COMPLEX INTERACTIONS BETWEEN PATERNAL AND MATERNAL EFFECTS: PARENTAL EXPERIENCE AND AGE AT REPRODUCTION AFFECT FECUNDITY AND OFFSPRING PERFORMANCE IN A BUTTERFLY
Abstract
Parental effects can greatly affect offspring performance and are thus expected to impact population dynamics and evolutionary trajectories. Most studies have focused on maternal effects, whereas fathers are also likely to influence offspring phenotype, for instance when males transfer nutrients to females during mating. Moreover, although the separate effects of maternal age and the environment have been documented as a source of parental effects in many species, their combined effects have not been investigated. In the present study, we analyzed the combined effects of maternal and paternal age at reproduction and a mobility treatment in stressful conditions on offspring performance in the butterfly Pieris brassicae. Both paternal and maternal effects affected progeny traits but always via interactions between age and mobility treatment. Moreover, parental effects shifted from male effects expressed at the larval stage to maternal effects at the adult stage. Indeed, egg survival until adult emergence significantly decreased with father age at mating only for fathers having experienced the mobility treatment, whereas offspring adult life span decreased with increasing mother age at laying only for females that did not experience the mobility treatment. Overall, our results demonstrate that both parents’ phenotypes influence offspring performance through nongenetic effects, their relative contribution varying over the course of progeny's life.
Nongenetic inheritance is defined as “any effect on offspring phenotype brought about by the transmission of factors other than DNA sequences from parents or more remote ancestors” (Bonduriansky and Day 2009). Nongenetic inheritance is now recognized to play a fundamental role in the evolution and population dynamics of species (Avital and Jablonka 2000; Räsänen and Kruuk 2007; Bonduriansky and Day 2009; Danchin and Wagner 2010). Parental effects represent a main component of such transmitted nongenetic phenotypic variance (Bonduriansky and Day 2009; Danchin and Wagner 2010; Wagner and Danchin 2010). Such effects occur when variation in parental phenotype, which may be caused genetically or in response to the environment the parents inhabit, affects offspring phenotype (Lacey 1998; Mousseau and Fox 1998a). Parental effects have been shown to affect offspring life-history traits in many organisms (Mousseau and Fox 1998a), as well as population dynamics in few empirical studies (Benton et al. 2008; Plaistow and Benton 2009; Venturelli et al. 2010). For example, Benton et al. (2008) found that maternal age of the founding cohort affected offspring life histories and population structure in the soil mite Sancassania berlesei, with effects lasting over multiple generations. Moreover, theoretical studies suggest that parental effects might influence evolution via natural selection (Mousseau and Fox 1998a; Badyaev and Uller 2009). Parental effects may influence evolution even at an ecological time scale (Carroll et al. 2007). Indeed, environmentally induced parental effects have already been shown to increase offspring fitness in response to abrupt changes in environmental conditions or to stressful events (Mousseau and Fox 1998a; Marshall and Uller 2007; Coslovsky and Richner 2011). For example, Coslovsky and Richner (2011) manipulated the perceived predation risk before and during ovulation in great tits, and found that offspring from the most exposed mothers were smaller and with longer wings, two traits likely to be selected for in response to predation. Parental effects may thus represent a source of rapid adaptive response in the context of global changes. Such transgenerational mechanism remains, however, often ignored when modeling species’ response to global changes.
Most studies dealing with parental effects have focused on maternal effects only (Mousseau and Fox 1998a). Because mothers may control the protein and RNA contents invested in their progenies, they can affect early gene expression in their offspring, including genes that affect major developmental trajectories (Johnstone and Lasko 2001; Bettegowda et al. 2008; Fox and Czesak 2000). However, the importance of paternal effects is now more widely recognized (Mousseau and Fox 1998a; Hunt and Simmons 2000; Roth et al. 2010; Curley et al. 2011). Although paternal effects were firstly thought to be significant only in species displaying paternal care (Falconer and Mackay 1996), there is now increasing evidence that paternal phenotype can also affect offspring phenotype in species without paternal care (Giesel 1986; Crill et al. 1996; García González and Simmons 2005; Bonduriansky and Head 2007; Priest et al. 2008; Smallegange 2011). For instance, variations in daughter fitness were partly explained by paternal effects in Drosophila melanogaster, a species without paternal care: using mothers that had mated at least once, Priest et al. (2008) showed that both the physical act of mating and exposure to male seminal fluid increased the fitness of daughters.
In most arthropods where paternal care is absent, paternal investment is often reduced to the so-called nuptial gifts, that is, transfer of nutrients to females during mating (Vahed 1998). Whereas many studies have examined how nuptial gifts affect life-history traits of the mating female (e.g., life span, fecundity) (Vahed 1998), few have assessed whether nuptial gifts also affect offspring traits (Fox and Czesak 2000; Priest et al. 2008). In butterflies, it has been shown that the quality of the spermatophore (e.g., its amount and types of nutrients), a form of nuptial gift, affects the number of eggs laid by females (Oberhauser 1997; Karlsson 1998; Stjernholm and Karlsson 2000). Nonetheless, studies dealing with paternal effects remain scarce and thus the contribution of paternal-associated nongenetic inheritance on evolution remains largely unexplored.
In butterflies, maternal effects have been shown to strongly impact offspring life history, and thereby could be a way to respond to stressful environmental conditions (parasites or predator exposure, extreme meteorological events, etc.) and rapid environmental changes such as habitat fragmentation (Gibbs and Van Dyck 2010). Indeed, such changes in environmental conditions are likely to affect resource distribution, and thus populations’ and individuals’ strategies of resource allocation. In Pararge aegeria, for example, Merckx et al. (2006) showed that butterflies from more fragmented habitats showed higher flight performances. In this framework, the oogenesis-flight syndrome hypothesis predicts that in flying insects, resources are traded off between flight and oogenesis (Johnson 1963; Hughes et al. 2003; Jervis et al. 2005, but see Hanski et al. 2006). We could expect stress-induced responses to be also resource demanding, and thus to indirectly affect the allocation of resources in oogenesis. Under these hypotheses, we expect more active flying females or females exposed to a stress to have fewer resources to allocate to other activities including reproduction. Such stress or flight-induced negative effects on maternal egg provisioning (e.g., number, size, and composition) and offspring growth has been demonstrated in the butterfly P. aegeria (Gibbs et al. 2010a,b). Although males are also expected to trade their resources between reproduction and other activities, it is currently not known whether increased mobility or stressful events affect nuptial gifts and offspring performance.
Apart from environmentally induced parental effects, parental age is one of the most studied factors that can generate parental effects. The effects of parental age on offspring phenotype have been largely investigated in the context of senescence where survival and/or reproductive output are expected to decline over time (Williams 1957; Rose 1991). In this context, it has been suggested that parental age at reproduction might have transgenerational effects on offspring phenotypes. Indeed, many empirical studies in the laboratory have shown that offspring life span decreases with increasing maternal age in a wide range of taxa (Mousseau and Fox 1998a; Priest et al. 2002; Tarin et al. 2005). In addition, maternal age can modify offspring life-history trajectories. For example, in the great tit, offspring from older mothers performed better early in life but suffered from an earlier onset of reproductive senescence (Bouwhuis et al. 2010). Parental age also interacts with other factors involved in the genesis of parental effects, such as environmental conditions likely to affect parental quality (e.g., a forced flight treatment likely to involve both a cost of the flight per se and a cost of the stress due to the test, Gibbs et al. 2010a). Interestingly, those factors might have nonadditive effects on offspring phenotypes. For instance, in the butterfly P. aegeria, the larval mass of offspring declined more steeply with maternal age when females had experienced a forced flight (Gibbs et al. 2010a). Several studies have shown parental effects to be related with environmental factors (see for a review Mousseau and Fox 1998a; Coslovsky and Richner 2011). As maternal and paternal effects depending on environmental factors may coexist with potential age effects, they are likely to coevolve with age effects. Although such processes have important implications on species evolution, they are still largely unexplored.
The objective of this study is to investigate whether parental age (both maternal and paternal), a mobility treatment in stressful conditions and their interaction influence offspring performance in the butterfly Pieris brassicae. Using butterflies raised under controlled conditions and a standardized mobility treatment under stressful conditions in the laboratory, we first tested the effect of the treatment on parents’ life span, mating success, and age at reproduction. Then, we tested whether the treatment and both maternal and paternal age at reproduction affected parents’ fecundity (number of eggs produced per pair) and reproductive success (number of adults produced per pair). Finally, we investigated whether the treatment, maternal age at laying, and paternal age at reproduction acted on offspring performance, (1) early in development (offspring survival and offspring development time until adult emergence), and (2) later in development (offspring life span once they had metamorphosed into adults).
Materials and Methods
BREEDING CONDITIONS
Experiments were performed at the Muséum National d’Histoire Naturelle in Brunoy, France, using butterflies from a breeding established in 2007. The stock originated from eggs collected in three distinct locations in Brunoy and Saint-Girons (France) and Mesnil-Eglise (Belgium) during the summer 2007. The three lineages were allowed to mix freely from the third generation of captive breeding. The butterflies used in this study belonged to the 20th generation of captive breeding (F20). We thus assumed other noncontrolled sources of parental effects arising from differences in the conditions of the source populations to have disappeared. During this experiment, both parents and offspring generations were reared in similar conditions. Larvae were reared on Brassica oleracea provided ad libitum in familial groups of up to 20 individuals, in 15 × 5 × 5 cm boxes in a climate chamber at 23 ± 1°C by day and 18 ± 1°C by night under photoperiod conditions (Light:Dark 14:10 h) that induce direct development. Adults were bred in 1 × 1 × 1 m cages under the same light conditions at 25 ± 1°C by day and 20 ± 1°C by night with a solution of 10% sugar in artificial flowers.
EXPERIMENTAL TREATMENT: FORCED MOBILITY IN STRESSFUL CONDITIONS
The experimental treatment was designed to induce potential metabolic costs expected to trade-off with life-history traits of butterflies. On the day after emergence, 447 individually marked butterflies from 30 different families were assigned to a manipulated group (N= 203, 106 males, 97 females) or a nonmanipulated reference group hereafter referred to as the control group (N= 244; 121 males and 123 females). Within each family, an equal number of individuals from each gender was allocated to each of the two groups. In the manipulated group, each butterfly was individually introduced one day after emergence within a 25 × 10 × 10 cm plastic chamber perforated at its base and fixed to a rapid agitator (Vortex Genie 2, Scientific Industries). The temperature of the chamber was kept constant at 25 ± 1°C. The butterfly was allowed to habituate to the chamber for 30 s, during which we verified that it was able to take-off from the bottom to the center of the chamber. We then turned the agitator on for 2 min, resulting in strong shaking of the chamber that impeded the butterfly to perch on the chamber's wall. Each butterfly could either fly in a reduced volume or lay uncomfortably at the bottom of the chamber in a reduced area strongly shaken, both behaviors likely to be resource demanding due to stressful conditions. Preliminary experiments showed that any test longer than 2 min was likely to damage butterfly's wings and legs. In natural populations, individuals of P. brassicae fly much longer than 2 min. Thus, our experimental treatment was not designed to induce a metabolic cost associated only with flight but rather to induce a cost due to a forced activity in stressful conditions. Such treatment may thus reflect what butterflies experience in the wild when moving in stressful conditions, such as escaping from predators or crossing unsuitable habitats during dispersal.
MATING DESIGN AND REARING OF THE OFFSPRING
From the second day after emergence, we allowed the F20 butterflies to mate for 2 h each day in a cage containing an equal proportion of manipulated and control individuals of both sexes. Once a pair had mated, we removed it immediately from the cage. Because of staggered emergences, newly emerged butterflies from both sexes and treatments were added in the cage on a daily basis to maintain equal proportions. This represented a total of 16 up to 40 individuals within the cage, with four to 10 manipulated individuals of each sex and four to 10 control individuals of each sex. We allowed mate choice to make the study more relevant to natural populations. The mating experiment lasted 22 days, as we did not dispose of enough individuals to continue the experiment in the same conditions on the 23rd day. Individuals that did not mate and were still alive on the 23rd day were excluded from the analyses. Females were isolated in a laying cage with cabbage as host plant, and artificial flowers containing a 10% sugar solution. Cabbage leaves were changed daily until the females died. Mated males were also isolated to impede multiple matings and thus avoid the unwanted effects of a decrease in spermatophore quality due to repeated mating events (Ferkau and Fischer 2006). Manipulating eggs and first instars larvae can induce high mortality in this species. Thus, to limit manipulation of those stages, offspring of a given clutch were reared together until they joined the third instar in 15 × 5 × 5 cm boxes, whatever their numbers in the clutch. Third instar caterpillars were then counted within each clutch and were divided into groups of up to 20 individuals in separate boxes. On the day of emergence, offspring were marked and released in 1 × 1 × 1 m cages, separating males from females to prevent mating.
PARENTS’ LIFE-HISTORY TRAITS
We recorded several life-history traits of the parents. We first recorded adults’ life span on a daily basis to quantify parent adult life span for each individual. We then recorded mating success for each individual as a binary variable, as well as age at mating for mated females and males. Finally, we counted the number of eggs produced per female on a daily basis to quantify the number of eggs produced per pair. We then counted the number of emerged adults to quantify the number of adults produced per pair as a measure of reproductive success. Almost all females laid new egg clutches over several days (some of them laid eggs on 14 different days). As a result, we obtained clutches laid at different ages for most of the females. Each individual of the offspring generation was thus characterized by both its mother age at mating and age at laying. We expected mothers’age at laying to have a stronger influence than mothers’age at mating on the information transferred to the eggs. We thus used age at laying in the models investigating potential maternal effects on offspring life history. For males, we expected age at mating to be of importance for the quality of spermatophores and thus used this variable in our analyses.
OFFSPRING LIFE-HISTORY TRAITS
We recorded three life-history traits in offspring. We first recorded survival until adult emergence for each egg on a daily basis and averaged egg survival over each clutch to estimate egg survival per clutch, referred to as egg survival. For those having survived until adult emergence, we recorded development time as the number of days between egg laying and adult emergence. Finally, we recorded offspring life span (in days) once offspring had reached the adult stage, hereafter referred to as offspring adult life span.
DATA ANALYSIS
Data analysis was performed using R 2.12 (The R Foundation for Statistical Computing, Vienna) and the R package “nlme” to compute linear mixed models.
Parents’ life-history traits-–We first tested the effects of the experimental treatment on parent's life-history traits, that is, parents’ adult life span, mating success, and age at mating, using generalized linear mixed models (GLMMs), with treatment as a fixed effect and family as a random effect. Sex was also added as a fixed effect. Moreover, mating success, likely to affect adult life span, was added as a covariate in the model with parents’ adult life span as response variable. We then used generalized linear models (GLMs) to test the effects of the treatment and parents’ age at mating on the number of eggs and the number of adults produced per pair. During the mating experiment, the number of potential partners varied across days between 8 and 20 (according to the number of adults available in our rearing, see below), which could have affected mating success. To test for this effect, each individual was characterized by the cumulative number of different available sexual partners over the time it spent in the mating cage. We then added this variable as a factor in the model testing the effect of the experimental treatment on mating success. This factor was, however, not significant and was thus discarded from the final model. Mating success was analyzed using a logit link specifying a binomial error, whereas adult life span and age at mating fitted a normal distribution.
Offspring’ life-history traits—We used GLMM to test the effects of the experimental treatment, mother age at laying, and father age at mating on egg survival, development time, and offspring adult life span, with identity of the mating pair of parents as a random effect. We used a Poisson distribution and a log link for the number of eggs and the number of adults produced per pair and corrected for overdispersion using a quasi-Poisson distribution with a log link when necessary. As mating success and egg survival are binomial variables, data were analyzed using a logit link specifying a binomial error.
For all analyses, we started with the saturated model, including all variables and two-way interactions, and subsequently dropped nonsignificant fixed terms (P > 0.05). We first dropped higher order terms when nonsignificant before considering any removal of main effect terms. For each analysis, we thus present all terms that were included in the final model. Post-hoc analyses of the significant effects were performed using GLMM with family or pair as a random effect. Results are given as mean ± SE.
Results
The 203 manipulated butterflies flew on average 60.23 s (standard error = 2.91 s, range = 0–120 s) over the 120 s test.
EFFECTS OF THE EXPERIMENTAL TREATMENT ON PARENTS’ LIFE SPAN, MATING SUCCESS AND AGE AT MATING
Manipulated butterflies lived shorter than control ones and were also less likely to mate (Table 1). Moreover, females lived longer than males and mated butterflies lived significantly longer than unmated ones (Table 1). To check whether mating success of manipulated individuals was reduced as an indirect effect of the treatment on life span, we added parents’ adult life span as a covariate in the model, and found indeed that life span significantly increased mating success (estimate = 0.101 ± 0.016; z= 6.122; P < 0.001), whereas treatment became nonsignificant (estimate =−0.399 ± 0.273; z=−1.463; P= 0.143). Age at mating did not differ between sexes (P= 0.770) nor between manipulated and control individuals (P= 0.243).
| Response variable | Explanatory variable | Estimate | t or z | P |
|---|---|---|---|---|
| Adult life span | Treatment | −2.818 ± 0.591 | −4.766 | <0.001 |
| Sex | −6.107 ± 0.684 | −8.923 | <0.001 | |
| Mating success | 6.368 ± 0.663 | 9.600 | <0.001 | |
| Mating success | Treatment | −0.622 ± 0.250 | −2.494 | 0.013 |
EFFECTS OF THE EXPERIMENTAL TREATMENT AND PARENTAL AGE ON REPRODUCTIVE OUTPUT
Twenty-eight percent of the butterflies mated in our experiment (62 pairs out of 220 potential pairs, Table 2). Those butterflies belonged to 25 different families (out of 30 initially present in our study), out of which 17 were equally represented across the four treatment-by-sex groups. The number of mating pairs was not significantly different among the four groups (Table 2; goodness-of-fit test: χ2= 4.968, df = 3, P= 0.174). In addition, both mother’ and father’ age at mating was not significantly different among the four groups (goodness-of-fit tests: χ2= 3.605, df = 3, P= 0.307 for mother age at mating; χ2= 1.061, df = 3, P= 0.787 for father age at mating). Both the number of eggs produced per pair and the number of adults produced per pair depended only upon the interaction between paternal treatment and paternal age at mating (Table 3). In mating pairs including a manipulated male, father age at mating significantly decreased the number of eggs per pair (Fig. 1A, Table 3; estimate =−0.206 ± 0.188; t=−2.214; P= 0.037) and the number of adult offspring produced per pair (Fig. 1B, Table 3; estimate =−1.261 ± 0.342; t=−3.685; P= 0.001). In contrast, in pairs including a control male, father age neither affected egg number per pair (Fig. 1A; estimate = 0.188 ± 0.117; t= 1.604; P= 0.119) nor number of adult offspring produced per pair (Fig. 1B; estimate = 0.195 ± 0.168; t= 1.165; P= 0.253).
| Father | Mother | Nmp | Noff |
|---|---|---|---|
| Control | Control | 19 | 583 |
| Control | Manipulated | 17 | 471 |
| Manipulated | Control | 18 | 173 |
| Manipulated | Manipulated | 8 | 179 |
| Response variable | Explanatory variable | χ2 | P |
|---|---|---|---|
| Egg number | Paternal treatment | 4.716 | 0.030 |
| Paternal age at mating | 5.549 | 0.018 | |
| Paternal treatment × paternal age at mating | 10.720 | 0.001 | |
| Adult number | Paternal treatment | 10.835 | <0.001 |
| Paternal age at mating | 2.977 | 0.084 | |
| Paternal treatment × paternal age at mating | 18.754 | <0.001 |
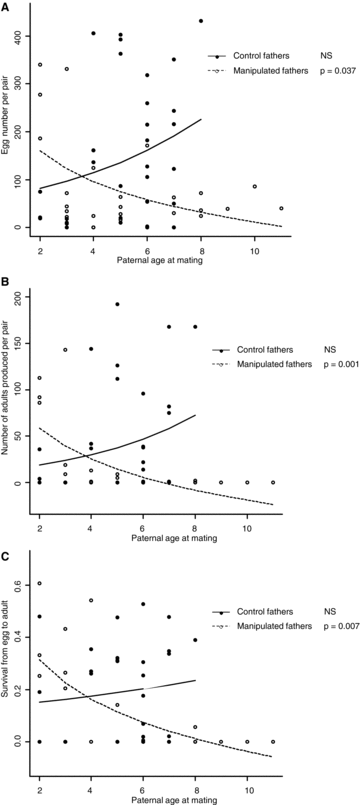
Effect of paternal treatment and paternal age at mating on reproductive output in Pieris brassicae measured as, (A) number of eggs produced per pair, (B) egg survival from egg laying to adult emergence averaged per clutch, and (C) number of adults produced per pair. Predicted relationships were obtained from generalized linear models with a Poisson distribution.
EFFECTS OF THE EXPERIMENTAL TREATMENT AND PARENTAL AGE ON OFFSPRING PERFORMANCE
Egg survival depended only upon the interaction between paternal treatment and paternal age at mating. In pairs including a manipulated male, increased paternal age at mating significantly decreased egg survival (Fig. 1C; estimate =−0.597 ± 0.200; t=−2.99; P= 0.007). In contrast, in pairs including a control male, paternal age had no effect on egg survival (Fig. 1C; estimate = 0.073 ± 0.144; t= 0.508; P= 0.616). Development time was significantly affected by the interactions between paternal treatment and maternal age at laying, as well as between paternal age at mating and maternal age at laying (Table 4). To assess the direction of those effects, we separated individuals into two groups, according to the age at laying of their mother. The median maternal age being 12 days old, we considered individuals whose mother was ≤ 12 days old (young mother group: 696 adult offspring) and individuals whose mother age was > 12 days old (old mother group: 665 adult offspring). In the young mother group, paternal age at mating and paternal treatment had no effect on development time (paternal age at mating: estimate = 0.041 ± 0.209; t= 0.197; P= 0.846; paternal treatment: 0.173 ± 0.949; t= 0.182; P= 0.858). In contrast, in the old mother group, development time increased with paternal age at mating (estimate = 0.571 ± 0.225; t= 2.534; P= 0.018) and in offspring whose father had been manipulated (estimate = 2.013 ± 0.836; t= 2.407; P= 0.024). Finally, offspring adult life span was significantly affected by an interaction between maternal age at laying and maternal treatment (Table 4). Indeed, increased mother age at laying significantly decreased offspring life span in offspring from control mothers (estimate =−0.256 ± 0.057; t=−4.505; P < 0.001), whereas the relationship was not significant in offspring from manipulated mothers (estimate =−0.018 ± 0.067; t=−0.266; P= 0.790) (Fig. 2).
| Response variable | Explanatory variable | χ2 | P |
|---|---|---|---|
| Egg survival | Paternal treatment | 5.904 | 0.015 |
| Paternal age at mating | 0.524 | 0.469 | |
| Paternal treatment × paternal age at mating | 8.024 | 0.005 | |
| Development time | Paternal treatment | 21.794 | <0.001 |
| Paternal age at mating | 18.840 | <0.001 | |
| Maternal age at laying | 5.769 | 0.016 | |
| Paternal treatment × maternal age at laying | 58.805 | <0.001 | |
| Paternal age at mating × maternal age at laying | 32.432 | <0.001 | |
| Offspring adult life span | Maternal age at laying | 21.209 | <0.001 |
| Maternal treatment | 0.633 | 0.426 | |
| Offspring sex | 86.622 | <0.001 | |
| Maternal treatment × maternal age at laying | 7.643 | 0.006 |
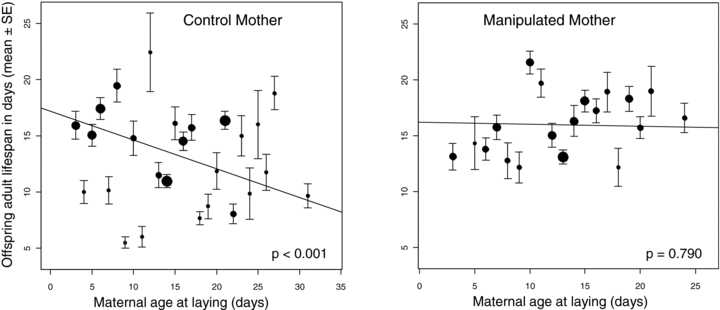
Relationship between offspring adult life span and maternal age at laying for offspring from (A) control females and (B) manipulated females. Circles are sized proportionally to offspring sample size, ranging from two offspring (e.g., life span of offspring from 9 days old control mothers at laying) to 90 offspring (e.g., life span from 21 days old control mothers at laying). For each mother's treatment, the linear relationship between offspring adult life span and maternal age at laying was plotted from the random slope model (see text). Error bars represent standard errors.
Discussion
PARENTS’ LIFE-HISTORY TRAITS ARE AFFECTED BY THE EXPERIMENTAL TREATMENT
Our results strongly suggest that the mobility treatment in stressful conditions induced metabolic costs that traded off with life-history traits in manipulated butterflies. Indeed, manipulated butterflies had a reduced life span, which indirectly reduced their mating success. Manipulated butterflies lived 3 days shorter than control butterflies. This corresponded to a reduction of life span of 28% (3 of 11 days) and 18% (3 of 17 days), respectively for males and females. Likewise, mating success was reduced by 22% in manipulated individuals. Although the experimental treatment was thus particularly costly for manipulated butterflies in our laboratory study, to what extent this type of resource allocation trade-off also occurs in natural populations is an open question. Indeed, the treatment might have affected parents and their offspring through a metabolic flight cost (which could be comparable to what occurs in nature) but also through the stress induced by the vortex test itself. Indeed, the vortex test implies that individuals have no other choice than flying or being strongly shaken in the confined device. As a result, even not directly comparable to a natural situation, this test is undoubtedly extremely stressful for individuals. We thus expect that the consequences of the vortex test are comparable to the consequences of a stress encountered in nature. We could have expected a cost of mating in individuals from the manipulated group, as the literature provides some evidence of mating cost in the butterfly Callophrys xami in conditions of nutritional stress (Cordero 2000). We found, however, that both mated females and males lived surprisingly longer than unmated ones, whether manipulated or not. Mate choice may explain this pattern provided that individuals of high-quality mated preferentially (see below for a detailed discussion on the potential role of mate choice in our study).
REPRODUCTIVE OUTPUT AND EXPRESSION OF PROGENY'S LIFE-HISTORY TRAITS ARE MODULATED BY BOTH MATERNAL AND PATERNAL EFFECTS
Our results show that paternal effects, through an interaction between paternal treatment and paternal age, were marked early in ontogeny, that is, from the egg stage until adult emergence. Indeed, the total number of eggs produced per pair, egg survival until adult emergence, and the total number of adults produced per pair significantly decreased with paternal age at mating but only for manipulated fathers. In contrast, maternal effects occurred late in progeny life history and always through an interaction. Indeed, we found that offspring development time increased with father age at mating and was longer for progenies from manipulated fathers, but only for offspring from old mothers at laying. Overall, paternal effects thus appear to strongly affect offspring early life history, and are likely to modulate the maternal effects demonstrated in previous studies (for instance in the butterfly P. aegeria, Gibbs et al. 2010a,b). It is thus of major importance to take into account both maternal and paternal effects when investigating the role of parental effects on phenotypic variation in populations and species evolution.
Parental effects shifted from male effects expressed at the larval stage to female effects expressed at the adult stage (Fig. 3). Indeed, maternal effects occurred late in progeny life history and always through an interaction. We found that offspring development time increased with paternal age at mating but only for progenies produced by manipulated males and old mothers at laying. Offspring adult life span was the only trait that variation was not dependent upon paternal age or paternal treatment. In the control group of females, offspring adult life span decreased with increasing maternal age at laying, whereas surprisingly no effect of female age was observed in the manipulated female group. Offspring from manipulated mothers lived even longer than those from control mothers (offspring mean life span from manipulated mothers = 16.05 ± 0.30 days; from control mothers = 14.43 ± 0.29 days). It is noteworthy that our results do not support the oogenesis-flight syndrome hypothesis (Hanski et al. 2006), which predicts that resources may be traded off between flight and oogenesis in flying insects. Indeed, manipulated females did not show reduced numbers of eggs and emerged adults as well as egg survival. The relative importance of paternal and maternal effects along the life cycle has been very poorly documented yet. Schmid and Dolt (1994) also found in the plant Solidago altissima that both paternal and maternal environments affected offspring life history at different stages in the life cycle. In contrast to our study, parental effects shifted from maternal to paternal effects across life history: as the maternal growth environment (e.g., soil or sand) affected early characters such as germination probability or seedling mass, whereas the paternal growth environment had effects later on traits such as leaf length and stem. Similarly, in the fly Telostylinus angusticollis, Bonduriansky and Head (2007) manipulated the parental larval diet quality and found that maternal diet quality affected offspring early life history (e.g., egg-size and development time) whereas paternal diet quality affected offspring traits later in the life cycle (e.g., offspring adult body size). Further empirical studies are required to better understand the relative importance of paternal and maternal effects, and the evolutionary role of these shifts from paternal to maternal effects or conversely.
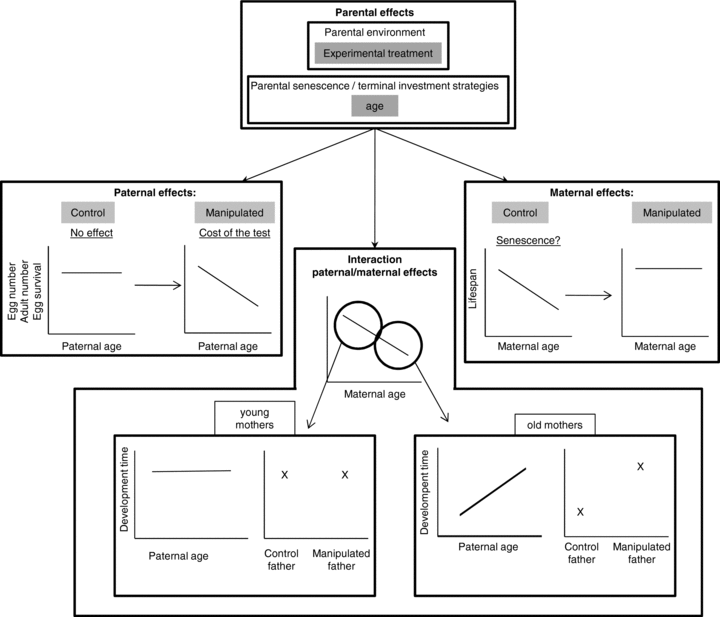
Diagram summarizing paternal and maternal effects acting on offspring life-history traits in Pieris brassicae.
TRUE PARENTAL EFFECTS OR MATE CHOICE?
In our experimental design, we allowed individuals to choose their mate to make this study more relevant to natural populations. The drawback of the design is that it becomes more challenging to disentangle the relative role of parental effects and mate choice in explaining the observed patterns. As mentioned above, manipulated individuals might have endured a large metabolic cost and may thus be of lower quality. Provided that sexual partners discriminate between manipulated and control individuals, the fact that both the number of eggs and of adults per pair and egg survival decreased with increasing paternal age in the manipulated group of fathers might reflect the fact that females preferentially mate with high-quality males. In butterflies, mate choice seems to be driven by females (Darwin 1874; Kemp 2007), although some studies have tested a potential choice by males (see for instance Knüttel and Fiedler 2001; Ellers and Boggs 2003). To our knowledge, the existence of mate choice has not been directly investigated in P. brassicae. Although mate choice by females might be stronger, as most females mate only once, whereas some males mate up to three times (Ducatez unpubl. data), and males are known to display a courtship behavior where they fly around the female that accepts or declines mating (Feltwell 1982; the underlying mechanisms driving acceptance or rejection are not known) it is not known yet whether males also select their mates. We could also expect males of P. brassicae to direct their court toward particular females. Thus, mate choice may also be driven by males in P. brassicae. In our study, mated females lived longer than unmated ones, suggesting that the resources contained in the spermatophores could be directly used by females. Such effect has been demonstrated in other butterflies from the genus Pieris, where increased resources in the spermatophores increased female life span (Stjernholm and Karlsson 2000). Increased life span in mated males was also observed in our study. This may reflect a choice by females of higher quality males as sexual partners, individuals of lower quality characterized here by their shorter life span remaining unmated. This partner selection could be due to a direct choice of females (which may reject males of poor quality), or either to a lower rate of activity in poor quality males that rend them less efficient when they are in competition with other males for female access and also less tough to harass females so as to optimize their mating probability. Overall, the increased life span of the mated males compared with unmated males may be an argument in favor of the existence of a mate choice by females in P. brassicae.
Although mate choice experiments are clearly needed to go further in our study, some of our data strongly suggest that mate choice alone is not likely to explain our results. First, the number of mated pairs did not differ between the four possible sex-by-treatment combinations, suggesting that neither males nor females preferentially chose high-quality partners. Note that the low number of pairs associating two manipulated parents, although not significantly different from the three other groups, is intriguing and would deserve further investigations. Second, under the mate choice hypothesis, we might have expected manipulated individuals to mate later. Age at mating did not differ between manipulated and control individuals as well as among the four groups. This again strongly suggests that mate choice alone is not likely to explain our results, although mate choice by females may coexist with parental effects.
“PATERNAL EFFECT”: A DIRECT EFFECT OF THE NUPTIAL GIFT OR OF POSTCOPULATORY MATERNAL ADJUSTEMENT IN RESPONSE TO MALE QUALITY?
Paternal investment, especially through nuptial gifts, have been shown to increase female life span and fecundity in several insect species (Vahed 1998; Savalli and Fox 1999; Stjernholm and Karlsson 2000), but few studies have tested for their effects on offspring phenotype (Fox and Czesak 2000). This study not only demonstrates the presence of paternal effects acting on offspring phenotype but also suggests that multiple sources of paternal effects (paternal age at mating and paternal exposure to a mobility treatment in stressful conditions) are at work to affect the offspring phenotypes in a nonadditive manner. Different mechanisms have been proposed to explain how paternal effects may be transmitted in species with nuptial gifts. First, males may directly modulate their paternal investment by controlling the quality and the quantity of reserves as well as the quantity of accessory products (e.g., hormones) contained in the spermatophores transmitted to female mates (García-González and Simmons 2005). Second, they may manipulate maternal resource allocation to eggs. Third, environment might induce epigenetic modifications in the germ line (e.g., changes in methylation patterns or chromatin structure), resulting in epigenetic “reprogramming” of sperm (Jablonka and Lamb 1998; Pembrey 2002; Pembrey et al. 2006). Alternatively, such a decrease in reproductive output and egg survival with increasing age at mating for manipulated fathers may be mediated by a maternal effect involving a postcopulatory female assessment of male quality, where females may differently invest in their offspring according to the quality of the nuptial gift. However, in natural populations, P. brassicae females very rarely mate with more than one male (Feltwell 1982, Christer Wiklund pers. comm.). This was also the case in other experiments that we conducted with our breeding in the laboratory, where only 2.8% of the mated females mated twice (Ducatez unpubl. data). This suggests that postcopulatory assessment of male quality is unlikely in this species.
WHICH MECHANISMS DRIVE THE EFFECTS OF AGE AT MATING ON REPRODUCTIVE OUTPUT AND OFFSPRING LIFE-HISTORY TRAITS: SENESCENCE OR VARIATION IN INDIVIDUAL QUALITY?
In our study, negative effects of parental age at mating on reproductive output and offspring life-history traits can either reflect senescence effects (e.g., a decrease of individuals’ quality with time) or variation in individual quality provided the existence of mate choice. As reported above, the number of eggs and of adults per pair as well as egg survival decreased with increasing paternal age only in the manipulated group of fathers. Paternal age at mating might thus be an indicator of how strongly manipulated fathers were negatively affected by the experimental treatment. Indeed, under this hypothesis, manipulated fathers poorly affected by the treatment are expected to mate earlier due to their higher quality. Such pattern might arise from two different mechanisms: first, strongly impacted males may have a lower courtship activity and may thus mate older. Second, as suggested earlier, females may discriminate males according to their quality, so that strongly impacted males would mate the latest. In contrast, under the senescence hypothesis, the effect of paternal age at mating in our study would reflect the decrease in father quality with increasing age.
Our results on the effects of maternal age at laying strongly suggest that senescence in females is likely to drive the observed patterns. First, offspring from old control mothers as well as offspring from old mothers and manipulated fathers lived shorter and had a longer development time, respectively. This suggests that only young females are able to limit the effects of paternal age and paternal treatment. Second, we tested for a change in offspring life history with mother age at laying within family, as family was added as a random effect. In the control mothers group, offspring adult life span thus decreased with maternal age at laying within a family. This age effect thus likely supports the senescence hypothesis, where female quality decreases with age. Such an effect of maternal age on offspring life span has already been documented in several insect species (Priest et al. 2002; Fox et al. 2003). Surprisingly, such senescence effect was not detected in the group of manipulated females, whereas we could have expected stronger senescence effects in the offspring of manipulated mothers. Indeed, stress (e.g., oxidative stress, nutritional stress, thermal stress…), and especially chronic stress, is expected to increase the senescence effects (Hughes and Reynolds 2005; Monaghan et al. 2008). However, the literature also provides some evidence of mild stress inducing protective mechanisms that extend life by altering the rate of senescence (Monaghan et al. 2008). Further experiments are needed to understand the underlying mechanisms behind the maternal effect we observed here.
Overall, our study underlines the importance of transgenerational effects of environmental conditions on individual performance that have the potential to influence population dynamics in butterflies. Investigations of both proximate and ultimate mechanisms driving parental effects are now needed to fully understand the evolutionary consequences of this nongenetic inheritance (Lacey 1998; Marshall and Uller 2007; Uller 2008; Bonduriansky and Day 2009). More generally, our results highlight the need to integrate parental effects arising from both sexes when studying species’ response to environmental changes.
Associate Editor: E. Svensson
ACKNOWLEDGMENTS
We thank K. Akkari and M. Peroz for breeding maintenance, and M. Congretel for experimental work. We thank C. Teplitsky for helpful discussions on the statistical analyses and four anonymous reviewers for suggestions that greatly improved the manuscript. This work was supported by grants from the Institut Fédératif de Recherche (IFR) 101 (Institut d’Ecologie: Biodiversité, Ecologie, Environnement) and the Agence Nationale de la Recherche (ANR) (open call project DIAME Dispersal and metapopulation to MB and DL, 6th extinction call project MOBIGEN to MB and HF). SD was supported by a PhD grant from the French Ministère de l’Enseignement Supérieur et de la Recherche.




