THE GEOGRAPHICAL MOSAIC OF COEVOLUTION IN A PLANT–POLLINATOR MUTUALISM
Abstract
Although coevolution is widely accepted as a concept, its importance as a driving factor in biological diversification is still being debated. Because coevolution operates mainly at the population level, reciprocal coadaptations should result in trait covariation among populations of strongly interacting species. A long-tongued fly (Prosoeca ganglbaueri) and its primary floral food plant (Zaluzianskya microsiphon) were studied across both of their geographical ranges. The dimensions of the fly's proboscis and the flower's corolla tube length varied significantly among sites and were strongly correlated with each other. In addition, the match between tube length of flowers and tongue length of flies was found to affect plant fitness. The relationship between flower tube length and fly proboscis length remained significant in models that included various alternative environmental (altitude, longitude, latitude) and allometric (fly body size, flower diameter) predictor variables. We conclude that coevolution is a compelling explanation for the geographical covariation in flower depth and fly proboscis length.
Coevolution has been a controversial concept within evolutionary biology. Although some have viewed it as a major factor shaping the world's biota (Darwin 1859; Boucher et al. 1982; Janzen 1983; Thompson 1989, 1994; Grimaldi 1999), others have pointed out that its role may have been overestimated (Janzen 1980; Schemske 1983). Its role in mutualistic relationships is viewed as particularly problematic, because these interactions, with the exception of brood site mutualisms such as those between figs and fig-wasps (Ansett et al. 1997), tend to be far less specialized than parasitisms that have received the most focus in studies of coevolution (Schemske 1983; Thompson 1994).
The concept of coevolution can be attributed to Darwin (1859), although its potential for broadly explaining patterns of trait evolution in interacting species was only recognized in the 1960s (Ehrlich and Raven 1964). Darwin (1862) used as a case-in-point the evolution of long-spurred flowers and long-tongued pollinators. His logical assumption was that selection should favor spurs that are longer than the pollinator's tongue. This results in more effective pollination because heads/bodies of insects make better contact with the reproductive parts of the flower when they are forced to insert their entire proboscis to obtain the nectar hidden in the depths of the flower. In turn, pollinators should evolve longer tongues to reach the nectar, which is otherwise hard to reach. This positive feedback system can lead to a coevolutionary arms race (cf. Jerison 1973; Benkman et al. 2003; Langmore et al. 2003), which, if not checked by opposing selective pressures, is capable of producing traits of extraordinary proportions. One famous putative example is the matching of the 30-cm long spur of a Malagasy orchid and the tongue of a hawkmoth Xanthopan morgani ssp. praedicta. But like most purported examples of coevolution, little evidence exists to support it (indeed, the orchid may have been too rare historically to exert much selective pressure on moth tongue length). Although selection on pollinator tongues is very difficult to study, there is mounting evidence from phenotypic studies supporting Darwin's mechanism for spur length evolution in plants (e.g., Nilsson 1988; Johnson and Steiner 1997; Alexandersson and Johnson 2002).
Some researchers have expressed doubt about the importance of coevolution as a major force shaping morphologies of interacting organisms such as plants and their pollinators (Schemske 1983). Indeed, some unlikely alternative hypotheses have been invoked to explain the evolution of long tongues in insects (e.g., that they function as an antipredation device against flower dwelling spiders—Wasserthal 1997). However, more recent studies suggest the paucity of good coevolutionary studies in the past may rather be linked to the variability of coevolutionary outcomes and interacting communities (e.g., Thompson 1999a, b, 2005). They suggest that evolutionary outcomes may change in space and time from being reciprocal (coevolutionary hot spots) to nonreciprocal cold spots (e.g., Gomulkiewicz et al. 2000). This insight has underlined the need for more multipopulation studies. Coevolutionary outcomes may also be affected by the abiotic environment and hence it is necessary to take into account multiple ecological effects when considering trait evolution.
We investigated the specialized mutualism between Zaluzianskya microsiphon (Scrophulariaceae) and the long-tongued fly Prosoeca ganglbaueri (Nemestrinidae). This fly pollinates ca. 10 plant species in the Drakensberg mountains of South Africa (Goldblatt and Manning 2000). Of those, Z. microsiphon that has nectar concealed at the tip of a long corolla tube is by far the most widespread and abundant member of this guild and fulfils the bulk of the fly's nectar requirements (Johnson et al. 2002; Anderson et al. 2005). Because this system has the potential to conform to a classical Darwinian coevolutionary scenario, we predicted that proboscides of the flies would covary with corolla tube lengths of Z. microsiphon over the geographical scale of their distribution ranges.
Materials and Methods
To establish whether there are geographical patterns of trait covariation, during January–March 2004 and 2005 we measured the proboscis and thorax widths of 3–60 P. ganglbaueri flies, flower depths and widths of 20–89 Z. microsiphon plants at 16 study sites covering an area of ca. 43,850 km2 in the Drakensberg mountains (Fig. 1; Table 1). We excluded an outlying population of Z. microsiphon which is genetically anomalous, known to form hybrids with a congener, and which possibly belongs to a different clade (Archibald et al. 2004, 2005). This population is also unique in that it occurs outside the range of P. ganglbaueri and is visited by two other nemestrinid fly species. Unlike this anomalous population, the Drakenberg populations of Z. microsiphon are not known to form hybrids and are likely to belong to a single clade (Archibald et al. 2004). The correlation between the mean flower corolla length and mean fly proboscis length among populations was explored using Pearson's correlation.
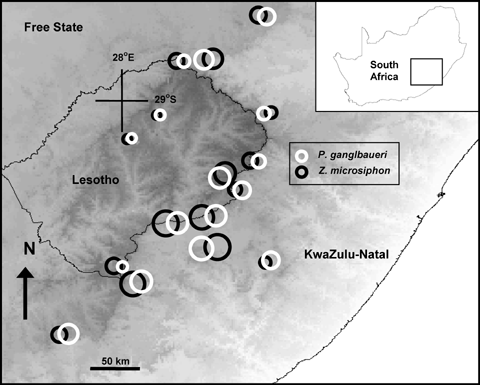
Variation in the functional proboscis length of P. ganglbaueri and the corolla length of its main food plant (Z. microsiphon) in 16 populations. Length of these traits are proportional to the diameter of the circles. The diameter of the circle in the boxed legend represents 25 mm.
| Population | Prosoeca proboscis length (mm) | Zaluzianskya corolla length (mm) | Latitude | Longitude | Altitude | Prosoeca thorax width (mm) | Flower width (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 36.5±0.8 (10) | 34.0±0.6 (23) | 27.96 | 29.67 | 1969 | 6.6±0.1 | 16.4±0.31 |
| 2 | 37.7±0.8 (28) | 42.2±0.5 (44) | 28.69 | 28.90 | 2181 | 6.7±0.1 | 14.6±0.4 |
| 3 | 26.2±0.4 (60) | 32.6±0.4 (59) | 28.74 | 28.89 | 2727 | 5.7±0.1 | 13.9±0.4 |
| 4 | 28.8±1.3 (5) | 25.2±0.4 (20) | 29.27 | 29.27 | 2411 | 4.8±0.2 | 18.4±0.3 |
| 5 | 20.4±0.5 (9) | 19.4±0.4 (20) | 29.46 | 28.47 | 2550 | 4.5±0.1 | 15.9±0.6 |
| 6 | 28.4±0.9 (10) | 32.8±0.5 (42) | 29.59 | 29.31 | 2500 | 4.8±0.1 | 22.0±0.6 |
| 7 | 21.9±0.5 (11) | 20.5±0.3 (20) | 29.67 | 28.37 | 2506 | 4.2±0.1 | 14.8±0.4 |
| 8 | 36.1±2.1 (17) | 27.8±0.4 (22) | 29.87 | 29.12 | 2415 | 6.3±0.2 | 24.1±0.3 |
| 9 | 44.4±0.5 (3) | 46.3±0.8 (20) | 29.87 | 29.72 | 2334 | 6.6±0.3 | 19.2±0.4 |
| 10 | 44.4±0.6 (20) | 51.8±0.5 (44) | 30.05 | 28.93 | 2275 | 6.4±0.1 | 27.3±0.8 |
| 11 | 44.8±1.0 (11) | 54.6±0.8 (20) | 30.14 | 28.69 | 1960 | 6.5±0.2 | 26.2±0.5 |
| 12 | 49.6±0.9 (21) | 54.5±0.4 (54) | 30.40 | 28.82 | 1818 | 5.8±0.1 | 24.6±0.7 |
| 13 | 35.9±1.3 (5) | 24.7±0.3 (30) | 30.66 | 29.57 | 2126 | 5.8±0.4 | 15.6±0.5 |
| 14 | 21.7±0.4 (18) | 33.8±0.4 (20) | 30.73 | 28.14 | 2450 | 5.3±0.1 | 22.5±0.7 |
| 15 | 45.7±1.7 (7) | 52.1±1.9 (20) | 30.76 | 28.21 | 1800 | 5.02±0.1 | 23.3±0.5 |
| 16 | 40.3±0.5 (9) | 32.7±0.4 (23) | 31.18 | 27.58 | 2227 | 6.5±0.1 | 20.1±0.5 |
Variation in Z. microsiphon corolla length and P. ganglbaueri proboscis length could potentially be explained by allometric relationships among traits on the same organism, instead of coadaptation, or alternatively by abiotic factors. Thus multiple regression was used to analyze simultaneously the effects and relative importance of abiotic and allometric predictor variables on flower corolla length and fly proboscis length. Abiotic variables considered were latitude, longitude, and altitude at each locality, acquired using GPS. Potential allometric traits considered were fly thorax width and flower diameter. The multiple regression with fly proboscis length as a response variable included Z. microsiphon corolla length, fly thorax width, latitude, longitude, and altitude as predictor variables. The multiple regression with Z. microsiphon corolla length as a response variable included fly proboscis length, flower diameter (widest point across the spreading corolla lobes), latitude, longitude, and altitude as predictor variables.
The above regression analyses rely on the assumption that trait values for populations are the outcome of independent evolution, and not spatially structured by gene flow or common descent. Therefore, to test whether corolla and tongue lengths are geographically structured, we used Mantel tests (1000 permutations) implemented in NTSYS (Rohlf 2000). A positive relationship between pairwise geographic distances and pairwise trait (tongue lengths and corolla lengths) differences would imply that trait values for populations are structured by gene flow or common descent. Alternatively, no relationship or a negative relationship would imply that trait values in each population are influenced more by local evolutionary processes than by gene flow or common descent.
To test whether plant phenotype was adapted to the pollinator environment, we bagged flower buds of Z. microsiphon in two populations situated approximately 30 km apart (Sehlabathebe Lodge where plants have short corollas and Ramas gate where plants have long corollas). Once these flowers had opened, we cut the inflorescences from both sites and presented the inflorescences from both populations to foraging flies at the Ramas gate site only. To avoid contamination of the local gene pool, we captured flies after they had visited a single flower on any of the experimental inflorescences. We did not notice any preference by the pollinators for any particular flower morph although this was not explicitly tested. After a single flower on each inflorescence had been visited by a fly, the cut inflorescences from both populations were placed in a nutrient-rich medium and any seeds that developed from the visited flowers were later counted. Zaluzianskya microsiphon is self-incompatible (Johnson et al. 2002), so any seeds that arise must be the consequence of effective cross-pollination. To control for the effects of possible resource limitation of seed production, we hand-pollinated a second flower on each inflorescence. Here we expected that if tube length was adapted to tongue length, then plants with tubes longer than the average pollinator tongue should set more seed than plants with shorter tubes.
Results
Proboscis length of P. ganglbaueri and corolla length of Z. microsiphon were found to be highly variable between populations (Fig. 1, fly proboscis F= 100.22, df = 15, P < 0.0001, flower corolla tube, F= 479.49, df = 15, P < 0.0001). For example, the shortest mean tongue length in any population was 20.4 ± 0.5 mm whereas the longest was 49.6 ± 0.9 mm (variability was similar for tube lengths). Population mean values for corolla length of Z. microsiphon and proboscis length of P. ganglbaueri were significantly correlated (r= 0.83, P < 0.001; Fig. 2).

The relationship between proboscis length P. ganglbaueri flies and the depth of Z. microshiphon flowers in 16 populations. Each symbol represents the mean trait value (se) per population. A fly from the shortest tongued and longest tongued population are included to show the magnitude of variation in the system.
In a multiple regression that included the environmental and allometric predictor variables, corolla tube length remained a significant predictor of fly proboscis length (Table 2). Similarly, in a second regression model, fly proboscis length significantly predicted flower corolla length, even when other predictor variables were included (Table 2).
| Response variable | Standardized partial regression coefficients | Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proboscis length | Corolla length | Thorax width | Flower width | Altitude | Latitude | Longitude | R 2 | F | P | |
| Proboscis length | – | 0.40* | 0.29* | – | −0.35* | 0.25 | 0.19 | 0.85 | 17.80 | 0.0001 |
| Corolla length | 0.74* | 0.36 | −0.007 | −0.30 | −0.21 | 0.69 | 7.60 | 0.003 | ||
- *P<0.05.
Differences in the mean tube and tongue lengths between populations were not significantly structured by geographical distance. This was evidenced by Mantel tests with negative slopes for correlations between pairwise geographical distances and pairwise differences in corolla lengths (r=−0.176, P= 0.06) or proboscis lengths (r=−0.068, P= 0.29).
When short-tubed plants of Z. microsiphon were moved to sites with long-tubed plants and long-tongued flies, their mean seed set (± SE) per flower was significantly lower (8.5 ± 4.2, n= 20) than in long-tubed plants (29.7 ± 4.2, n= 20) that were identically exposed to fly visits (t= 2.04, P= 0.048). This was probably a result of a poor fit between pollinator and flower and not resource limitation as both short- and long-tubed control hand-pollinated (n= 20 per treatment) flowers had a threefold higher seed set than flowers exposed to single pollinator visits.
Discussion
The patterns of trait covariation documented in this study are consistent with population-level coevolution between P. ganglbaueri flies and Z. microsiphon flowers. This relationship remains significant when other environmental and allometric variables are considered simultaneously in multiple regression models (Table 2). Our experiments, and those of others (e.g., Nilsson 1988; Johnson and Steiner 1997; Alexandersson and Johnson 2002) show that pollinator proboscis length can exert strong selection on flower length, but the converse—selection on fly proboscides—is much harder to demonstrate. Although maneuverability between flowers is reduced by long tongues in bees (Harder 1983), it is logical to assume that the negative effects of long tongues are balanced by the strong positive effects of being able to obtain more nectar from long-tubed flowers. Hence, it is reasonable to expect selection to favor longer proboscides in P. ganglbaueri when the nectar of Z. microsiphon, its major food plant, is hidden in corolla tubes that exceed the length of the fly's proboscis.
The regression models suggest that altitude and fly thorax width might also influence fly proboscis length (Table 2). Thus, although coevolution remains the most compelling explanation for these data, it is impossible to exclude a role for sequential trait evolution (Jermy 1976), in this case if flower length is unilaterally adapted to fly proboscis length, which in turn, is modified according to other selective pressures or allometric relationships. For example, abiotic factors such as low temperatures and strong winds may constrain the evolution of fly proboscis length at high altitudes. Thus, abiotic factors and coevolution probably act together to shape morphological traits.
The lack of a positive relationship between pairwise geographic distance and pairwise trait differences (Fig. 1) is consistent with a geographical mosaic of coevolution where local adaptation at the population level plays a major role in shaping trait values (Thompson 1996b; Thompson and Cunningham 2002). As spatial proximity among populations is usually a good proxy for genetic relatedness and likelihood of gene flow (e.g., Wright 1943; Malécot 1955; Kimura and Weiss 1964), the results of the Mantel tests suggest that neither gene flow nor common descent plays a major role in shaping the trait values of populations. This nonstructured pattern, together with occasional trait mismatches at some sites (1, 2) strongly suggest that the outcomes of coevolutionary relationships can be spatially variable, which is one of the predictions of the geographical mosaic theory of coevolution (Thompson 1999b, 2005).
Coevolutionary theory (see Thompson 1999b) predicts that variability in the composition of interacting communities (e.g., the plant community visited by the fly) may affect the morphological end products of the coevolutionary process (in this case tongue and corolla length). For example, the coevolutionary process between P. ganglebaueri and Z. microsiphon may be constrained if there are alternative short-tubed flowers available as nectar sources for the fly. In contrast, simpler communities, lacking these short-tubed nectar plants, may allow escalatory coevolution between the fly and Z. microsiphon. Variability in a system, such as this one, could also be generated by abiotic factors that constrain how far the coevolutionary process may proceed.
In a few populations we found that plant and pollinator traits were mismatched (Fig. 1). Although these mismatches are predicted by the geographical mosaic theory of coevolution (Thompson 1999b, 2005). Ridenhour and Nuismer (2007) argue that they should be rare when coevolution is escalatory. In this case, we suspect that the occasional mismatches arise when plant communities are relatively recent assemblages or when there have been recent range shifts in fly populations.
To our knowledge, this is the first study showing that morphological traits of a nectar-producing plant and its pollinator are correlated across a broad geographic range in a manner consistent with pairwise coevolution. In an earlier study, Steiner and Whitehead (1990, 1991) showed that leg lengths of oil-collecting bees correlated with the spur lengths of the oil-producing flowers that they pollinate. However, Steiner and Whitehead did not explicitly test alternative explanations for these patterns or the fitness consequences of the traits. Toju and Sota (2006a, b) studied coevolution in an antagonistic system in which weevils use their rostrums to bore through the thick defensive pericarp of Japenese Camelia fruit to oviposit near the plant's seeds. In this antagonistic system there was very strong matching of these coadapted traits in each population. These studies suggest that coevolution can lead to trait diversification across populations of interacting species (cf. Thompson 1999b; Thompson and Cunningham 2002).
Associate Editor: J. Kohn
ACKNOWLEDGMENTS
Financial support was granted by the NRF and University of KwaZulu-Natal. J. Jersakova provided assistance in the field. A. Ellis, D. Hansen, and J. Terblanche helped provided valuable discussion and/or helped with statistical analysis. J. Manning shared his knowledge on collecting sites.




