EVIDENCE FOR COEVOLUTION OF SOCIALITY AND RELATIVE BRAIN SIZE IN THREE ORDERS OF MAMMALS
Abstract
As the brain is responsible for managing an individual's behavioral response to its environment, we should expect that large relative brain size is an evolutionary response to cognitively challenging behaviors. The “social brain hypothesis” argues that maintaining group cohesion is cognitively demanding as individuals living in groups need to be able to resolve conflicts that impact on their ability to meet resource requirements. If sociality does impose cognitive demands, we expect changes in relative brain size and sociality to be coupled over evolutionary time. In this study, we analyze data on sociality and relative brain size for 206 species of ungulates, carnivores, and primates and provide, for the first time, evidence that changes in sociality and relative brain size are closely correlated over evolutionary time for all three mammalian orders. This suggests a process of coevolution and provides support for the social brain theory. However, differences between taxonomic orders in the stability of the transition between small-brained/nonsocial and large-brained/social imply that, although sociality is cognitively demanding, sociality and relative brain size can become decoupled in some cases. Carnivores seem to have been especially prone to this.
The “social brain hypothesis” has been proposed as an explanation for the unusually large brains in relation to body size found in primates (Byrne and Whiten 1988; Dunbar 1992, 1998; Barton 1996; Barton and Dunbar 1997). In essence, it argues that large brains are necessary to manage social relationships between individuals. Dunbar (1992) and Barton (1996) were able to show that relative neocortex size correlated with social group size, and interpreted this as implying that larger groups require more computational power in cognitive terms to manage their larger number of possible social interactions and relationships. The general relationship between sociality and relative brain size has since been extended to a number of other mammalian taxa, including carnivores (Dunbar and Bever 1998) and ungulates (Pérez-Barbería and Gordon 2005).
Although the consistency of these results provides strong evidence for a relationship between sociality and relative brain size, the results themselves do not explicitly show how the two traits are coupled over evolutionary time. Recently developed statistical methods (Pagel 1999a,b) now make it possible to infer ancestral traits and so to evaluate the pattern of correlated evolution between two traits. Using these tools, we examine the explicit evolutionary hypothesis of whether changes in sociality and relative brain size are correlated over time, and if so, whether there is a close coevolutionary relationship, or whether the causal link is less direct resulting in lagged evolutionary coupling between these two variables. The distinction is important because the two alternatives offer different hypotheses as to the nature of the relationship between sociality and relative brain size.
One possibility is that the two traits are tightly coupled, whereby changes in sociality are critically dependent on proportional changes in relative brain volume (or the volume of key brain components). In this case, we expect to see both traits appearing, and potentially being lost, simultaneously over time. A tight coevolutionary relationship between the traits should cause them to act as a ratchet for each other, whereby increasing one leads to an evolutionarily synchronous increase in the other. Thus, across a particular phylogeny, social species should also have large brains relative to body mass, whereas nonsocial species should have small brains.
The second possibility is that changes in one variable may be facilitated by changes in the other, but may not be completely evolutionarily dependent on them. In this case, the sequence of change tells us something about the nature of this relationship. If relative brain size changes precede changes in sociality, this suggests that relative brain size is responding to some other variable (e.g., the need to solve some ecological problem such as foraging efficiency [Martin 1984]), and that changes in relative brain size make possible, but perhaps do not determine in a causal sense, changes in sociality. Conversely, if changes in sociality precede changes in relative brain size, this would suggest that greater sociality selects for larger relative brain size, but sociality per se may not be completely dependent on having a large brain. In this case, alternative ways of coping with the stresses of larger groups must exist, and the cognitive strategies implied by the social brain hypothesis may not be necessary for social evolution. However, because the kinds of processes that buffer animals against the costs of group-living in this case are likely to be simple bootstrapping mechanisms rather than sophisticated solutions to these problems, the social brain hypothesis would predict that the nature of sociality in these intermediate transition states is likely to be very different from that which we find in the end state of large-brained/social species. In such “transition” states, we would expect social groups to be more fragmentary and less cohesive.
To test whether the evolution of sociality and relative brain size are coupled, we have selected three focal groups (primates, carnivores, and ungulates) where a relationship between relative brain size and sociality has been demonstrated (Dunbar 1992, 1998; Pérez-Barbería and Gordon 2005) and use statistical methods (Pagel 1992) that allow us to determine whether coevolution is synchronous or lagged. In all three orders, there is marked variation in sociality and relative brain size between the species, thus providing sufficient statistical power for comparative analysis. In these analyses, we use relative brain size (indexed as the residual from the common regression line for absolute brain size regressed on absolute body size), because absolute brain size is invariably strongly correlated with body size in most taxa.
Methods
DEFINITION OF VARIABLES AND DATA COLLECTION
We collected information on brain size and body mass for a total of 206 species representing four orders (Perissodactyla, Artiodactyla, Carnivora, and Primates). Of these, 78 species were ungulates (from Shultz and Dunbar [2006]), 86 were carnivores, and 42 primates, comprising 34%, 35%, and 15% of the total extant species of each of these three taxonomic groups, respectively (Nowak 1999) (see Fig. 1). The subset of species included in the analysis for each group was restricted to those for which information on brain mass, body mass, and a measure of sociality were available.
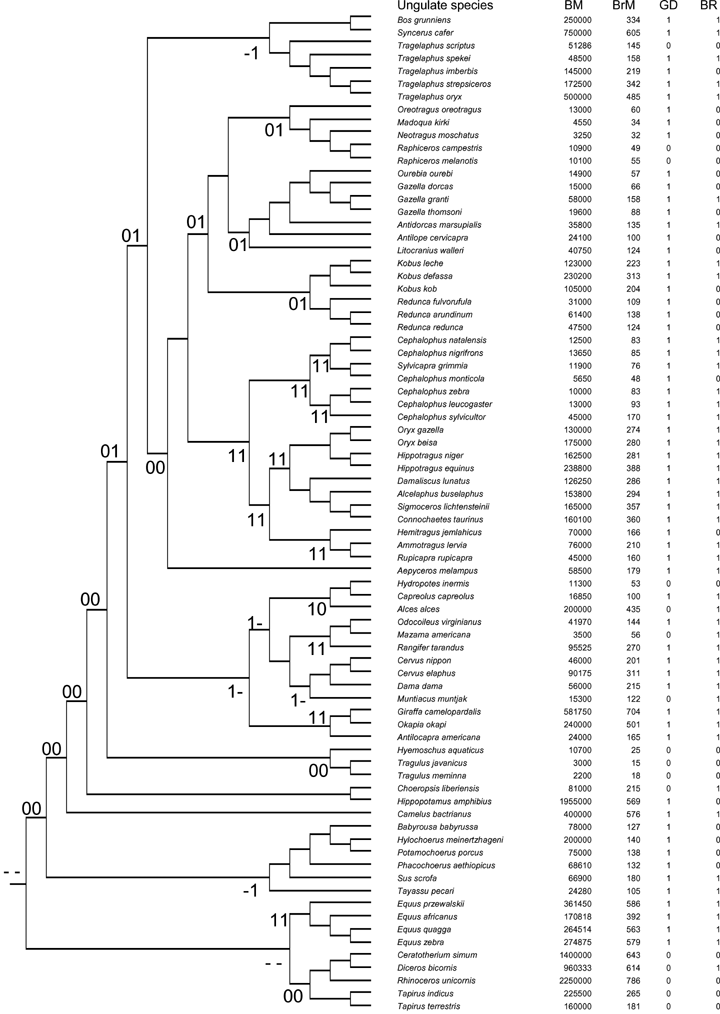
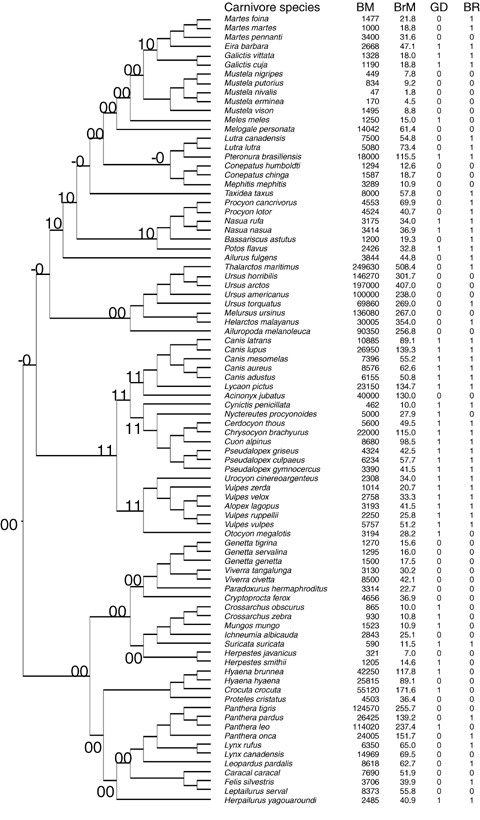
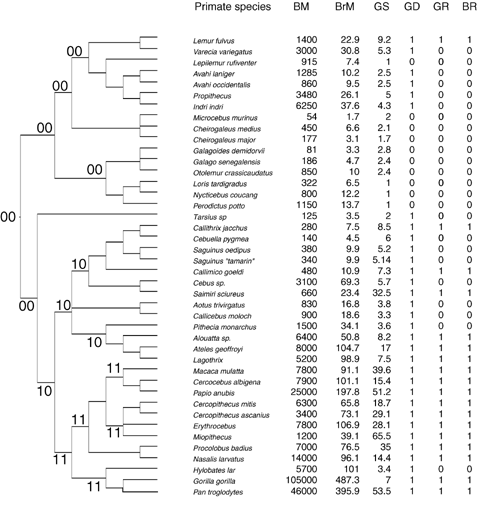
Phylogenetic relationships between species of ungulates (A), carnivores (B) and primates (C). The branch lengths in the phylogenetic trees are arbitrary units using Pagel's algorithm (Pagel 1992). Body mass and brain mass (in grams). Acronyms: BM, body mass; BrM, brain mass; GS, average group size; GD, sociality classification following descriptions of the social behavior of the species (0, nonsocial; 1, social); GR, primates sociality classification based on the ratio of a species' geometric mean group size relative to that for the whole taxonomic Order (see Methods); BR, small (0) versus large (1) brained species based on the residuals of the regression of total brain mass against body mass controlling for phylogeny (see Methods); the ancestral states at the nodes estimated as likelihoods are indicated by two digits close to the correspondent node, the first digit indicates the relative brain size character (0: small-brained species; 1: large-brained species), the second digit indicates the state of sociality (0: nonsocial; 1: social), a hyphen indicates a similar likelihood between states 0 and 1 of a character. To avoid confusion, we have retained the original taxonomic names for primate species given by (Stephan et al. 1981).
RELATIVE BRAIN SIZE
The data for primate brain size and body size were taken from Stephan et al. (1981) and carnivore brain/body data were collated from Kruska (1988) and Dunbar and Bever (1998); ungulate brain/body size data were taken from the following sources: Haarmann (1975), Kruska (1973), Jerison (1973), Oboussier (1966). Brain mass was based on fresh tissue weight (in grams). These sources provide both body size and brain size from the same individuals (see Fig. 2). In some cases, body size estimates may differ from published body size averages for the species; this is unavoidable because, to calculate residuals (see below), brain and body estimates must be from the same individuals.
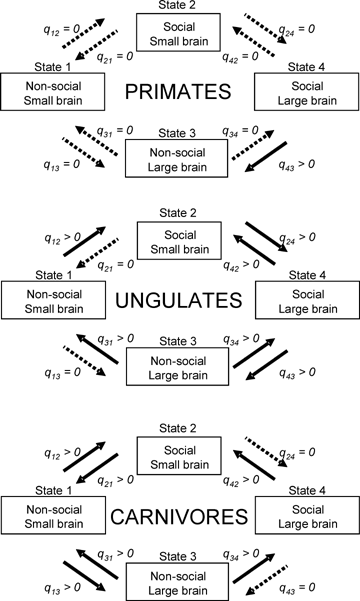
Flow diagrams for decoupled evolution between sociality and relative brain size. We assumed that the ancestral state is a solitary and small brain for body size (State 1), with social/large-brain as the final state (State 4). The significant transitions between states are shown with solid arrows, nonsignificant transitions are shown with dotted arrows. See Table 2 for details and definition of acronyms.
Brain-body residuals values (which we refer to as “relative brain size”) were calculated separately for each order using a phylogenetically controlled generalized least squares regression (PGLS) of log10-transformed brain size on log10-transformed body mass. Residual brain size was estimated separately for each taxon as we were primarily interested in the relationship between relative brain size and sociality within, not between, taxonomic groups. PGLS analyses allow the degree of phylogenetic autocorrelation to be identified and controlled for (Harvey and Pagel 1991). Through simple optimization, Pagel's λ estimates the degree of phylogenetic autocorrelation in a model, where λ= 0 when there is no autocorrelation and λ= 1 when the data follow the assumptions of Brownian motion (Paradis et al. 2004). The PGLS approach was executed in R (Grafen 1989) using the APE (Analysis of Phylogenetics and Evolution) package (Ihaka and Gentleman 1996) and code provided by R. P. Duncan.
The statistical analysis required for hypothesis testing (DISCRETE) requires that traits being tested for correlated evolution are categorical. Thus species with residual values larger than zero were categorized as large-brained, whereas those with negative residual values were classed as small-brained.
INDICES OF SOCIALITY
For all three orders, the primary criterion for sociality was that adults formed regular associations (with a minimum group size of two adult individuals). All individuals concerned need not invariably travel as a cohesive group, but they should at least associate regularly. Carnivore sociality assessments were based primarily on Gittleman (1989), with missing species based on descriptions of social behavior and group size in Nowak (1999), whereas for primates we used the categorizations given by Smuts et al. (1987). For some species of ungulates, no quantitative information on group size was available; additionally, group size in ungulates can vary greatly within species across the year, from a few animals to thousands (as during migratory movements). Following Nowak (1999) and Brashares et al. (2000), we defined solitary species as those where individual animals were observed alone during most of the year, whereas social species were defined as those which were typically observed in groups.
For primates, we also used a second criterion to define the degree of sociality. In practice, most primates are social and live in relatively cohesive groups (76% of species in the database are classified as social; nonsocial species were almost exclusively represented by nocturnal strepsirhines: see Table 1); as a result, there is insufficient variation in sociality within these two grades to provide enough statistical power to test for correlated changes. As an alternative index of the relative degree of sociality, we used the ratio of a species' geometric mean group size relative to that for the primate sample as a whole (see Smuts et al. 1987; Dunbar 1992). (We use the geometric mean because it provides a better estimate of the central tendency when distributions are highly skewed, as is invariably the case with group size.) Individual primate species were then categorized as “more social” if their species-specific mean group size was larger than the overall geometric mean, and “less social” if it was smaller than the overall geometric mean.
| Relatively small brained | Relatively large brained | Fisher's exact | Proportion social | ||
|---|---|---|---|---|---|
| Carnivores | Nonsocial | 31 | 17 | 0.001 | 0.44 |
| Social | 10 | 28 | |||
| Ungulates | Nonsocial | 11 | 5 | 0.03 | 0.79 |
| Social | 24 | 38 | |||
| Primates | Nonsocial | 9 | 1 | <0.001 | 0.76 |
| Social | 8 | 25 | |||
| Less social | 15 | 9 | 0.001 | 0.44 | |
| More social | 2 | 17 | |||
PHYLOGENETIC ANALYSIS
Ungulate phylogeny was constructed from Gatesy et al. (1997), Murphy et al. (2001), and Flagstad et al. (2001). For primates, we used Purvis's (1995) composite phylogeny, based mainly on molecular data, whereas for carnivores we used the composite phylogeny compiled in Bininda-Emonds et al. (1999). None of these studies use information on body size or brain size to construct the phylogenetic trees. As branch lengths were not available for all species of ungulates and carnivores, we applied Pagel's algorithm (Pagel 1992) to estimate branch lengths using PDAP 5.0 software (Garland et al. 1992). Previous studies of ungulates have demonstrated that the results are robust regardless of the use of alternative algorithms to estimate branch lengths (Pérez-Barbería et al. 2002, 2004; Pérez-Barbería and Gordon 2005). Although estimates of branch lengths are available for primates (Purvis 1995), for consistency we used the arbitrary units calculated by Pagel's algorithm for all three taxa. The phylogenetic trees for ungulates, carnivores, and primates used in the analyses are given in Figure 1.
HYPOTHESIS TESTING WITH PHYLOGENETIC CONTROL
First, we classified species in each taxonomic group into 2 × 2 contingency tables (small-brained/large-brained and social/nonsocial) and used Fisher's exact test to evaluate the association between relative brain size and sociality. This also allowed us to determine whether there are biases in the distribution of species across binomial classes. Second, to test our main hypothesis that there is a close coevolutionary relationship between relative brain size and sociality, we used Pagel's (1992) discrete variables method (DISCRETE), which also allows us to introduce phylogenetic control. Each discrete variable (i.e., brain size relative to body mass, and sociality) can adopt two states characterized by binary values (i.e., brain size: 0 = species that are small-brained relative to body mass, 1 = those that are large-brained relative to body mass; sociality: 0 = nonsocial species, 1 = social species).
Identifying patterns of evolution assumes that we can identify an ancestral state for the pair of traits. We assume this to be small-brained/solitary [0,0] for all three orders. Because the fossil record indicates that brain volumes have increased through time in many mammalian orders (Jerison 1973), and present day insectivores, which resemble the earliest known placental mammals that are small-brained and small-bodied (Stephan et al. 1981), parsimony would enjoin us to assume that the primitive mammalian state is solitary/small-brained. To provide some analytical evidence for the assumed ancestral states, especially because gregariousness cannot be directly observed in the fossil record, we used DISCRETE to estimate the ancestral estates at the root of each phylogenetic tree (Pagel 1992). The model provides support for the assumption that the ancestral states of characters were small-brained and nonsocial in primates and carnivores: the predicted basal ancestral states for relative brain size and sociality were small-brained and nonsocial with probabilities of 0.74 and 0.53, respectively, for primates and 0.56 and 0.78, respectively, for carnivores. In ungulates, the ancestral states could not be defined with confidence as the probabilities of each of the possible states were around 50%. However, ancestral ungulates were small-brained (Jerison 1973) and, as the alternatives to small-brained and asocial did not receive stronger support, we argue that we are justified in making the same assumption for this order, if only for reasons of parsimony.
The DISCRETE method compares two phylogenetic models that are fitted to the data by maximum likelihood. The first model allows independent evolution of the two binary values across the branches of the tree. Each variable can undergo two alternative evolutionary transitions (forward, 0 → 1, or backward, 0 ← 1), which requires four parameters to be estimated. In the second model (correlated evolution), the two variables evolved in a correlated fashion, and four possible states of two binary values are possible (1 = 0,0; 2 = 0,1; 3 = 1,0; 4 = 1,1), with each of the transitions being defined by a transition rate parameter, qi,j, that denotes the rate of change from state i to state j (where i and j refer to the beginning and end character states of each transition (e.g., q1,4= 0,0 → 1,1). Allowing one of the variables to change state in any branch of the tree yields eight possible transitions (see Fig. 2), and the model thus requires 8 qi,j parameters to be estimated because transitions of both variables are estimated simultaneously as combinations of single-state transitions.
The relative fit of the correlated evolution model L(C) and the independent evolution model L(I) was assessed by comparing their log-likelihoods using a likelihood ratio statistic 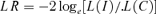 . For this test, the most conservative assumption is that the degrees of freedom equal the number of parameters (i.e., 4). However, simulation models indicate that the appropriate degrees of freedom may in fact be three or even two; if the value for the chi-square test exceeds the value for four degrees of freedom, then it is certainly safe to reject the null hypothesis, but significant results based on smaller numbers of degrees of freedom should still be given credence (Pagel 1997). For each of the three taxonomic groups we compared L(C) with the respective L(I). If L(C) was not significantly larger than L(I), we concluded that there was no evidence for correlated evolution between relative brain size and sociality; however, if L(C) was significantly larger than L(I), we accepted that there was evidence of correlated evolution between characters.
. For this test, the most conservative assumption is that the degrees of freedom equal the number of parameters (i.e., 4). However, simulation models indicate that the appropriate degrees of freedom may in fact be three or even two; if the value for the chi-square test exceeds the value for four degrees of freedom, then it is certainly safe to reject the null hypothesis, but significant results based on smaller numbers of degrees of freedom should still be given credence (Pagel 1997). For each of the three taxonomic groups we compared L(C) with the respective L(I). If L(C) was not significantly larger than L(I), we concluded that there was no evidence for correlated evolution between relative brain size and sociality; however, if L(C) was significantly larger than L(I), we accepted that there was evidence of correlated evolution between characters.
DISCRETE can also test whether the rate of either forward or backward change through any of the intermediate states is significantly different from zero. If there is tight coupling over evolutionary time between the two traits, we assume that there will be no significant pathways that link the ancestral and final states through intermediate states. To do this, we forced the parameter for the rate of transition between the ancestral state of small-brained/nonsocial species (0,0) to each intermediate state (e.g., small-brained/social; large-brained/nonsocial) to zero (q1,2= 0; q1,3= 0) (or a nominal value close to zero if there were computational problems with the algorithm: i.e., nominal qi,j= 1 ×10−7) and compared the fit of the constricted model with the fit of the full model. This was then repeated for each of the eight possible transitions between ancestral and final states for the two traits. The test statistic follows a chi-squared distribution with one degree of freedom (Pagel 1997). It is important to be clear that identifying transitional states in this second analysis does not undermine any claim, based on the first analysis, that there is an overall pattern of correlated evolution. Instead, by highlighting possible exceptions to tight coupling, this analysis allows us to say something about the potential direction (or primary driver) of the overall correlation. Examples of this procedure can be found in Cezilly et al. (2000) and Pérez-Barbería et al. (2001, 2002). The method is implemented in the programme DISCRETE (available from Mark Pagel, School of Animal and Microbial Science, University of Reading, Whiteknights, Reading, UK).
Results
CORRELATED EVOLUTION AND ANCESTRAL STATES OF RELATIVE BRAIN SIZE AND SOCIALITY
There was a significant association between relative brain size and sociality in all three taxa (Table 1). For primates, it is apparent that the majority of the species fall into the social category, thus supporting the use of the second criteria for the relative degree of sociality. This second criteria of sociality also yields a strong association between the two traits.
We then used a DISCRETE analysis as a second step to confirm that there is significant correlated evolution between relative brain size and sociality, independent of any phylogenetic confounds. This was confirmed for carnivores (L(I)=−91.18; L(C) =−83.68; χ4= 15.0; P= 0.005) and, although less strongly, ungulates (likelihood ratio statistic, L(I) =−74.84;L(C) =−70.67;χ24= 8.37, P= 0.08;χ23= 8.37, P= 0.04). For primates, the significance of correlated evolution of relative brain size with sociality was conditional upon the criterion used for sociality. When sociality was defined as a simple function of solitary versus social species, no significant evidence of correlated evolution of characters was detected (L(I) =−24.86; L(C) =−20.85; χ24= 7.86, P= 0.10), probably due to the very limited power of the analysis when there is a strongly biased distribution in the number of species that are large-brained/social (Table 1). However, when sociality was defined using the geometric mean group size to differentiate “more-social” from “less-social” species, then correlated evolution with relative brain size was significant (L(I) =−38.86; L(C) =−29.59; χ24= 18.58, P= 0.001).
EVOLUTIONARY STATE TRANSITIONS
We next tested for evidence of lagged coevolution of traits within the taxa. In ungulates and carnivores, there were significant backward and forward transitions between most intermediate states (Fig. 2, Table 2). However, the existence of these transitions indicates that there is no consistent transitional pathway between the ancestral and final states. Rather, they suggest that ungulates and carnivores have a degree of flexibility in decoupling the association between relative brain size and sociality. It should be emphasized that, despite these significant transitions, overall there was very strong evidence for correlated evolution between relative brain size and sociality in carnivores; these significant transitions represent exceptions to (and not evidence against) the overall pattern of the traits being coupled over evolutionary time. Primates, in contrast to the other groups, appear to show a much more tightly coupled link between relatively large brains and “more-social” grouping characteristics.
| Taxa | L(C8) | Alternative models † | L(C7) | Likelihood ratio statistic | P |
|---|---|---|---|---|---|
| Ungulates | −70.65 | q 12=0 | −73.82 | 6.35 | 0.01 |
| q 13=0 | −70.65 | 0 | 1 | ||
| q 21=0 | −72.23 | 3.17 | 0.08 | ||
| q 24=0 | −72.77 | 4.24 | 0.04 | ||
| q 31=0 | −72.59 | 3.89 | 0.05 | ||
| q 34=0 | −75.39 | 9.47 | 0.002 | ||
| q 42=0 | −72.65 | 3.99 | 0.05 | ||
| q 43=0 | −74.45 | 7.60 | 0.006 | ||
| Carnivores | −83.68 | q 12=0 | −87.15 | 6.94 | 0.008 |
| q 13=0 | −94.52 | 21.68 | 0.000 | ||
| q 21=0 | −88.79 | 10.22 | 0.001 | ||
| q 24=0 | −83.74 | 0.12 | 0.729 | ||
| q 31=0 | −86.26 | 5.16 | 0.023 | ||
| q 34=0 | −88.63 | 9.90 | 0.002 | ||
| q 42=0 | −88.21 | 9.06 | 0.003 | ||
| q 43=0 | −83.67 | 0.02 | 0.888 | ||
| Primates | −29.59 | q 12=0 | −31.00 | 2.80 | 0.59 |
| q 13=0 | −29.41 | 0.35 | 0.99 | ||
| q 21=0 | −30.13 | 1.09 | 0.90 | ||
| q 24=0 | −31.95 | 4.73 | 0.32 | ||
| q 31=0 | −29.45 | 0.28 | 0.99 | ||
| q 34=0 | −33.55 | 7.92 | 0.09 | ||
| q 42=0 | −33.21 | 7.25 | 0.12 | ||
| q 43=0 | −35.24 | 11.30 | 0.02 | ||
Discussion
The results confirm a coevolutionary relationship between sociality and large relative brain size in all three mammalian orders. However, the pattern of coevolution appears to vary between the three orders. This raises several issues.
First, it seems that, broadly speaking, the selection pressures forcing these two traits into a coevolutionary relationship are consistent across a wide variety of mammalian taxa. We suggest that this may be because, in general, sociality is cognitively demanding, such that the transition from a solitary to a social way of life (or from less to more social in the case of primates) is facilitated by a corresponding increase in computational power (i.e., relative brain volume) (Barrett et al. 2003). This might be because it is necessary to track the behavior of other group members more closely to avoid becoming separated from the group, or because individuals need to compromise on personal foraging or time budget demands to maintain social cohesion, or because it is necessary to come up with cognitive or social strategies to reduce the natural costs of group-living (i.e., direct and indirect foraging competition, social harassment, stress due to crowding, etc). There is little doubt that group-living offers not only biological but also ecological advantages for the group members: these include protection against predators and optimizing food or breeding opportunities (Caraco et al. 1980; Wrangham and Rubenstein 1986; Fryxell 1991; Chapman et al. 1995; Farnsworth et al. 2002).
Second, the analysis of transitional states implies that, despite this overall consistency, the selection pressures favoring sociality may not necessarily have been uniform across the three groups. The generally accepted adaptive benefits of sociality are improved resource acquisition, predation avoidance, and/or offspring care (Pullian and Caraco 1984; Clutton-Brock 1991). Because the three orders differ markedly in their diets and hence foraging strategies (Mace et al. 1981; Martin 1984; Nowak 1999; Fish and Lockwood 2003; Pérez-Barbería and Gordon 2005), it is difficult to see any obvious ecological commonalities that might explain sociality evolving across all species as a mechanism to improve resource acquisition. As individuals of most species are potential prey at some point in their lives, it might be possible to argue that predation risk is sufficiently universal to act as a selection force of this kind, although the intensity of predation risk varies widely across both social and nonsocial species. An alternative explanation is that there may not be a particular ecological selection force driving the evolution of sociality in all taxa (Wrangham and Rubenstein 1986); rather, the precipitating factor could vary across orders. In primates and ungulates, for example, antipredator strategies seem to have been the principal factor selecting for group-living (Nelson and Mech 1991; Bleich et al. 1997; Reale and Festa-Bianchet 2003; Dunbar and Shultz 2007), but in many carnivores (especially the canids) the major benefit of sociality is either cooperative hunting or cooperative rearing of young or both (Macdonald 1983). The issue may, rather, be that many different ecological problems can be very effectively solved by forming groups; however, living in groups creates ecological and reproductive costs that have to be resolved, and it is in the modulating of these that the social brain plays its role. In other words, sociality is not an epiphenomenal outcome of cognition (relative brain size) whose evolution has been driven by responses to solving ecological problems on the basis of individual-based learning. Rather, the increase in relative brain size is the outcome of socially based ecological problem solving (irrespective of whether the principal problem in question is foraging-related or predation-related).
Third, the three taxa differ in the extent to which the two traits can change independently of each other. Primates are characterized by a very low rate of independent transitions. In contrast, carnivores and ungulates exhibited a very different pattern: in these two taxa, almost all the intermediate transitions were significant, suggesting much greater flexibility in the extent to which the two traits can be decoupled. Note that, although it seems to have been relatively easy for carnivores to switch between the ancestral state (small-brained/asocial) and both the intermediate states (small-brained/social and large-brained/asocial), the pattern of transitions shown in Figure 2 implies that only the second of these provides a possible transitional route to the final state of large-brained/social; the transition from small-brained/social to large-brained/social is unlikely in Figure 2. This suggests that although it has been possible to increase relative brain size independently of sociality (perhaps reflecting the cognitive demands of a change in hunting strategy) and then evolve sociality off the back of an enlarged brain, it has not been possible to evolve a large brain off the back of a prior change to sociality. This is almost certainly because the type of sociality that evolves in such cases does not involve intense levels of coordination and/or bonding between group members. In such cases, the switch to sociality may be driven by the need for effective antipredator strategies, especially in small-bodied species, rather than by the demands of cooperative hunting. The European badger (Meles meles) may be an example.
There are several possible explanations for the existence of these intermediate state transitions in carnivores and ungulates. Although our results clearly indicate that the evolution of sociality in both taxa is generally correlated with the evolution of large brains, a large brain may not necessarily be obligatory for sociality. The specific nature of the social system (in terms of the nature of intragroup relationships) may be an important factor determining the minimum brain size necessary to maintain those relationships. Identifying the species that are involved in the state changes in Figure 2 and their social systems may prove revealing as to what specific social factors drive relative brain size. The ungulate species that are small-brained and social (see Fig. 1A), for example, include species such as gazelles and other antelope, which tend to have a very fluid and changing group structure (Rahmani 1990; Lawes and Nanni 1993). In the ungulate phylogenetic tree, these species constitute a well-defined clade showing consistency in the state of the traits (small-brained/social) in all parent nodes. Such cases may be characterized by less-stable, less-cohesive form of sociality.
An alternative possibility is that some carnivore and ungulate species might have gone through secondary selection for solitary living, giving rise to reversal transitions from large-brained/social to large-brained/nonsocial. The only examples of extant large-brained/nonsocial species in our ungulate dataset (Mazama mazama, Alces alces, Tragelaphus scriptus, and Muntiacus muntjak) are unrelated species that occupy closed habitats; similarly, in carnivores, most of the 17 species that are large-brained and asocial occur in closed or dense bush habitats, or are burrowers. These species might have been subjected to evolutionary trade-offs that resulted in a secondary reversion to a nonsocial state conditioned by habitat. However, cases like this should always prompt us to ask whether our knowledge of a species' social behavior is detailed enough to have allowed us to classify them correctly. Among primates, for example, some species that live in spatially dispersed social systems nonetheless have intense social bonds (e.g., chimpanzees, orangutans, possibly some prosimians); in the absence of detailed knowledge of individual social relationships, these species were traditionally assumed to be asocial, but subsequent research has forced a re-evaluation in several cases (Smuts et al. 1987).
Finally, the fact that a more sophisticated definition of sociality was needed in the case of primates seems to underline the widely, but perhaps only informally, recognized fact that primates seem to exhibit an unusually bonded form of sociality compared to other vertebrate taxa (Byrne and Whiten 1988; Harcourt 1992). Primate sociality seems more demanding cognitively, and these demands increase with group size, indicating that there is something particularly complex about primate social behavior. Further work comparing the specific relationships between the social structure of groups with brain architecture across taxonomic groups is needed to identify what aspects of social behavior in primates are similar and which are different from those of other taxonomic groups.
Associate Editor: C. Janis
ACKNOWLEDGMENTS
RD is supported by a British Academy Research Professorship, and SS is funded by the British Academy. The Scottish Executive Environment and Rural Affairs Department funded part of this research.




