A PHYLOGENETIC TEST FOR ADAPTIVE CONVERGENCE IN ROCK-DWELLING LIZARDS
Abstract
Phenotypic similarity of species occupying similar habitats has long been taken as strong evidence of adaptation, but this approach implicitly assumes that similarity is evolutionarily derived. However, even derived similarities may not represent convergent adaptation if the similarities did not evolve as a result of the same selection pressures; an alternative possibility is that the similar features evolved for different reasons, but subsequently allowed the species to occupy the same habitat, in which case the convergent evolution of the same feature by species occupying similar habitats would be the result of exaptation. Many lizard lineages have evolved to occupy vertical rock surfaces, a habitat that places strong functional and ecological demands on lizards. We examined four clades in which species that use vertical rock surfaces exhibit long hindlimbs and flattened bodies. Morphological change on the phylogenetic branches leading to the rock-dwelling species in the four clades differed from change on other branches of the phylogeny; evolutionary transitions to rock-dwelling generally were associated with increases in limb length and decreases in head depth. Examination of particular characters revealed several different patterns of evolutionary change. Rock-dwelling lizards exhibited similarities in head depth as a result of both adaptation and exaptation. Moreover, even though rock-dwelling species generally had longer limbs than their close relatives, clade-level differences in limb length led to an overall lack of difference between rock- and non–rock-dwelling lizards. These results indicate that evolutionary change in the same direction in independent lineages does not necessarily produce convergence, and that the existence of similar advantageous structures among species independently occupying the same environment may not indicate adaptation.
Convergent evolution has long been recognized by the similarity of unrelated groups that occupy similar ecological niches. Few would dispute that the streamlined body form of ichthyosaurs, sharks, and dolphins is convergent, nor would they deny convergence of wolves and thylacines or a myriad of other examples. However, overall similarity alone does not necessarily indicate convergence, because similarity may reflect inheritance from a common ancestor rather than convergent evolution (i.e., the similarity may be plesiomorphic rather than homoplastic). For example, the similar body form of many salamanders and lizards results not from convergence, but from retention of the ancestral tetrapod body plan (Benton 1997). Moreover, even the occurrence of species with independently derived similarity in similar environments may not indicate convergent adaptation. An alternative explanation is that species may evolve their phenotypic similarities for different reasons, but once the traits have evolved, they may permit subsequent occupation of the same habitat. In this case, the convergence would be the result of repeated exaptation (Gould and Vrba 1982; Arnold 1994).
The solution to this problem is to consider similarity of form in a historical context; phylogenetic analysis can distinguish ancestral from derived similarity and can distinguish among several different routes to derived similarity (Wake 1991). Character evolution is now routinely studied using phylogenetic methods with the goal of distinguishing symplesiomorphy from derived similarity (Brooks and McLennan 2002); similarly, since the work of Felsenstein (1985) and Harvey and Pagel (1991), statistically based phylogenetic comparative methods are now widely employed to investigate whether character evolution is correlated with changes in other characters. Surprisingly, however, the two approaches have not been integrated and rigorous investigations of the evolution of overall similarity are rare (e.g., Stayton 2006). Here we illustrate how a statistical approach using a fully resolved phylogeny with branch lengths proportional to elapsed time can be used to examine the evolution of convergent features.
Our specific focus is on a putative case of convergent evolution among rock-dwelling lizard species. In southern Africa, the cordylid genus Platysaurus is comprised of approximately 15 species commonly called “flat lizards” for their extreme dorsoventral flattening. These long-legged lizards run rapidly and with great agility across vertical rock walls and sleep, and sometimes seek refuge, in very narrow crevices (Branch 1998; Losos et al. 2002; Whiting et al. 2003). A distantly related cordylid species, Pseudocordylus capensis, occupies similar habitats and exhibits the same morphological features, although it is not as dorsoventrally flattened as the flat lizards. In Brazil, the iguanid lizard Tropidurus semitaeniatus is so morphologically similar to Platysaurus—despite being extremely distantly related (Townsend et al. 2004)—that at first glance museum specimens can be mistaken for each other (Vitt and Pianka 2003). Tropidurus semitaeniatus is also a rock-dwelling lizard, and although its natural history is not as well known, it appears to be quite similar ecologically to Platysaurus (Vitt 1991; Vitt and Pianka 2003). Two other clades of iguanid lizards also contain rock-dwellers; Petrosaurus (four species) and Anolis bartschi are in different subfamilies from each other and from T. semitaeniatus, but share similar habits and much the same morphology, although they are not as extremely dorsoventrally compressed as the flat lizards.
This putative case of convergence raises two questions:
- 1
Are these species truly convergent? That is, does quantitative analysis confirm that these distantly related species are more similar morphologically to each other than they are to other, more closely related, species?
- 2
To the extent that they are convergent, did the similarity evolve simultaneously with the evolutionary transition to using rocky habitats, or did the morphological features evolve prior to the ecological ones?
Materials and Methods
TAXON SAMPLING
We chose five rock-dwelling species in four lizard clades: Ps. capensis and Platysaurus capensis, in Cordylidae; Petrosaurus thalassinus, in subfamily Phrynosomatinae, Iguanidae; A. bartschi, in Polychrotinae, Iguanidae; and T. semitaeniatus, in Tropidurinae, Iguanidae. The genera Platysaurus and Petrosaurus contain multiple species, all of which are rock-dwellers; these species vary somewhat in size, but are otherwise fairly homogeneous in body proportions and quite different morphologically from other members of their respective clades (Broadley 1978; Grismer 2002). We randomly chose one species of each of these genera from among those available in the museum collections we used. The other three rock-dwelling species are not part of larger rock-dwelling clades.
For each clade, we included 8–15 non–rock-dwelling species chosen from among those available in museum collections to sample the phylogenetic and ecomorphological spectrum of that clade and to include the sister taxa of rock-dwelling lineages. We chose an adult specimen of each species from the collections of the American Museum of Natural History; the California Academy of Sciences; the Ellerman Museum, University of Stellenbosch; the Museum of Natural History of the University of Kansas; the Museum of Vertebrate Zoology, University of California, Berkeley; the National Museum of Natural History, Smithsonian Institution; and the Transvaal Museum.
MORPHOLOGICAL MEASUREMENTS
We radiographed specimens and took skeletal measurements using a video imaging system (Morphosys; Meacham and Duncan 1990) connected to a personal computer. We measured the following skeletal elements on the right side of the specimen, unless bones were broken or did not lay flat on the radiograph film, in which case we took the measurement of the bone of interest from the left side: humerus, ulna, femur, tibia, fourth metacarpal, and fourth metatarsal. We took each measurement twice and averaged them. If the two measurements were not within 5% of one another, we took a third measurement and used the average of the closest two.
We took the following external measurements using a ruler and calipers: snout-vent length (SVL); length of the third and fourth toes on the forefoot, measured from the point of connection of the third and fourth toes to the most distal point on the claw; length of the fourth toe on the hindfoot, measured from the point of connection of the toe to the foot to the most distal point on the claw; and head depth, measured directly behind the eyes at the deepest point of the head. Head depth is strongly correlated with body depth, which was not included due to difficulty of accurate measurement. We took toe measurements on the right side of the body. We took measurements at least twice and natural log transformed all measurements for statistical analysis.
PHYLOGENY RECONSTRUCTION
To examine whether changes associated with transitions to rock-dwelling differed from changes that occurred along other branches of the phylogeny, we estimated phylogenies for each of the four clades sampled in this study. Previously published DNA sequences were available for most species and GenBank accession numbers for DNA sequences used in tree reconstruction are listed in online Supplementary Appendix S1. Three of the four datasets (Anolis, tropidurines, and phyrnosomatines) were comprised of mtDNA sequences encoding part of ND1, tRNAIle, tRNAGln, tRNAMet, ND2, tRNATrp, tRNAAla, tRNAAsn, tRNACys, tRNATyr, and part of COI (Harmon et al. 2003; Schulte et al. 2003; Kizirian et al. 2004). The cordylid dataset (Melville et al., in review) did not include ND1, tRNAIle, tRNAGln, and only part of tRNAMet, but included all other regions. Ambiguously aligned (by eye) base pairs were removed prior to analyses using the criterion that a region was considered ambiguously aligned if more than one alternative placement of a nucleotide was possible in length-variant noncoding and tRNA stem regions. This criterion is easily implemented by eye, whereas computer-algorithm-based alignment software perform poorly when attempting to identify ambiguously aligned regions (Kjer et al. 2007). All alignments including exclusion sets have been submitted to TreeBase (study accession number S1841; matrix accession numbers M3385, M3386, M3387, and M3388). No molecular data were available for three tropidurine species for which we had morphological data. Using the phylogeny of Frost et al. (2001) as a guide, we substituted two closely related species for which molecular data were available (T. torquatus for T. etheridgei and T. azureus for T. flaviceps) and made T. semitaeniatus the sister taxon to the clade containing T. hispidus and T. torquatus, with the node uniting them placed half the distance to the node connecting this clade to its sister taxon.
We generated phylogenetic hypotheses using maximum likelihood (ML) analyses in PAUP*4.0 (Swofford 2002). We chose ML because model-based tree estimation procedures do not minimize branch lengths like maximum parsimony and account for multiple overlapping substitutions yielding more accurate branch length estimates. We conducted Bayesian analyses with MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) using identical molecular evolutionary models, 107 generations, and default priors. Results of these analyses were identical or congruent with those obtained using ML for these DNA sequence regions (results not shown); the only differences among the four phylogenies was that in the phylogenetic hypotheses of cordylids and phrynosomatines, several nodes were collapsed as unresolved in the Bayesian trees that are resolved in ML trees. All these branches are comparatively very short in the ML analyses. Simultaneous optimization of model parameters and phylogenetic hypotheses for these datasets were computationally impractical. Therefore, we conducted searches using a successive approximations approach (Swofford et al. 1996), as outlined by Sullivan et al. (2005), for each dataset independently. We initially used Modeltest version 3.7 (Posada and Crandall 1998) to find the best-fitting model of sequence evolution for a tree estimated using neighbor joining (NJ). For most datasets, this model was the General Time Reversible (GTR) model with proportion of sites invariant (I) and rate heterogeneity approximated by a Γ distribution (Γ). However, to maintain consistency among all analyses, and because model underparameterization can affect branch length estimates (Revell et al. 2005), the GTR + I +Γ model was assumed for all ML analyses. We estimated branch lengths for the final trees using fixed optimal ML parameters in PAUP.
The approximate relative age for the root of each of the four clades was estimated using a larger mtDNA dataset for squamate reptile families and several fossil calibrations (J. Schulte, unpubl. data). Prior to divergence time estimation, we determined whether molecular evolutionary rates were variable among lineages. The likelihood scores of the best overall ML topology and the best ML topology with a molecular clock enforced were calculated in PAUP* and subsequently used to perform a likelihood ratio test. The test statistic [−2 ln Λ, in which Λ is the likelihood ratio: L(clock)/L(nonclock)] is expected to be χ2 distributed with n− 2 degrees of freedom where n is the number of sequences (Muse and Weir 1992). All phylogenetic hypotheses used for divergence time estimation significantly reject the assumption of rate homogeneity. Therefore, methods that allow evolutionary rate heterogeneity among lineages are appropriate for these phylogenies. We used the penalized likelihood method of Sanderson (2002) as implemented in r8s version 1.71 (Sanderson 2003). This method is robust to modest model violations, is easily implemented in a single software package, and performs as well as or better than other rate heterogeneity methods such as Bayesian and nonparametric rate smoothing in numerous empirical and simulated datasets (Sanderson 2002; Pisani et al. 2004; Ho et al. 2005; Linder et al. 2005). Cross-validation determined the optimal smoothing parameter for each tree, as outlined in Sanderson (2002) and implemented in r8s. We calculated the relative depths of each tree by setting the total depth of the tropidurine phylogeny to 1.00 and scaling the remaining three trees accordingly. The resultant root ages of each tree were as follows: tropidurines 1.00 (as stated, above); phrynosomatines 1.28; Anolis 1.51; cordylids 1.55.
STATISTICAL ANALYSES
We used squared-change parsimony (Huey and Bennett 1987; Maddison 1991) to reconstruct the ancestral states at all nodes for the 11 continuous characters in each of the four phylogenies. Squared-change parsimony has previously been shown to provide the ML states at ancestral nodes (Maddison 1991). We then calculated the multivariate changes along all internodes in each phylogeny, standardizing for differences in branch lengths by dividing changes by the square root of the branch length. We removed the effect of changes in size by calculating the residuals from the linear regressions of changes in all variables on changes in SVL.
We calculated principal components (PC) scores from a PC analysis on size-removed changes in all variables, retaining those axes with an eigenvalue greater than one. We then conducted separate Multivariate Analyses of Variance (MANOVAs) on the 10 size-adjusted morphological variables and on the PC scores from the first two PC axes, comparing changes on branches of the phylogeny on which the transition to rock-dwelling occurred against changes on all other branches. Given that these MANOVAs were significant, we then conducted Analyses of Variance (ANOVAs) on changes in each variable separately.
Statistical evaluation of analyses based on the changes inferred on all branches of a phylogeny is problematic both because changes along all branches are nonindependent and because phylogenies contain more branches than the number of species, leading to an inflation in the degrees of freedom. For this reason, we calculated significance values of the MANOVAs and ANOVAs using two different approaches
- 1
We permuted the non–rock to rock lizard transitions (the grouping variable for the MANOVAs and ANOVAs) randomly among branches in the phylogenies with the constraint that rock transitions could only be placed on terminal branches because all transitions to rock-dwelling occur on terminal branches of the real phylogenies (see Fig. 1). We performed 1000 random permutations, each time calculating F-statistics for the MANOVAs and univariate ANOVAs. Significance was evaluated as the fraction of times the F-value from the permutation exceeded the observed value for F.
- 2
We simulated character evolution by correlated Brownian motion for the 11 morphological characters. The covariance matrix used for the Brownian motion simulations was the variance–covariance matrix of independent contrasts calculated separately for each phylogeny and then pooled across phylogenies. Pooling was performed by calculating the element-by-element weighted average of the matrices in which weights were determined by the number of contrasts contributing to the estimation of each matrix (the number of taxa in the clade minus one [Manly 1997]). This provides an unbiased estimate of the Brownian motion rate matrix (Garland et al. 1999). We performed 1000 Brownian motion simulations, each time calculating F-statistics for the MANOVA and univariate ANOVAs. We also conducted Brownian motion simulations of the first two PC scores. In this case, the generating variance–covariance matrix for the simulations was a 2 × 2 diagonal matrix with the diagonal composed of the first two eigenvalues from the PC analysis on the correlation matrix of the changes along all branches. Statistical significance was calculated as described above. Simulation tests reported in online Supplementary Appendix S2 reveal that Type I error for both of these statistical methods is appropriate.
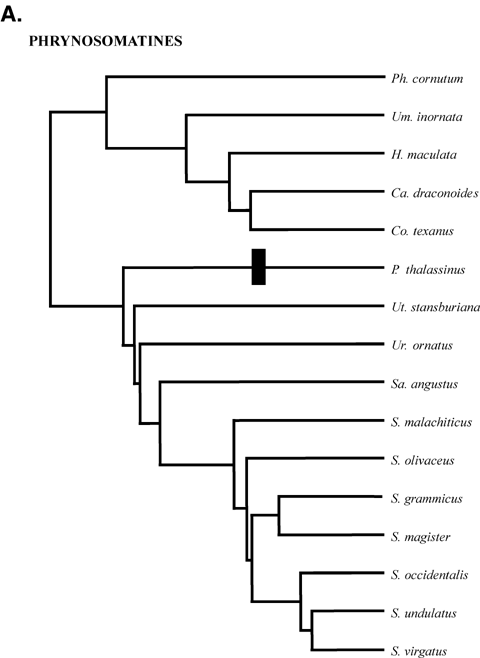
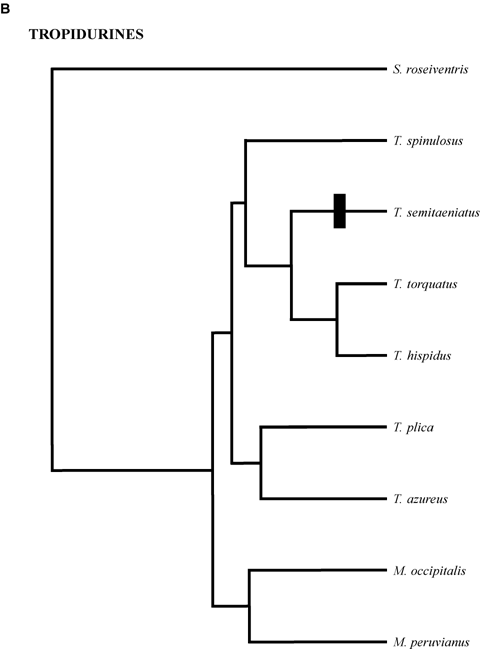
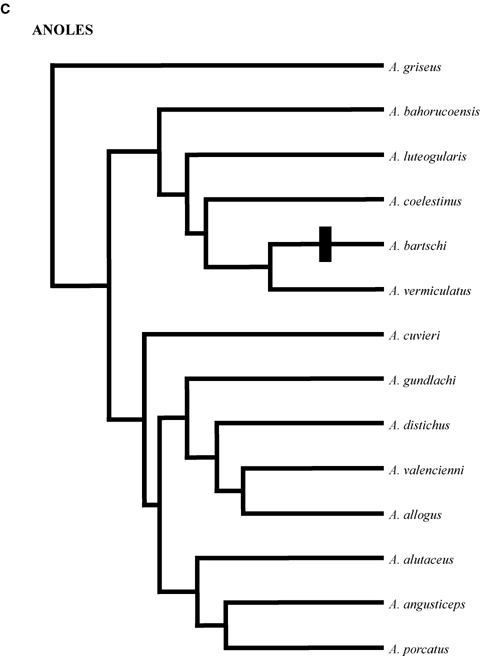
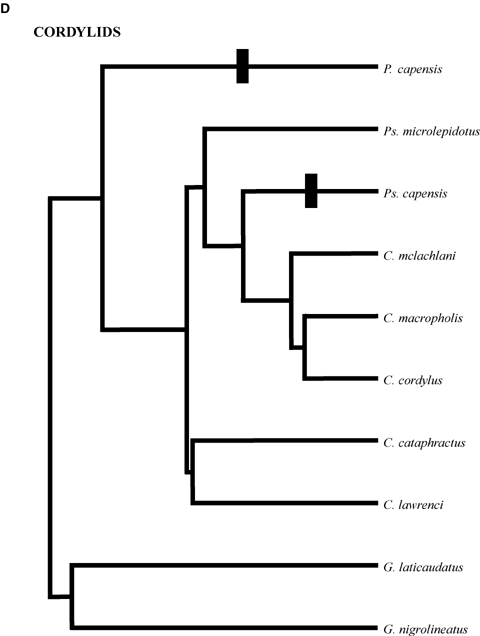
Phylogenetic relationships of clades. (A) Phrynosomatines. Generic abbreviations: Ca =Callisaurus; Co =Cophosaurus; H =Holbrookia; P =Petrosaurus; Ph =Phrynosoma; S =Sceloporus; Sa =Sator; Um =Uma; Ur =Urosaurus; Ut =Uta. The sand lizard clades comprises Callisaurus, Cophosaurus, Holbrookia, Phrynosoma and Uma; (B) Tropidurines. M =Microlophus; S =Stenocercus; T =Tropidurus; (C) Anoles. A =Anolis; (D) Cordylidae. C =Cordylus; G =Gerrhosaurus; P =Platysaurus; Ps =Pseudocordylus. All trees are scaled to have the same total depth. Estimated relative total depths are (A) 1.28, (B) 1.00, (C) 1.52, and (D) 1.55. Black bars indicate the evolution of rock-dwelling.
We also conducted a sensitivity analysis to examine whether error resulting from the measurement of only a single specimen per species could influence our results. In general, one would not expect random error produced by measuring a single individual to lead to detection of an apparent pattern when none really exists; quite the contrary, such random error should act to make detection of real patterns more difficult (i.e., it should increase Type II, rather than Type I, error). This expectation has been confirmed by simulation (Harmon and Losos 2005). However, in some circumstances, error in estimation of species' mean values can lead to somewhat inflated Type I errors in phylogenetic comparative analyses; in particular, such error is possible if most of the variation in the traits in question occurs within, rather than between, species. Harmon and Losos (2005) suggest that inflation of Type I error is not likely to be a problem if >50% of trait variation is partitioned among, rather than within, species.
To examine whether using only a single specimen per species could have led to inappropriate rejection of the null hypothesis, we sampled multiple individuals of two of the clades in this study, Phrynosomatinae and Anolis (specimens of some of the species in the other two clades were not readily available; mean number of individuals per species = 11.2, range: 5–15, only one species with a sample size < 8). Treating these two clades separately, we conducted ANOVAs on SVL and on size-corrected values (size removed by calculating residuals on a regression in which all data for a variable in a clade were used in a single regression against SVL) for all other morphometric measurements. For all traits except metacarpal length in Phrysomatinae, >50% of the variation was partitioned among species, and for most traits >85% was partitioned among species.
To further investigate whether using a single specimen biased our results, we calculated species' mean values for the multiple measurements and re-ran all of the analyses using these values. Results, reported in online Supplementary Appendix S3, are nearly identical to those produced using species' values based on a single individual.
In addition, we note that some authors have suggested that extremely short terminal branches in a phylogeny may lead to statistical problems if species' values are measured with error (Purvis and Webster 1999; Felsenstein 2004). Inspection of the phylogenies for the clades in this study does not reveal any extremely short terminal branches; indeed, the rock-lizard transitions tend to be on longer branches, which would tend to minimize change inferred to have occurred on them as the changes are standardized by branch length.
Our comparative methodology relies explicitly on an assumption of evolution by Brownian motion. This is a common assumption in phylogenetic comparative analyses (e.g., Felsenstein 1985; Garland et al. 1993), and other comparative methods have been shown to be robust to violations in this assumption (at least to the extent that Type I error was not severely increased; Díaz-Uriarte and Garland 1996). We show in online Supplementary Appendix S4 that trait evolution is generally consistent with a Brownian motion model.
McPeek (1995) suggested a modification of the independent contrasts method that calculates ancestor–descendant contrasts on focal branches of a phylogeny and compares them to sister-taxon contrasts calculated throughout the rest of the tree. Although at first glance this would seem to be an appropriate method for our study, we did not use McPeek's (1995) method, which is actually primarily a test for an increased rate of evolution along specific branches, for reasons elaborated in online Supplementary Appendix S5.
Results
The first two PC axes on nonphylogenetically corrected data explain 75.5% of the variance (Table 1). The first axis loads strongly for all limb elements, whereas the second axis loads most strongly for head depth and, to a somewhat lesser extent, for a contrast between proximal and distal limb elements. Rock-dwelling species differ from other species (Fig. 2; MANOVA on morphometric variables, F10,38= 8.05, P < 0.001; MANOVA on PC scores for first two axes, F2,46= 4.46, P= 0.017). Given the significant MANOVA results, we examined variables individually. Univariate analyses indicate that the difference between rock-dwelling species and others results primarily from differences in head depth (Table 2).
| Variable | PC Axis | |
|---|---|---|
| 1 | 2 | |
| Head depth | 0.335 | 0.448 |
| Humerus | 0.821 | −0.239 |
| Ulna | 0.886 | −0.257 |
| Metacarpal | 0.757 | 0.228 |
| Manus III | 0.837 | 0.445 |
| Manus IV | 0.834 | 0.387 |
| Femur | 0.865 | −0.358 |
| Tibia | 0.886 | −0.385 |
| Metatarsal | 0.836 | −0.219 |
| Pedal IV | 0.797 | 0.301 |
| % variance explained | 64.1 | 11.4 |
| Eigenvalue | 6.41 | 1.14 |
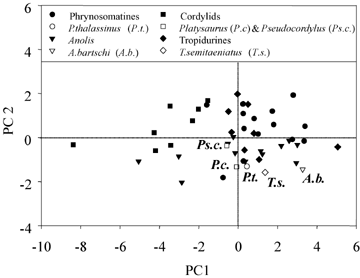
Principal components analysis on species data. Results of this analysis are presented in Table 1. Platysaurus species is P. capensis, and Pseudocordylus species is Ps. capensis.
| Variable | F-ratio* | P-value |
|---|---|---|
| PC 1 | 0.681 | 0.413 |
| PC 2 | 8.178 | 0.006 |
| Head depth | 18.47 | <0.001 |
| Humerus | 1.495 | 0.223 |
| Ulna | 1.955 | 0.169 |
| Metacarpal | 1.668 | 0.203 |
| Manus III | 0.009 | 0.926 |
| Manus IV | 0.155 | 0.695 |
| Femur | 3.994 | 0.052 |
| Tibia | 0.655 | 0.423 |
| Metatarsal | 0.630 | 0.431 |
| Pedal IV | 0.027 | 0.871 |
- *1,47 degrees of freedom
Phylogenetic analyses provide similar results with regard to the PC analysis, but evolutionary trends differ from the nonphylogenetic results. The first two PC axes account for 74.0% of the variance (Table 3). The first axis loads strongly for changes in all limb elements, whereas the second axis loads primarily for changes in head depth and, to a lesser extent, for a contrast between hand and foot versus other limb elements. Branches on which transitions to rock-dwelling occurred differ from those not involving such transitions (MANOVA on morphometric variables, F= 6.61; permutation test, P= 0.001; simulation test, P < 0.001; MANOVA on PC scores for first two axes, F= 15.47; permutation test, P= 0.001; simulation test, P < 0.001). Analyses of variance (ANOVAs) indicate that the transition to rock-dwelling was associated with differences on both PC axes, a decrease in head depth, and increases in humerus, ulna, metacarpal, femur, tibia, and metatarsal lengths. Changes in digit lengths did not differ among branches of the phylogeny (Table 4).
| Variable | PC Axis | |
|---|---|---|
| 1 | 2 | |
| Head depth | −0.011 | 0.779 |
| Humerus | 0.741 | −0.090 |
| Ulna | 0.836 | −0.195 |
| Metacarpal | 0.821 | 0.031 |
| Manus III | 0.781 | 0.514 |
| Manus IV | 0.752 | 0.470 |
| Femur | 0.837 | −0.344 |
| Tibia | 0.857 | −0.399 |
| Metatarsal | 0.847 | −0.210 |
| Pedal IV | 0.755 | 0.353 |
| % variance explained | 58.2 | 15.8 |
| Eigenvalue | 5.82 | 1.58 |
| Variable | F-ratio | Permutation | Simulation |
|---|---|---|---|
| P-value | P-value | ||
| PC 1 | 9.03 | 0.039 | 0.017 |
| PC 2 | 17.94 | 0.004 | 0.001 |
| Head depth | 20.69 | 0.004 | <0.001 |
| Humerus | 10.58 | 0.022 | 0.009 |
| Ulna | 9.19 | 0.035 | 0.013 |
| Metacarpal | 10.37 | 0.027 | 0.015 |
| Manus III | 0.13 | 0.77 | 0.78 |
| Manus IV | 0.88 | 0.45 | 0.46 |
| Femur | 16.81 | 0.004 | <0.001 |
| Tibia | 8.44 | 0.034 | 0.018 |
| Metatarsal | 9.44 | 0.033 | 0.017 |
| Pedal IV | 0.34 | 0.63 | 0.62 |
Figure 3 indicates that the transition to rock-dwelling did not always result in the same changes. In particular, most lineages evolved longer limbs (positive values on PC 1), but not all rock-dwelling lineages evolved exceptionally flatter heads (negative values on PC 2). Relatively large decreases in head depth occurred on the branches leading to P. capensis, T. semitaeniatus, and P. thalassinus. By contrast, the branches leading to A. bartschi and Ps. capensis did not experience exceptional change in head depth.
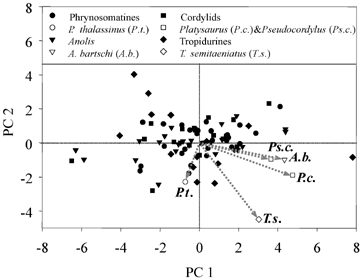
Principal components analysis on morphological change on branches. Although head depth loads most strongly on PC 2, some other variables have relatively high loadings (Table 3). Broken arrows are shown to indicate the direction and magnitude of change along each branch leading to a rock-lizard taxon. Platysaurus and Pseudocordylus species are as indicated in Figure 2.
Discussion
The five focal taxa of this study are found almost exclusively on rocky substrates, where they often move up, down, and along vertical surfaces and use narrow crevices as retreats (Vitt 1981; Novo Rodríguez and Estrada 1986; de Lisle 1991; Schwartz and Henderson 1991; Grismer 2002; Losos et al. 2002; L. J. Vitt, pers. comm.; J. B. Losos, unpubl. data). Phylogenetic analysis confirms that occupation of these habitats by these five taxa has generally involved evolutionary change in the same direction, toward decreased head height and increased limb length. These changes are statistically greater in magnitude than those that occurred on branches of the phylogeny on which a transition to rock-dwelling did not occur.
Nonetheless, evolutionary changes in the five lineages that moved to rock-dwelling have not occurred entirely in parallel. Four of the lineages have evolved longer hindlimbs, but Petrosaurus actually experienced a slight decrease in limb length. Similarly, although all five lineages evolved flatter heads and relatively longer hand and foot elements, the magnitudes of these changes were small in two of the lineages, A. bartschi and Ps. capensis (Fig. 3).
Why have not A. bartschi and Ps. capensis evolved flatter heads and relatively longer hand and foot elements (the variables that load on PC 2), and why has Petrosaurus evolved shorter limbs? One possibility is that these species interact with their environment differently than do the other rock-dwellers we studied. In particular, perhaps selection has favored increased limb length, but not decreased head depth, in the first two species, and decreased head depth and shorter limb elements in the latter. Although a possibility, the species do not differ from the others in any obvious manner in the way in which they use rock habitats (Vitt 1981; Novo Rodríguez and Estrada 1986; de Lisle 1991; Losos et al. 2002; J. B. Losos, unpubl. data).
A second possibility is that the lineages have occupied similar selective environments, but because their ancestral starting points were not the same, different patterns of evolutionary change were required to bring the species to comparable endpoints. Figure 2 illustrates that the value for A. bartschi for PC 2 is nearly as low as that for the other rock-dwelling species. Although rock-dwelling species in general have significantly lower values on PC 2 than non–rock-dwellers (Table 2), anoles as a group—including non–rock-dwellers—have low values on PC 2 as well (Fig. 2), perhaps because almost all anoles are arboreal and move on vertical surfaces to some extent (none of the other focal clades in this study is composed primarily of arboreal species; these trends can also be seen specifically in residual head depth; see online Supplementary Appendix S4, Table 4). Consequently, the reason that the branch leading to A. bartschi does not exhibit the large evolutionary decrease exhibited by the other lineages is that anoles may be pre-adapted for using rock surfaces (i.e., the flat head and relatively long hands and feet of A. bartschi are exaptive for rock-dwelling), and thus little evolutionary change was required when this species evolved rock-dwelling habits. This explanation, however, does not pertain to Ps. capensis, which has an intermediate value for PC 2 (Fig. 2; although a relatively flat head, see online Supplementary Appendix S4, Table 4), but has not experienced much evolutionary change in that respect (Fig. 3). Unfortunately, the natural history of Ps. capensis, like that of most African reptiles, is poorly known; a better understanding is required to guide further research into this question.
The opposite situation exists for limb length. In contrast to head depth, comparison of the limb lengths of rock-dwellers and non–rock-dwellers shows no statistical difference (Table 2). However, this nonsignificant overall relationship masks the fact that an evolutionary trend exists; comparison of each rock-dwelling species to other members of its clade indicates that the rock-dwellers consistently exhibit longer legs than most or all of their relatives (Fig. 2), as one would expect based on the analysis using changes inferred from the phylogeny (Fig. 3). The lack of an overall difference between rock-dwellers and non–rock-dwellers results because of among-clade differences in limb lengths; for example, phrynosomatines have longer limbs than cordylids. As a result, non–rock-dwelling phrynosomatines have limbs as long as or longer than rock-dwelling cordylids.
In this light, the lack of an evolutionary increase in limb length by the rock-dwelling P. thalassinus is interpretable; because other phrynosomatines, particularly the sand lizard clade (sister to all other phrynosomatines), have long limbs (Irschick and Jayne 1999), the ancestor of P. thalassinus may have had long limbs and thus would have been pre-adapted for rock-dwelling.
ADAPTATION AND EVOLUTION
These results highlight that adaptation is both a process and an end result and reflect upon the much-debated distinction between convergent and parallel evolution (reviewed in Wake 1991; Gould 2002; Donoghue 2005; Stayton 2006). Lineages with different ancestral morphologies can evolve in different directions toward the same adaptive phenotype; this corresponds to the classic definition of convergent evolution. Alternatively, lineages may begin with the same ancestral morphology and may evolve in the same direction toward the same endpoint; this would correspond to some definitions of parallel evolution, although more restrictive definitions additionally require that the evolutionary change be accomplished by similar alterations in the developmental program (Wake 1991; Gould 2002).
A less-discussed possibility, but one that probably occurs frequently, is when lineages start with different morphologies and evolve in parallel. In this case, parallel patterns of adaptive evolution may not produce highly similar outcomes, as has occurred with limb length of rock-dwelling lizards and head shape in herbivorous lizards (Stayton [2006]; this corresponds to the third type of convergence mentioned in Stayton's paper).
This discussion makes clear that comparative studies can benefit from considering both species' phenotypes and the patterns of evolutionary change. Had we looked only at the phenotypes of extant taxa, we would have recognized that distantly related species had convergently evolved extremely flattened heads, but the consistent evolutionary increase in limb length in rock-dwelling taxa would not have been obvious (see Stayton [2006] for a similar example). Conversely, had we focused solely on patterns of evolutionary change, we would have overlooked the similarity between A. bartschi and other rock-dwellers and would not have been alerted to the importance of exaptation in this lineage.
FUNCTIONAL DEMANDS ON ROCK LIZARDS
The use of rocky surfaces poses a number of challenges to lizards. First, rocky habitats are often very exposed, with little vegetative cover. As a result, rock-dwelling lizards often use two tactics to avoid predators. The first is to retreat into narrow crevices into which the predators cannot follow. The second tactic is to run rapidly and nimbly away from predators. Both of these tactics are utilized by the focal species in our study.
The use of steep surfaces provides a number of additional challenges to rock-dwelling lizards (Cartmill 1974, 1985). Unlike moving on a horizontal surface, locomotion on vertical surfaces is constrained by the lack of underlying support to counteract gravity. Individuals thus must minimize the floating phase of locomotion in which no limbs are in contact with the surface. Moreover, when the forelimbs lose contact (or the hindlimbs if the lizard is moving downward), the center of gravity must be kept close to the surface to prevent the animal from pivoting around its hindlimbs (or forelimbs) and toppling backward away from the surface (Cartmill 1974, 1985; Arnold 1998b; Vanhooydonck et al. 2002). In addition, to enter and move within a crevice, lizards must minimize their body height.
The biomechanical solutions to these problems are straightforward in some respects. The obvious solution to using narrow crevices is to evolve a dorsoventrally compressed body form, a response that has evolved many times in lizards (Arnold 1998a, 2002). Many species amplify this strategy by evolving morphological adaptations to wedge themselves into place by expanding the height of their heads or bodies to prevent being extracted (Arnold 1998a, 2002). A flattened body form may have the further advantage of making a lizard less conspicuous on a flat surface (Vitt 1981).
In addition, by changing the orientation of the limbs, lizards can reduce body height, which facilitates movement within narrow spaces and keeps the center of gravity near the surface when moving on an incline. With the exception of chameleons (Higham and Jayne 2004), which are specialized for gripping narrow perches, lizards commonly change limb posture to decrease their height in response to increased steepness of the locomotor surface (Jayne and Irschick 1999; Spezzano and Jayne 2004). The proximal limb elements of lizards are capable of considerable rotation about their long axis (Barclay 1946; Brinkman 1981), and this long axis rotation can change the orientation of the lower limb from nearly perpendicular to nearly parallel to the locomotor surface, resulting in substantial decreases in the distance of the body from the surface (Jayne and Irschick 1999; Spezzano and Jayne 2004). With increased incline, pelvic rotation of some species also increases (Jayne and Irschick 1999), which suggests an increased reliance on movements in a horizontal plane for increasing propulsive forces. The orientation of the foot of lizards also may change in a manner that reduces vertical movements. If the toes of the foot are pointed forward, then straightening the ankle during the propulsive phase of a stride will tend to elevate the limb and the body. However, when some lizards are on inclined surfaces the foot has a significantly more lateral orientation than when moving on a horizontal surface, and this can reduce the tendency for the body to increase its distance from the surface during the step cycle (Jayne and Irschick 1999).
Besides falling backwards off a vertical face when running uphill, another difficulty is long-axis roll when moving up or down on a steep or vertical surface. The tendency for long-axis roll in these circumstances potentially could be reduced by increasing the lateral distance from the hip to the foot either by having a longer femur or keeping the femur nearly perpendicular to the body. Orienting the long axis of the foot more laterally also may help to reduce long-axis roll of the entire animal in these circumstances.
On open horizontal surfaces, the maximum speeds of lizard species are positively correlated with overall limb length and stride length (distance traveled between successive footfalls [Losos 1990; Irschick and Jayne 1999; Vanhooydonck et al. 2006]). When animals run at high speeds in open areas, a large fraction of stride length is usually attained during a suspended phase in which neither of the limbs within a girdle touch the ground, and this is especially true for the high speed locomotion of terrestrial lizards (Irschick and Jayne 1999). However, the loss of ground contact and the vertical displacement associated with doing so are likely to be detrimental for movement on steep inclines or within the limited vertical space in rocky crevices. Yet, as a result of their overall sprawling limb posture, many of the key movements that contribute to forward displacement while a foot is on the ground can still occur while keeping the animal close to the locomotor surface. As a combined result of pelvic rotation and femur retraction in the horizontal planes, the length of the femur should have a central role for contributing to long step length, which could facilitate attaining rapid speeds while remaining close to the locomotor surface.
In summary, lizards on inclined surfaces orient their limbs more laterally to minimize distance from the surface. In such a posture, longer limbs would be beneficial to prevent rolling around the long axis of the body, and longer femurs would provide greater speed. In support of these predictions, not only do limb lengths in general increase in rock-dwelling species, but the femur also exhibited the strongest effects (Table 4).
Many climbing species move in all orientations, from head upward to head downward and occasionally even upside-down. In such positions, the forelimbs are necessary to keep the lizard from toppling from the surface and may provide more of the propulsive force (Arnold 1998b; Zaaf et al. 2001). Consequently, Zaaf and Van Damme (2001; see also Arnold 1998b) predicted that the fore- and hindlimbs will be more similar in length in climbing species than in nonclimbers. However, this prediction is not upheld in the clades we studied; in all four clades, the ratio of forelimb:hindlimb length was intermediate for our focal species.
A further difficulty with locomotion on steep rock surfaces is that most lizards gain purchase by using claws and the smooth surface of rocks may provide limited footholds (Vanhooydonck and Van Damme 2001). The claws of rock-dwelling lizards might also be expected to differ among climbing and nonclimbing species, but the ecological morphology of lizard claws has received little study (Arnold 1998b; Zani 2000).
ROCK-DWELLING LIZARDS IN OTHER CLADES
We focused on species that frequently use vertical surfaces and narrow crevices. In the four clades we examined, these species showed a general trend toward increased limb length and decreased body height. As mentioned above, many other lizards that use narrow crevices have evolved a flattened body form.
By contrast, trends with regard to limb length are more mixed. Comparisons among several sets of closely related tropidurines reveal that taxa that have occupied rocky habitats have longer limbs than their non–rock-dwelling relatives (Vitt 1993; Vitt et al. 1997). A number of geckos (Ptyodactylus, Asaccus, Agamura, and Hemidactylus lemurinus) that are rock-wall specialists also have extremely long legs, although others do not (E. N. Arnold, pers. comm.). Although a comparative study of geckos found no difference in limb dimensions between climbing and ground-dwelling species, the only species listed as using vertical rock walls, Rhoptropus boultoni, had the second longest proximal limb elements (Zaaf and Van Damme 2001; the long-legged gekkonid species listed above were not included in this study).
Two additional species that use flat and steep surfaces have long limbs. First, is a cordylid not sampled for this study, Cordylus peersi. Many cordylids are heavily armored, with bony osteoderms and large spines on the head and body. Most of these species rarely use vertical surfaces and have relatively short legs. The one exception is C. peersi, which is usually found on vertical surfaces and has limbs substantially longer than other armored cordylids (C. peersi is the outlier in figure 5c of Losos et al. 2002).
The second example is the species exhibiting the highest value of limb length of all species in this study (Fig. 2). This tropidurine species, Tropidurus plica, is also a vertical habitat specialist, although it lives on very large trees, rather than rock surfaces (Vitt 1991).
On the other hand, lacertid lizards that use stone walls and rocky habitats do not have longer limbs than lacertids that use other habitats (Arnold 1998b; Vanhooydonck and Van Damme 1999; but see Kramer 1951). Some other rock-dwelling species also do not have particularly long legs, including Xantusia henshawi, the skinks Mabuya sulcata and M. laevis, and the chuckwalla, Sauromalus obesus, and its ecological doppelgänger, the Andean genus Phymaturus. Several of these species do not stray far from refuges, so sprint speed may not be important for them, but why the skinks and lacertids, which do move rapidly in pursuit of prey and to escape predators, have not evolved longer legs is an enigma. To some extent, the situation with lacertids parallels that for Petrosaurus in that many lacertids live on the ground in open habitats and rely on rapid movements; these species generally have longer limbs than do climbing lacertids (Arnold 1998b; Vanhooydonck and Van Damme 1999). Arnold (1998b) and Vanhooydonck and Van Damme (1999) differ on whether lacertids that climb steep surfaces have longer limbs than lacertids that move through dense vegetation.
Arnold (1998b) showed that specialized climbing lacertid lizards move their hindlimbs forward to a much lesser extent when running up a vertical surface than do ground-dwelling species when running on a horizontal surface, thus producing shorter strides, even in proportion to their shorter legs. He suggested that climbing species use a “low gear” to combat the opposing force of gravity as they move upward. Whether this low gear locomotion led to the evolution of shorter legs, or whether the causality runs in the reverse direction, remains to be determined. In summary, more research is needed to understand why vertical climbing frequently, but not always, leads to the evolution of long legs.
More generally, this leads to the question: if long limbs often are beneficial on flat, vertical surfaces, why do not all long-legged rock-dwelling lizards have equally long hind limbs (Fig. 2)? A variety of explanations are possible, including
- 1
The requirements of environments may differ. Some places may select for greater speed compared to others.
- 2
Limb length is not the only phenotypic feature that affects locomotion. Some clades may achieve the same functional outcome with shorter limbs, but greater muscle mass, more efficient enzymes, or stronger claws (e.g., Vanhooydonck et al. 2006). Moreover, as discussed above, stride length is affected both by the length of proximal limb elements and the orientation of distal limb elements. Consequently, the same stride length can be achieved by species with different limb lengths—more lateral orientation of the distal limb elements can compensate for shorter proximal limb lengths.
- 3
Limbs are involved in other activities besides running, such as jumping. As with sprinting, long legs generally enhance jumping performance (Losos 1990; Toro et al. 2003). At least some of the focal species in this study also jump frequently (Grismer 2002; Vitt and Pianka 2003). Possibly, differences in selection for jumping ability or other limb-related performance capabilities are responsible for differences in limb length among rock-dwelling lizards.
In summary, evolutionary transition to the use of steep surfaces generally leads to similar patterns of adaptive morphological change in many lineages. Nonetheless, the extent and direction of evolutionary change varies among lineages, at least partly as a result of the extent to which a lineage's ancestral state was already well-suited for using such habitats. Phylogenetic analysis can help identify the extent to which parallel changes occur among species occupying the same habitats, but nonphylogenetic comparisons provide an important and complementary perspective.
Associate Editor: K. Schwenk
ACKNOWLEDGMENTS
We are particularly grateful to B. Jayne for extensive guidance concerning lizard biomechanics and E. N. Arnold for discussions on lizard diversity. We thank the museums listed above and their curators and staff for the loan of specimens; J. Wiens for statistical advice; R. Glor, K. Nicholson, T. Sanger, K. Schwenk, P. Wainwright, L. Vitt and two anonymous reviewers for constructive comments on a previous draft; and the National Science Foundation (DEB 9982736) for support.




