THE COST OF REPRODUCTION INDUCED BY BODY SIZE AT BIRTH AND BREEDING DENSITY
Abstract
Body size at birth has implications for the quality of individuals throughout their life. Although large body size is generally considered an advantage, the relationship between body size at birth and long-term fitness is often complicated. Under spatial or temporal variation in environmental conditions, such as the seasonally changing densities of Fennoscandian vole populations, selection should favor variation in offspring phenotypes, as different qualities may be beneficial in different conditions. We performed an experiment in which a novel hormonal manipulation method was used to increase phenotypic variance in body size at birth in the bank vole (Myodes glareolus). The effects of body size on the future fitness of young males and females were then studied at varying population densities in outdoor enclosures. Our results show that small body size at birth and high breeding density increase the survival costs of reproduction. However, there was no interaction between the effects of body size and density on survival, which suggests that the fitness effects of body size were strong enough to persist under environmental variation. Moreover, litter size and the probability of breeding were more sensitive to variation in breeding density than offspring size. Therefore, it is unlikely that individual fitness could be optimized by adjusting offspring body size to the prevailing population density through adaptive maternal effects. Our results highlight the significance of the costs of reproduction in the evolution of life-history traits, and give strong experimental support for the long-term fitness effects of body size at birth.
Body size at birth has a great significance for individual fitness as many life-history traits, for example age at maturity and fecundity, are size dependent. In mammals, birth size is determined during early ontogeny, but it has implications for the quality of individuals throughout their life (Desai and Hales 1997; Lindström 1999; Metcalfe and Monaghan 2001; Lummaa and Clutton-Brock 2002; Ozanne and Hales 2004). In addition to its genetic background (e.g., Mappes and Koskela 2004), body size at birth is a strongly maternally derived trait (Desai and Hales 1997) and, at least in some species, it is known to be under the control of the mother's endocrine system (Oksanen et al. 2002). Therefore, environmental factors affecting the mother's maturation age, condition, or diet may have substantial influence on the body size at birth of her offspring. Through maternal effects, the mother may modify the phenotype of her offspring, and it has been suggested that maternal effects may influence also larger-scale processes such as population dynamics, expression of adaptive phenotypic plasticity, and character evolution (Rossiter 1996; Mousseau and Fox 1998; Dufty et al. 2002; Sheldon 2002).
Although large body size is generally considered an advantage, there are several factors that may constrain the evolution of body size and maintain the genetic variation. Most importantly, there is a well-established trade-off between offspring number and size implying that at some point it is more beneficial to produce one more offspring than to increase their size and quality (Smith and Fretwell 1974). When based on negative genetic correlation, as in the case of the bank vole (Myodes glareolus) (Mappes and Koskela 2004), the evolutionary significance of this trade-off is undeniable.
Numerous studies have focused on finding experimental evidence for the fitness effects of the trade-off between offspring number and size frequently reporting smaller mean juvenile body size in litters that have been experimentally enlarged and larger mean juvenile body size in litters that have been experimentally reduced (e.g. Mappes et al. 1995a; Koskela et al. 1998, 1999; Humphries and Boutin 2000). The long-term fitness effects of body size and growth during early development, however, are more diverse and often inconsistent (e.g. Koskela 1998; Festa-Bianchet et al. 2000; Neuhaus 2000; Oksanen et al. 2001; McAdam and Boutin 2003).
In nature, the long-term effects of body size at birth may be obscured by environmental factors that influence the expression of the trade-off as well as its further effects on individual fitness (van Noordwijk and de Jong 1986; Zera and Harshman 2001). Czesak and Fox (2003) report an example of environmental effects on genetic relationship among traits in the seed beetle (Stator limbatus) in which the magnitude of the genetic trade-off between egg size and lifetime fecundity differs between environments. Sinervo et al. (2000) have shown that the reproductive success of the two female morphs of side-blotched lizard (Uta stansburiana) characterized by their different offspring size-related reproductive tactics depends on the population density. Oksanen et al. (2003) manipulated offspring number and size simultaneously and showed that the short-term benefits of large body size at birth depend upon the immediate rearing environment in the bank vole. Moreover, the effects of body mass and population density on reproductive success and reproductive costs have been studied in big horn sheep (Ovis canadensis) by Festa-Bianchet et al. (1998). Their results suggest that the costs of reproduction are affected by an interaction between body mass and population density. These are examples of context-dependent benefits of body size suggesting that spatially or temporally varying environment may play a major role in maintaining genetic variability in body size and in the allocation of resources between offspring number and size. Theoretical studies have highlighted this possibility even earlier by showing that in temporarily varying environments selection should favor variation in offspring phenotypes as different qualities may be selected for in different environmental conditions (Cohen 1966; Den Boer 1968; Gillespie 1974, 1977; Ancel Mayers and Bull 2002).
In natural vole populations, life-history traits show both genetic variation (Mappes and Koskela 2004) and phenotypic plasticity (Agrell et al. 1992; Ergon et al. 2001; Norrdahl and Korpimäki 2002; Koivula et al. 2003). We created phenotypic variation in the birth size of bank vole offspring by using a hormonal manipulation method. The manipulation generates a greater than normal follicular ovulation and therefore by increasing the litter size, causes a simultaneous decrease in mean offspring body mass at birth (Sinervo and Licht 1991; Oksanen et al. 2002). To test the prediction that large body size at birth is more advantageous at high densities, where resource competition between individuals is intense (Bujalska 1988; Koskela et. al. 1999; Prévot-Julliard et al. 1999), we studied the effects of body size at birth on the probability of breeding and survival and the reproductive success of the females at varying population densities. Evidence for density-dependent selection on body size at birth would suggest that context dependence plays a role in maintaining genetic variation in body size in bank vole populations. Moreover, density dependence would enable the evolution of adaptive environmentally induced maternal effects, that is, phenotypic adjustment of offspring number and size to prevailing population density (Lacey 1998).
Materials and Methods
STUDY SPECIES
The study species, the bank vole (M. glareolus), is a small rodent species common in northern Europe (Stenseth 1985). The main habitats are forests and fields, and the diet consists of forbs, shoots, seeds, berries, and fungi (Hansson 1985). The density of breeding females is limited through their territoriality (Bujalska 1985; Koskela et al. 1997), and in high densities the maturation of young females is suppressed by social interaction among females (Kruczek and Marchlewska-Koj 1986; Marchlewska-Koj 1997). The patterns and amplitude of density variation show considerable geographical variability, and both stable and cyclic populations are found (Hansson and Henttonen 1985). In our study area, females give birth to a maximum of four litters during the breeding season, which lasts from late April to September. The litter size ranges from two to ten offspring (Koivula et al. 2003).
Nursing period lasts around 20 days, after which the pups reach total independence from their mother. Females do not distinguish their own pups from foreign ones, which enables manipulating and cross-fostering new-born offspring (Mappes et al. 1995a). Large body size at birth and at weaning has been shown to improve the probability of maturation and survival (Mappes et al. 1995b; Koskela 1998; Mappes and Koskela 2004). Bank voles have good trappability, and they are not sensitive to disturbance, which allows monitoring populations by live trapping.
MANIPULATION OF BODY SIZE AT BIRTH
Exogenous gonadotropin hormones were used to generate the ovulation of an increased number of follicles (Muños et al. 1995) in mature bank vole females. The females were originally captured at the study site, Konnevesi central Finland (62°37%N, 26°20%E), and were housed in laboratory for at least two weeks before the experiment started. The treatment forces the females to produce large litters with small individual offspring (Oksanen et al. 2002). Gonadotropin from human menopausal urine (hMG) (Sigma Chemical Co., St. Louis, MO) was used to generate a greater than normal follicular ovulation and human chorionic gonadotropin (hCG) (Sigma Chemical Co.) to induce ovulation. The manipulation was performed in the laboratory, where the animals were kept on a 16:8 light/dark photoperiod and given a continuous supply of food pellets and water. Females were subcutaneously injected every 12 h a total of five times with 0.4 IU hMG dissolved in 0.1 mL isotonic sodium chloride. The dose was calculated on the basis of the FSH content. Twelve hours after the last injection the females were subcutaneously injected with 6 IU of hCG dissolved in 0.1 mL isotonic sodium chloride. After the last injection, the females were paired to randomly chosen mature males by placing the pair in the same cage for a week. Another set of females was sham injected six times with isotonic sodium chloride to control for both the hMG and hCG injections of the treated females, and paired to randomly chosen males.
Both sets of females were observed twice a day for parturition. The time period from the pairing to the birth of the pups was on average 19 ± 1.5 days for the sham-injected group and 18 ±0.5 days for the hormone-injected group. When a birth was detected, pups were individually marked (toe codes) and sexed and their body mass was measured with electronic scales. Sham-injected females (n= 45) were divided into two groups for cross-fostering. Litters of females in the first group (n= 24) were replaced with offspring that were originally produced by the hormonally treated females (n= 17; mean litter size 10.24 ± 0.48, range: 7–13; mean body mass 1.50 ± 0.04 g), and in the second group (n= 21) the litters were replaced with offspring from other sham-injected females (n= 37; mean litter size 5.05 ± 0.18, range: 3–8; mean body mass 1.83 ± 0.03 g). The cross-fostering therefore produced experimental litters consisting of either small- or normal-sized offspring originating from several different birth mothers. The litter size of foster females was not changed. Thus the maximum number of offspring raised by any female was eight pups. This procedure enabled separating the effects of body size at birth from the effects of litter size and the quality of maternal care after gestation. The foster females nursed their litters in the laboratory until the offspring reached the age of 20 days. Juveniles were then weighed, separated from the foster mother, and released in outdoor enclosures.
STUDY DESIGN
The experiment was carried out during April–October 2000. The study site consists of 11 identical 0.2-ha outdoor enclosures situated in a fallow field. Enclosure fences are constructed of 1.25-m high galvanized sheet metal, which is embedded 0.5 m into the ground. The fences prevent the emigration and immigration of the voles, but permit possible predation by avian species. Juveniles from the two body size manipulation groups were divided in the enclosures in varying densities. Individuals originating from the same foster litter were assigned into different density treatments and replicate enclosures to randomize the effects of common rearing environment. At the beginning of June, 84 female juveniles (ca. 30-day old) were introduced into the enclosures (4, 6, 8, 10, or 12 females per enclosure), followed by 84 male juveniles (ca. 37-day old) one week later (4, 6, 8, 10, or 12 males per enclosure). The density was defined as the total number of individuals in the enclosure, which was 8, 12, 16, 20, or 24. Each density was assigned to two enclosures except for density 8, which were assigned to three enclosures to balance the total number of individuals per density treatment. One half of the males and females in each enclosure originated from the litters of the hormone-treated mothers and the other half from the litters of the sham-injected mothers. After the first week, we conducted a check-up trapping, and added 11 juvenile females into the enclosures to compensate for the individuals who appeared missing. We also released 22 older mature males, two per enclosure, to stimulate the maturation of the young females. These males were removed from the enclosures in the beginning of July, after the younger males had reached sexual maturity, and were not subject to any analyses.
MEASURES OF FITNESS
To monitor the animals, 20 multiple-capture live traps, baited with oats, sunflower seeds, and a piece of potato, were distributed in each enclosure in a 5 × 4 grid with 10 m separating each trap. The traps were set three times during two days (morning–evening–morning), once every week throughout the breeding season to monitor the survival and maturation of the individuals. Females that were found to be pregnant with their first litter were transferred to the laboratory on average three to four days prior to the birth of the pups to measure offspring number and size. Maturation age was defined as the age at which the female first gave birth and was therefore calculated only for breeding females. The mothers and their newborn offspring were immediately returned to the enclosures in their breeding cage with the cover left open, so that the mother could carry the pups to her nest. Each female was assigned a breeding status “breeding” or “nonbreeding” depending on if she did or did not produce at least one litter during the breeding season.
The breeding status of males was determined according to their plasma testosterone level (ng/mL) at the age of ca. 60 days. Retro-orbital blood samples were collected in heparinized capillary tubes (inner diameter: 0.5–0.6 mm, outer diameter: 1.3–1.4 mm) and centrifuged at 12,000 ×g for 5 min in a haematocrit centrifuge (Heraeus Biofuge haemo, Kendro Laboratory Products, Osterode, Germany) to separate the serum from the blood cells. Testosterone level was measured using a radioimmunoassay kit (TESTO-CTK, DiaSorin, Byk-Sangtec Diagonstica GmbH & Co, Dietzenbach, Germany). Five hundred microliter of 125I-labeled testosterone was added to the tubes coated in a testosterone antiserum raised in rabbits. Fifteen microliter of the blood plasma samples or 50 μl of the standards were then added to the tubes. The mixtures were equilibrated at 37°C for 3 h, during which time labeled testosterone and the testosterone contained in the standards or samples compete for a fixed and limited number of antibody-binding sites. After 3 h the incubation mixture was carefully aspirated so that no trace remained, and the radioactivity of the tubes was measured in a Gamma counter. The amount of radioactivity measured is inversely related to the amount of unlabeled testosterone in the samples and standards. The amount of testosterone in the samples was therefore determined by interpolation from the standards calibration curve.
Males with testosterone level of 2.10 ng/mL or lower were assigned a breeding status “nonbreeding” and males with testosterone level of 3.8 ng/mL or higher were assigned a breeding status “breeding.” The partition was based on a naturally occurring gap in the distribution of the measured testosterone levels and our previous study on bank vole testosterone levels in enclosure environment. The maturation of male bank voles can be deduced from testes size by visual observation. In our previous dataset, the testes size correlated positively with the plasma testosterone level (Spearman's r= 0.456, n= 37, P= 0.005 (T. Mappes and E. Koskela, unpubl. data).
At the end of the breeding season (at the beginning of October), just before winter and snowfall, all individuals were trapped and transferred to the laboratory. Survival was measured as a binary variable taking the values 0 or 1 (equivalent to “dead” or “alive”) depending on the result of the trapping. As only a small proportion of offspring survive over the winter to the next breeding season (Oksanen et al. 2002), reproduction and survival during the first breeding season are valid measures of the long-term fitness of an individual.
DATA ANALYSIS
The data were analyzed using SAS version 9.1 software. The probability of breeding and the probability of surviving to the end of breeding season were analyzed with PROC GLIMMIX procedure with binomial distribution and logit link function. The effects on maturation age, litter size, offspring size and testosterone level were analyzed with PROC MIXED procedure. Sex (female/male), breeding status (breeding/nonbreeding), and treatment group (hormone/sham) were used as fixed factors and body mass at birth and breeding density as continuous covariates. In the analysis of mean offspring body mass, litter size was included as an additional covariate. The effect of study enclosure was included in the models as a random factor. We started from a model that included all the above-mentioned factors as main effects and their two-way and three-way interactions. The model was then hierarchically simplified. Independent samples t-test and the Levene's test for equality of variances were performed in SPSS 14.0 for Windows.
The possible effects of common rearing environment were controlled for by cross fostering the litters and distributing the individuals originating from the same foster litter into different density treatments and replicate enclosures. Moreover, the effect of enclosure was added in the models as a random factor. The individuals were therefore treated as independent observations, and the unit for sample size is an individual, except for the analyses concerning the characteristics of the first litters produced by the females, in which the unit for sample size is a litter. Some of the females that were suspected to be missing and therefore replaced with additional individuals (see the study design) later proved to be alive. Therefore the population densities changed slightly at the beginning of the breeding season (± 2 individuals at maximum), and the samples sizes in statistical presentations are not completely in accordance with the ones described in the original study design (original n= 168; final n= 176).
Results
BODY SIZE
The birth size of individuals originating from the litters of hormone-treated mothers was significantly smaller than the birth size of individuals originating form the litters of sham-injected mothers (mean body mass at birth ± SE, sham: 1.84 ± 0.02 g; hormone: 1.48 ± 0.02 g; independent samples t-test: t= 13.421, n= 176, P < 0.001). When combined, the birth sizes of the offspring originating from both groups formed a continuous distribution of body sizes with relatively large phenotypic variance compare to the natural variation (Levene's test for equality of variances between offspring born to sham-treated mothers and offspring from both two groups: F= 17.249, n= 265, P < 0.001; Fig. 1). The difference in mean body mass between the manipulation groups persisted to the age of 20 days, when the offspring reach independence from their mother (mean body mass at 20 days ± SE, sham: 11.4 ± 0.1 g, hormone: 10.2 ± 0.1 g, independent samples t-test: t= 6.234, n= 176, P < 0.001), and even to the end of the breeding season (mean body mass at fall ± SE, sham: 21.7 ± 0.5 g, hormone: 19.2 ± 0.4 g, independent samples t-test: t= 3.732, n= 78, P < 0.001).
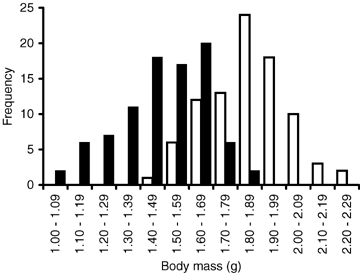
The frequency distribution of body mass at birth. Open bars represent the offspring of sham-treated mothers and black bars the offspring of hormone-treated mothers.
BREEDING
The probability of breeding was negatively related to the breeding density. Moreover, individuals originating from the litters of the hormone-treated mothers were less likely to breed than individuals originating from the litters of the sham-treated mothers. The significant effect of sex suggesting that females were more likely to breed than males, however, may be caused by the different methods of determining the breeding status (Table 1, Fig. 2).
| Estimate | SE | F | df | P | |
|---|---|---|---|---|---|
| Intercept | 3.165 | 1.802 | – | – | – |
| Sex (S) | 1.349 | 0.387 | 12.13 | 1, 146 | <0.001 |
| Treatment (T) | 1.156 | 0.532 | 4.71 | 1, 146 | 0.031 |
| Body mass (B) | −1.480 | 1.0517 | 1.98 | 1, 146 | 0.161 |
| Density (D) | −0.241 | 0.084 | 8.24 | 1, 9.0 | 0.018 |
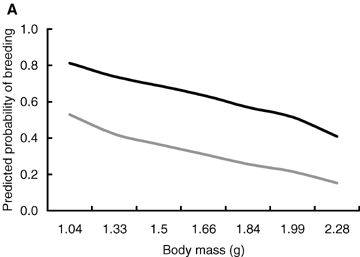
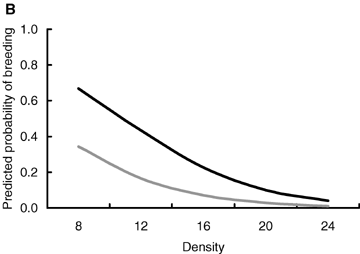
Predicted probability of breeding in relation to (A) body mass at birth and (B) breeding density, that is, the number of individuals in the enclosure. Black line, females; gray line, males. The figure is produced from the model presented in Table 1.
The majority of females produced at least one litter during the 4-month long breeding season (reproducing: 63%, nonreproducing: 37%, n= 92). Maturation age of the females, that is, age at which they first gave birth (range: 54–100 days, mean = 64, SD = 10.3) was not related to the breeding density. However, there was a marginally significant interaction between the effects of treatment group and body mass at birth (Table 2). Among the offspring of sham-treated mothers, maturation age was negatively related to body mass at birth, whereas among the offspring of hormone-treated mothers this relationship seemed to be opposite. The size of the first litter decreased with increasing breeding density (range: 3–7 pups, mean = 4.82, SD = 1.05; Table 2) whereas the mean body mass of the offspring decreased with the size of the litter (range: 1.51–2.21 g, mean = 1.83, SD = 0.17; Table 2). The plasma testosterone level of the males (range: 0.10–16.80 ng/mL, mean = 2.947, SD = 4.095) tended to be lower in high densities, but was not affected by the body mass at birth (Table 2).
| Fixed effects | Estimate | SE | F | df | P | |
|---|---|---|---|---|---|---|
| Maturation age | Intercept | 37.129 | 13.314 | – | – | – |
| Treatment (T) | 33.039 | 16.129 | 4.20 | 1, 42.4 | 0.046 | |
| Density (D) | 1.293 | 1.117 | 1.34 | 1, 8.58 | 0.278 | |
| Body mass (M) | 11.385 | 6.445 | 0.11 | 1, 43.5 | 0.743 | |
| T × M | −19.221 | 9.616 | 3.99 | 1, 42.5 | 0.052 | |
| Litter size | Intercept | 5.856 | 1.205 | |||
| Treatment (T) | 0.154 | 0.367 | 0.18 | 1, 49.3 | 0.676 | |
| Density (D) | −0.086 | 0.025 | 11.71 | 1, 10.4 | 0.006 | |
| Body mass (M) | 0.185 | 2.720 | 0.07 | 1, 41.3 | 0.797 | |
| Offspring size | Intercept | 2.202 | 0.219 | |||
| Treatment (T) | 0.012 | 0.052 | 0.05 | 1, 47.7 | 0.816 | |
| Density (D) | −0.002 | 0.004 | 0.33 | 1, 12.2 | 0.577 | |
| Body mass (M) | 0.104 | 0.105 | 0.99 | 1, 47.5 | 0.325 | |
| Litter size | −0.102 | 0.019 | 26.42 | 1, 46.5 | <0.001 | |
| Testosterone | Intercept | 12.807 | 4.680 | – | – | – |
| Treatment (T) | 1.445 | 1.665 | 0.75 | 1, 50.3 | 0.389 | |
| Density (D) | −0.459 | 0.216 | 4.52 | 1, 6.59 | 0.073 | |
| Body mass (M) | −3.907 | 2.997 | 1.70 | 1, 51.9 | 0.198 |
SURVIVAL
The effects of body mass at birth and population density on the probability of survival were dependent on the reproductive state (Table 3). Among breeding individuals, the probability of surviving to the end of the breeding season was positively related to body mass at birth irrespective of the breeding density (Fig. 3A) suggesting that breeding entailed a survival cost that was most prominent in individuals that had small body size at birth. The survival probability of breeding individuals also decreased with increasing breeding density irrespective of their body size at birth (Fig. 3B). Breeding at high densities was therefore costly in terms of survival, and the probability of facing the cost was equal among all breeding individuals.
| Estimate | SE | F | df | P | |
|---|---|---|---|---|---|
| Intercept | −5.402 | 2.314 | – | – | – |
| Sex (S) | −0.192 | 0.671 | 2.78 | 1, 142 | 0.097 |
| Breeding (B) | 5.398 | 2.890 | 2.92 | 1, 142 | 0.089 |
| Treatment (T) | −0.124 | 0.525 | 0.06 | 1, 142 | 0.813 |
| Body mass (M) | 4.935 | 1.517 | 3.98 | 1, 142 | 0.047 |
| Density (D) | −0.224 | 0.100 | 0.85 | 1, 142 | 0.357 |
| M × B | −5.402 | 1.659 | 10.59 | 1, 142 | 0.001 |
| D × B | 0.315 | 0.144 | 4.73 | 1, 142 | 0.031 |
| S × B | −1.047 | 0.852 | 1.51 | 1, 142 | 0.221 |
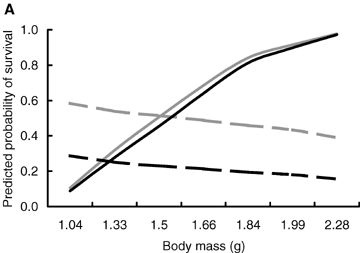
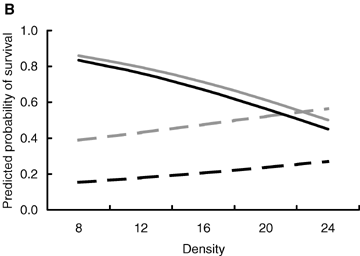
The predicted probability of surviving to the end of the breeding season in relation to (A) body mass at birth and (B) breeding density, that is, the number of individuals in the enclosure. Solid lines breeding individuals; dashed lines, nonbreeding individuals; black lines, females; gray lines, males. The figure is produced from the model presented in Table 3.
Discussion
We studied the effects of body size at birth and breeding density on the fitness characteristics of a laboratory-raised population of bank voles in seminatural enclosure environment. Body size at birth was manipulated with a novel hormonal manipulation method, which enables influencing body size during the gestation. The experiment therefore includes all the effects of the body size including the prenatal ones. This is an advantage compared to more traditional manipulations that are performed after the birth of the pups (e.g., Mappes et al. 1995; Humphries and Boutin 2000; Oksanen et al. 2001, 2003). The further effects of body size at birth are often closely connected to the age, size, or condition of the mother (Rossiter 1996). In our experiment, the effects of mother quality on offspring performance were minimized by experimentally manipulating offspring size and by distributing the offspring among several foster mothers who nursed them to independence. However, it should be kept in mind that the manipulations of endocrine system may also have unpredictable effects on individual physiology, which is important to take into account in the analysis and interpretation of the data.
BODY SIZE, DENSITY AND THE SURVIVAL COST OF REPRODUCTION
The experiment revealed that the significance of body size at birth for the future prospects of individuals was substantial, as the costs of reproduction in terms of survival were higher in individuals that had a small body mass at birth. In small mammals that continue to grow during pregnancy, there is a trade-off in resource allocation between reproduction and growth, and therefore the costs of reproduction may be even more pronounced in individuals with small body size at birth (Heino and Kaitala 1999). Another possible mechanism for the detected survival cost of reproduction is compensatory growth that may produce costs that are manifested in adult life (Metcalfe and Monaghan 2003; Ozanne and Hales 2004). However, in our study population, the difference in mean body size between the body size manipulation groups was still significant at the end of the experiment suggesting that all individuals with small body size at birth did not grow significantly faster than individuals in the control group after reaching independence from the mother.
The survival costs of reproduction were higher also in high breeding densities compared to low breeding densities. Population density and other density-related factors such as availability of food resources and free breeding territories have been previously found to affect several traits in small mammals (e.g., maturation, initiation of breeding, reproductive success, spacing behavior, somatic growth) (Bujalska 1988; Koskela et al. 1998, 1999; Prévot-Julliard 1999; Eccard and Ylönen 2001). Therefore, the reasons contributing to the increased probability of cost of reproduction in high-density populations are likely to be diverse.
THE EFFECTS OF BODY SIZE MANIPULATION AND DENSITY ON MATURATION AND REPRODUCTION
The age when sexual maturity is reached is generally expected to be negatively related to body size at birth (Roff 1992) and positively related to population density (Gadgil and Bossert 1970). In our experiment, body size at birth did not have influence on maturation age, testosterone level, or the probability of breeding. Breeding density, on the other hand, had a significant negative effect on the probability of breeding, and also the plasma testosterone levels of the males tended to be higher in lower densities, although the effect was only marginally significant. The maturation age of the females, however, was not affected by breeding density.
In natural bank vole populations where different age classes coexist, the social interactions between mature and immature individuals play an important role in the competition over free territories especially when population density is high (Gilbert et al. 1986; Prévot-Julliard et al. 1999). As our experiment was designed for studying the effects of body mass and population density per se, the study populations consisted exclusively of young males and females whereas older mature individuals were absent. The results therefore imply that population density can affect the probability of breeding irrespective of age-related social interactions.
The prediction that small individuals should reach sexual maturity at an older age is well grounded when the small body size at birth is caused by undernutrition of the mother prior to or during pregnancy (Wade and Schneider 1992). In our experiment, however, the mothers had high-quality food continuously available during the pregnancy, and the decrease in offspring body size was caused by the manipulation of the mother's endocrine system. The effects of body size at birth on maturation age, testosterone level, or the probability of breeding therefore are not confounded by factors such as undernutrition. However, the significant effects of hormonal manipulation method (hormone vs. sham) in the analyses of the probability of breeding and maturation age suggest that the hormone treatment may also have confounded the results. Even then, as the significant effect of breeding density on the probability of breeding and the effects of body mass at birth and breeding density on the probability of survival were observed even when controlling for hormonal manipulation in the analyses, we feel confident that our results represent the true effects of studied factors.
The size of the first litter produced by the females decreased with increasing breeding density irrespective of the female's body mass at birth. The effect was remarkably strong considering that the first females gave birth at the age of 54 days, which means that the mating had taken place almost immediately after the animals were released into the enclosures. The mean offspring body mass at birth was negatively related to litter size. These relationships suggest that litter size is more sensitive to environmental variation than mean offspring body size at birth. Moreover, the well-established trade-off between offspring number and size seems to have an overriding role as a determinant of mean birth size.
ADAPTIVE MATERNAL EFFECTS AS DETERMINANTS OF BODY SIZE
It has been suggested that in predictably varying environments, such as in the seasonally changing population densities of Fennoscandian vole populations (Hansson and Henttonen 1985), maternal effects may function as an adaptive mechanism for adjusting the phenotype of the offspring to the prevailing environmental conditions (Lacey 1998). Our results do not support the prediction of density-dependent selection on body size at birth. Moreover, we found no evidence for density effects on the birth size of next generation whereas the size of the first litter clearly decreased with increasing breeding density. Adjusting the reproductive effort to the prevailing conditions through offspring number rather than offspring size would therefore seem to be a more feasible strategy for the bank vole females. An earlier study by Koskela et al. (1999) shows that the short-term benefits of reduced litter size are not dependent on density, and therefore suggests that adjusting litter size to prevailing density does not increase short-term fitness. However, several studies in Microtus species suggest that density-dependent effects on recruitment rate are more common than density-dependent effects on survival (e.g., Ostfeld et al. 1993; Ostfeld and Canham 1995; Aars and Ims 2002).
IMPLICATIONS ON THE TRADE-OFF BETWEEN OFFSPRING NUMBER AND QUALITY
Optimal reproductive effort in a single reproductive attempt is generally determined by the two major life-history trade-offs: trade-off between offspring number and quality (e.g., Smith and Fretwell 1974; Mappes and Koskela 2004) and cost of reproduction (e.g., Williams 1966; Oksanen et al. 2002; Koivula et al. 2003). Earlier studies in birds and mammals have concluded that the costs of increased maternal effort are more likely directed to offspring than to parents, and therefore suggest that the intergenerational trade-off between offspring number and quality is the most significant one (Lindén and Møller 1989; Mappes et al. 1995). In accordance with more recent findings (Oksanen et al. 2002; Koivula et al. 2003), our results suggest that the cost of reproduction has a greater potential for modifying the evolution of life-history traits than previously recognized. In addition, our results show that the increased investment in offspring number may predispose not only the mother but also her offspring to the costs of reproduction. It therefore seems that the effects of the two trade-offs on the evolution of optimal body size at birth are cumulative, and that the costs of reproduction can be regarded as both an intraindividual and intergenerational trade-off (Stearns 1989).
On the other hand, our results do not show evidence for immediate decline in offspring quality as a consequence of small body size at birth. The finding that the long-term fitness effects of body size at birth (i.e., the cost of reproduction) were not density dependent may therefore have significant implications on the adaptive evolution of optimal offspring number and size. If there should be strong density-dependent selection on litter size, the negative genetic correlation between offspring number and size (Mappes and Koskela 2004) would not act as a constraint to the adaptive evolution.
Conclusions
Based on the abundant evidence of the effects of population density on various life-history traits in small rodents (e.g., Ostfeld and Canham 1995; Tkadlec and Zejda 1998; Prévot-Julliard 1999; Ergon et al. 2001), it is evident that population density can influence the expression of fitness-related traits. We studied how body size at birth impacts the long-term fitness of individuals living under variation in population density. Our results show that both small body size at birth and high breeding density have a substantial long-term effect on individual fitness, that is the increased probability of the survival cost of reproduction. However, we did not find evidence for interaction between the effects of body size and density on survival, that is, the density dependence of selection acting on body size at birth, which suggests that the costs of reproduction were generated by two different mechanisms. The lack of density dependence implies that the long-term fitness effects of body size at birth are not sensitive to variation in population density. Therefore, it seems unlikely that predictable fluctuations in vole density would contribute to the maintenance of genetic variability in body size at birth, or to the expression of phenotypic plasticity through adaptive maternal effects. However, a larger-scale study that includes the actual fluctuations in population density, as well as other possible density-related changes, for example, the availability of food or parasite load, would be needed to confirm the validity of this reasoning. Most importantly, our results show that the impact of body size during early development on the long-term fitness can be strong and persists under environmental variation.
Associate Editor: K. Hughes
ACKNOWLEDGMENTS
We thank H. Kokko, J. Kotiaho, L. Lindström, for valuable comments, O. Huitu and S. Helle for help with statistics and Konnevesi Research Station and the Experimental Animal Unit of University of Jyväskylä for providing the facilities. This study was financially supported by the Academy of Finland (grant numbers: 104568, 108955 to T.A.O.; 71425, 72896 to M.K.; 100143, 103148, 78777 to E.K. and 63789, 202166, 206091 to T.M.) and the Finnish Graduate School in Evolutionary Ecology.




