking differences in gene expression in subsequent phases of lower jaw development in both turtles and crocodiles (Fig. 8B). Both Pitx2 and Fgf8, which are expressed in dental epithelium and regulate early phase of tooth development (Mucchielli et al. 1997; Neubüser et al. 1997; Tucker et al. 1998; Lin et al. 1999), showed almost identical expression patterns in embryos of toothed crocodiles and toothless turtles at stage 15. Furthermore, Pax9, Bmp4, and Msx1, which are expressed in cranial neural crest-derived dental mesenchyme and function in odontogenesis (Satokata and Maas 1994; Neubüser et al. 1997; Jernvall et al. 1998; Peters et al. 1998; Tucker et al. 1998; Zhang et al. 1999, 2000; Chen et al. 2000; Han et al. 2003; Nakatomi et al. 2010; Zhou et al. 2011), also displayed similar patterns of expression.
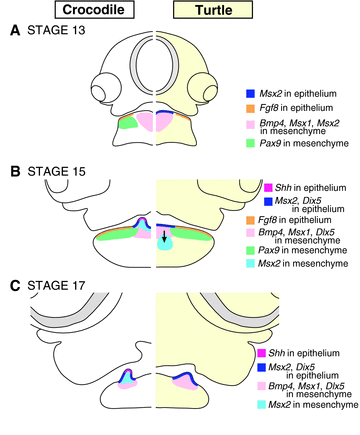
Schematic illustration showing alteration of developmental program that potentially leads to tooth loss in turtles. Expression domain of representative genes in the lower jaw of (A) stage 13, (B) stage 15, and (C) stage 17 crocodile (left panel) and turtle (right panel) embryos. For detail of gene expression pattern, see the main text.
In crocodile embryos at late stage 14 or 15, a pair of tooth germs that evaginate into the oral cavity was observed in the anterior part of the mandible (Fig 3A). At the apical domain of the epithelium that covers the tooth germ, focal expression of Shh was detected as reported in alligator embryos at older embryonic stages (Harris et al. 2006) (Fig. 3C; Fig S1D). Also, expression of Ptc1 and Msx2 was detected at both the apical epithelium of the tooth germs and the intervening mesenchyme. Shh is a well-known molecular marker of the oral epithelium where tooth development is initiated in embryos of various vertebrate taxa (Fraser et al. 2006, 2008; Buchtová et al. 2008; Vonk et al. 2008; Smith et al. 2009b,c). In mouse odontogenesis, Shh is expressed in the dental epithelium including enamel epithelium in later stages (Hardcastle et al. 1998; Dassule et al. 2000; Cobourne et al. 2001, 2004; Gritli-Linde et al. 2002). Besides, in crocodile embryos at stage 15, expression of Ptc1 and Msx2 was detected in both the ectoderm-derived dental epithelium and neural crest-derived dental papilla (Jowett et al. 1993; Bidder et al. 1998; Jernvall et al. 1998; Zhang et al. 1999; Khan et al. 2007).
However, in turtle embryos, expression of Shh was never detected in any sites of mandibular oral epithelium throughout embryogenesis, although expression of other tooth developmental genes such as Fgf8, Pax9, Bmp4, Msx1, and Dlx5 was maintained in the tissue of the lower jaw as in mice and crocodiles, displaying spatially comparable expression patterns (Fig. 8B and C). In turtle embryos older than stage 17, a pair of tissue outgrowths overlaid by thickened epidermis was clearly recognized on the oral side of the mandible. Judging from the concentration of the cells that express Bmp4 and Msx1 in the tissue outgrowths, Dlx5 expression in both the epithelium and the mesenchyme of the tissue outgrowths, and expression pattern of Pax9 at the vicinity of the tissue outgrowths, it seems this structure is partly comparable to the first-generation crocodilian tooth that shows similar expression patterns for these genes. However, expression of Shh was never detected in the thickened epidermis over the tissue outgrowths. Also, the gene encoding the Shh receptor, Ptc1, was only detected in some mesenchymal cells that were distributed inside of the tissue outgrowths, and not expressed in the thickened epidermis. Previous loss-of-function or gain-of-function experiments have revealed that Shh signaling plays a fundamental role in epithelial cell proliferation and differentiation during vertebrate odontogenesis (Hardcastle et al. 1998; Dassule et al. 2000; Gritli-Linde et al. 2002; Buchtová et al. 2008; Fraser et al. 2008). For example, treating lungfish embryos with cyclopamine, a chemical inhibitor of Shh signaling, resulted in complete loss of dentition, possibly due to subsequent loss of tissue–tissue interaction between ectoderm-derived dental epithelium and neural crest derived dental mesenchyme (Smith et al. 2009b). Therefore, it is possible that impairment of Shh signaling at the putative odontogenic region of oral epithelium in early stage of odontogenesis prevents turtles from accomplishing further epithelial-mesenchymal interactions that are necessary to form a regular type of tooth.
Furthermore, in turtle embryos, Msx2 was not detected in the mesenchymal cells that fill the internal domain of the tissue outgrowth, although Msx2 was focally expressed in thickened epidermis that covers the outgrowth. This is in stark contrast to the condition in crocodile embryos where Msx2 was detected at both the epithelium and the mesenchyme of the tooth germs. Because we detected intensive expression of the odontoblast marker Col1a1 in the mesenchyme of crocodile tooth germs, it seems these cells are biochemically equivalent to the dental papilla formed in other vertebrate lineages (Schwarz et al. 1990; Braut et al. 2003). However, we could not observe a condensation of the mesenchyme that expresses both Msx2 and Col1a1 like dental papilla in crocodile embryos, in the internal domain of the tissue outgrowth formed in late-stage turtle embryos. In early-stage turtle embryos, Msx2 was expressed in the mesenchyme widely distributed at the medial portion of the mandible (Fig. 5I), as in crocodile embryos. However, expression of Msx2 was downregulated in the mesenchyme just ventral to Msx2-positive oral epithelium in subsequent stages of turtle embryogenesis (Fig. 6I). Although it is known that Bmp4 induces Msx2 expression in dental mesenchyme during mouse embryogenesis (Vainio et al. 1993), similar expression patterns of Bmp4 were observed in the mandibular development of both toothed crocodiles and toothless turtles. Therefore, regulatory alteration of other signaling molecules such as FGFs that also potently induce Msx2 expression in dental mesenchyme (Chen et al. 1996) might lead to an early-stage arrest of odontoblast development in turtle embryos.
In chicks, which are also toothless, the rudimentary epithelial thickenings that resemble dental placodes in mammalian embryos are transiently formed in the oral cavity of early-stage embryos (around stage 27) (Chen et al. 2000). Interestingly, unlike in turtles, expression of Shh and Ptc1 are activated in the presumptive dental lamina of the mandible of later-stage chick embryos (by stage 31) (J. A. Helms et al. 1997, pers. obs.). The arrest of avian odontogenesis is probably the result of inactivation of Bmp4 in the oral epithelium, which activates the expression of Msx1 and Msx2 in dental mesenchyme in toothed tetrapod embryos such as mice (Chen et al. 2000). The study of chicken mutant, talpid2 (ta2) in which tooth rudiments are formed at the anterior part of both the upper and lower jaws suggests that the arrest of odontogenesis in avian embryos resulted from a loss of contact between the epithelial signaling center and the underlying competent mesenchyme driven by unknown genetic mechanisms (Harris et al. 2006; Louchart and Viriot 2011). In the present study, we found that expression of Shh is not activated in the oral epithelium of the mandible throughout turtle embryogenesis and the expression of major tooth developmental genes (e.g., Pitx2, Fgf8, Pax9, Bmp4, Msx1, Dlx5) is spatially maintained in toothless turtle embryos as in toothed crocodile embryos. These results support our hypothesis that primary cellular and molecular mechanisms that lead to tooth agenesis are different between birds and turtles (Fig. 9).
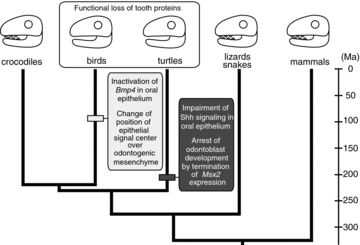
The convergent evolution scenario of tooth loss in birds and turtles supported by our data. Evolutionary events that caused tooth loss are mapped on the branch of bird and turtle lineages as horizontal bars. In turtles, edentulism appears to have been brought about by impairment of Shh signaling in oral epithelium and early-stage arrest of odontoblast development by termination of Msx2 expression in dental mesenchyme around 220 Ma. On one hand, similar phenotype might be acquired by inactivation of Bmp4 in oral epithelium (Chen et al. 2000) and change of relative position of epithelial signaling center over odontogenic mesenchyme (Harris et al. 2006) between 125 and 65.5 Ma (Louchart and Viriot 2011) in modern birds. After losing serially formed teeth through a different developmental program, two lineages might achieve a similar phenotype through secondary function loss of tooth structural proteins such as amelogenin. The divergence times of each amniote lineage are from Shedlock and Edwards (2009).
Even if the initial step for losing teeth is different between birds and turtles, similar phenotypes might be brought about through regulatory changes of downstream molecules that are common in two lineages. In turtles, Shh signaling is inactivated in the oral epithelium, although other tooth developmental genes such as Pitx2, Msx2, and Dlx5 are broadly expressed there without a gap in the anterior-posterior direction. This might explain why turtles have a longitudinally extended ridge of the beak, instead of serially formed teeth, on the jaws. In birds, Shh is expressed at the presumptive dental epithelium broadly in anterior-posterior direction, without a gap (J. A. Helms et al. 1997, pers. obs.). This might correlate with the fact that birds have a longitudinal ridge over the jaws, like turtles. After losing serially formed teeth through a different developmental program, both birds and turtles achieved a similar phenotype: a horny beak on their jaws. Once birds lost the capability to form tooth germs, mutations apparently accumulated in the downstream genes such as the genes encoding the tooth proteins such as dentine (dentin sialophosphoprotein [DSPP]) and all three enamel proteins (amelogenin [AMEL], ameloblastin [AMBN], and enamelin [ENAM]) probably due to a lack of selective pressures on these genes. Eventually, AMEL and DSPP became nonfunctional genes, and ENAM and AMBN disappeared from the genome through chromosomal rearrangement (Sire et al. 2008; Al-Hashimi et al. 2010). Similarly, Amelogenin has not been isolated from the genome of turtles, implying substantial alteration of its structure or disappearance from the genome (Girondot and Sire 1998). Such secondary function loss of structural protein coding genes might correlate with keratinization of the oral epithelium observed in both taxa (Lee 1997) (Fig. 9). Our results demonstrate that the cellular and molecular mechanisms that regulate early-stage arrest of tooth development are diverse in tetrapods, and downstream molecules or pathways may respond to preceding alteration of developmental program in the same way thereby resulting in convergent phenotypic evolution of craniofacial morphology.
Associate Editor: P. D. Polly
ACKNOWLEDGMENTS
We appreciate staff of Sriracha Crocodile Farm & Product Co., Ltd., especially N. Thongprasert in collection of fertilized eggs of crocodiles. We also thank M. Klomtun, P. Pinweha, E. Threenet for their kind help in collection of crocodile eggs and D. Polly, A. Louchart, and M. Smith for thoughtful comments and suggestions to improve this paper. MT thanks H. Wada who allowed the use of facilities for experiments and analyses, H. Ono who kindly gifted chick Msx1 probe, and R. Schneider and M. Brandley for critical reading and editing of a draft of the manuscript. This study was partly supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan to MT (22770077). The authors declare no conflict of interest.




