PROMISCUITY DRIVES SEXUAL SELECTION IN A SOCIALLY MONOGAMOUS BIRD
Abstract
Many socially monogamous species paradoxically show signs of strong sexual selection, suggesting cryptic sources of sexual competition among males. Darwin argued that sexual selection could operate in monogamous systems if breeding sex ratios are biased or if some males attract highly fecund females. Alternatively, sexual selection might result from promiscuous copulations outside the pair bond, although several recent studies have cast doubt on this possibility, in particular by showing that variance in apparent male reproductive success (number of social young) differs little from variance in actual male reproductive success (number of young sired). Our results from a long-term study of the socially monogamous splendid fairy-wren (Malurus splendens) demonstrate that such comparisons are misleading and do not adequately assess the effects of extra-pair paternity (EPP). By partitioning the opportunity for selection and calculating Bateman gradients, we show that EPP has a strong effect on male annual and lifetime fitness, whereas other proposed mechanisms of sexual selection do not. Thus, EPP drives sexual selection in this, and possibly other, socially monogamous species.
Sexual selection operates through variance in mating success (Bateman 1948; Arnold 1994), yet many socially monogamous species with seemingly low variation in mating success show signs of strong sexual selection (Andersson 1994). Indeed, in many birds elaborate male ornaments and sexual dimorphism are pronounced despite the fact that most species are socially monogamous (Lack 1968). This pattern raises an important evolutionary paradox: if all males obtain one and only one mate in socially monogamous systems, what are the causes of variance in mating success that could generate sexual selection?
Darwin (1871) recognized this paradox and suggested two possible sources of sexual selection in monogamous systems. First, adult breeding sex ratios might often be biased, such that some males obtain mates whereas others do not (Price 1984; Dearborn et al. 2001; Kvarnemo et al. 2007). Second, some males might attract higher-quality females that are able to produce more offspring, and thereby gain a reproductive advantage over other males (Fisher 1958; Kirkpatrick et al. 1990). More recently, genetic studies of wild populations have suggested a third possible solution to the paradox: extra-pair paternity (EPP), which results from copulations between males and females who are not socially paired with each other. Such promiscuous copulations are common in birds (Griffith et al. 2002), and can potentially lead to strong sexual selection (Webster et al. 1995).
Although EPP is generally assumed to be an important source of sexual selection on males (e.g., Møller and Ninni 1998), a number of results from recent studies have challenged this view. First, although some comparative studies have supported an association between promiscuity and sexual dichromatism in birds (Møller and Birkhead 1994; Owens and Hartley 1998), other analyses have found this association to be weak (Dunn et al. 2001). Second, theoretical studies have shown that EPP will often have little effect on the opportunity for sexual selection, particularly if there is a negative covariance between extra-pair and within-pair reproductive success (Webster et al. 1995) or if ecological factors limit the number of extra-pair females a male can court (Webster et al. 2001; Pedersen et al. 2006). Third, several detailed empirical studies have shown that variance in apparent male reproductive success (number of social young) differs little from variance in actual male reproductive success (number of young sired), suggesting that EPP has little effect on the opportunity for sexual selection (e.g., Dunn et al. 2001; Webster et al. 2001; Whittingham and Dunn 2005). Indeed, Freeman-Gallant et al. (2005) demonstrate that evidence for an effect of EPP on the opportunity for selection comes mainly from studies in which relatively few young have been assigned to their sires. These results cast doubt on the importance of EPP and suggest that the mechanisms originally proposed by Darwin may be more important sources of sexual competition in monogamous systems.
The role of EPP in sexual selection remains unclear because few studies have assigned most extra-pair young to their sires (see Freeman-Gallant et al. 2005), and because few studies have contrasted EPP with other potential sources of sexual selection (see Dearborn et al. 2001). In this study we examined the effects of EPP on male mating and reproductive success in a population of splendid fairy-wrens (Malurus splendens, Maluridae), and compare these effects to those of other potential sources of variation. Splendid fairy-wrens breed cooperatively and are socially monogamous (Van Bael and Pruett-Jones 2000), but plumage dichromatism and male reproductive anatomy (Tuttle et al. 1996; Rowe and Pruett-Jones 2006) suggest strong sexual selection. EPP rates are high in this species (55.4% of all broods contain extra-pair young, Webster et al. 2004; see also Brooker et al. 1990), and may be a key source of sexual selection. However, breeding sex ratios are biased toward males, with many adult males remaining as subordinate auxiliaries on their natal territories, and differences in female quality might also exist. Thus, in this population sexual selection could be generated by variation in pairing success, mate quality, or EPP.
Materials and Methods
GENERAL FIELD METHODS
We studied a population of splendid fairy-wrens at the Brookfield Conservation Park, approximately 100 km northeast of Adelaide, South Australia, from 1992 through 1998. We captured all resident adults in the population, collected blood samples for genetic analysis, and applied colored leg bands for individual identification. We never observed unbanded individuals in our study site other than rare dispersing females early in the breeding season, and are therefore confident that we had marked and collected samples from all breeding adults.
Social associations and breeding status were determined by direct observation: auxiliary males were males without a social mate who remained on their natal territories, breeding males were those males with a social mate, and successful males were those breeding males who produced one or more social young (see below). We found nests by following adults and by searching in appropriate habitat, and we visited nests once every three days to monitor breeding activity. Predation pressure was high in our study area, with the principle nest predators being gray currawongs (Strepera versicolor) and other large birds as well as snakes (Van Bael and Pruett-Jones 2000). Fairy-wrens are very small (ca. 8–9 g) and seem ineffectual at driving away these predators (K.A. Tarvin and S. Pruett-Jones, pers. obs.). To increase sample sizes, we protected some nests with wire mesh cages that allowed the parents to pass through but kept out larger predators. We banded nestlings and collected blood samples for genetic analysis when they were six-days old. Although a small number of nests may have been produced outside of the main breeding season, we are confident that we sampled virtually all nests that produced young because adults were never observed with unbanded fledglings (see Van Bael and Pruett-Jones 2000). A severe drought in 1994 caused widespread reproductive failure in our study population, and data from that year were excluded from these analyses.
GENETIC ANALYSIS OF PARENTAGE
We determined parentage of offspring by comparing genotypes at six microsatellite loci following standard methods detailed in Webster et al. (2004). We used a likelihood approach (Marshall et al. 1998) to determine paternity by comparing nestling genotypes to adult male genotypes (see Webster et al. 2004). This approach was unambiguous in the majority of cases, and we were able to assign paternity to 96% of 447 nestlings (Webster et al. 2004).
Annual reproductive success for each male was calculated as the number of young that reached the age at which we collected blood samples (day 6); many but not all of these nestlings fledged (Van Bael and Pruett-Jones 2000). Apparent reproductive success was the number of young produced on a male's territory (regardless of paternity), and actual reproductive success was the total number of young a male sired on all territories. For each male, the number of mates was estimated as the number of social mates (0 or 1) plus the number of extra-pair females with whom the male sired offspring.
To calculate “lifetime” reproductive success, we summed reproductive success across years for each male. Although this measure might underestimate lifetime reproductive success for some males (i.e., those who sired young before the study began or after it ended), it should accurately reflect lifetime reproduction in most cases because > 63% of males started and completed their entire reproductive life spans during our study. We calculated the number of mates obtained during each male's lifetime as we did for annual reproductive success, but for simplicity assigned just a single social mate to each breeding male because males have only a single social mate during any given year. To ensure that our results were not influenced by inclusion of males with life span that extended beyond the study period, we repeated analyses of lifetime reproductive success using only the 63% of males for whom we had complete lifetime data; results from these analyses were consistent with those for the entire dataset and are not reported here.
STATISTICAL ANALYSIS OF SELECTION
The “opportunity for selection” is given by the variance in reproductive success standardized by dividing by the mean squared, and is a measure of the maximum possible strength of selection (Arnold and Wade 1984). We partitioned the total opportunity into component parts using the approach of Webster et al. (1995). We also calculated Bateman gradients (or “sexual selection gradients”), which are a key descriptor of the strength of sexual selection (Arnold 1994; Mills et al. 2007), by regressing male reproductive success on number of mates. Analyses are based on a sample of 48–79 males per year, including auxiliaries (9–28 per year), for a total of 204 males. For the analyses of lifetime reproductive success, we regressed total number of young sired on lifetime number of mates, breeding life span (number of years as a breeder), and both (for the latter, using a Type III multiple regression model). For all regression analyses we log-transformed our data to fit assumptions of linear regression (i.e., residuals distributed normally and homoscedastically). Regressions for Bateman gradients were forced through the origin, but other regressions were not.
ESTIMATING THE EFFECTS OF PREDATION ON THE FITNESS ADVANTAGE OF EPP
In this study population most cases of nest failure were caused by predation (Van Bael and Pruett-Jones 2000). We estimated whether predation affected the fitness advantage of males who sired extra-pair young using a bootstrapping procedure. Using pooled data for all breeding males across years, we randomly selected with replacement a set of 226 males who sired extra-pair young and another set of 68 males who did not sire extra-pair young (sample sizes as in the original dataset). We then calculated the standardized fitness differential between males who did and did not sire extra-pair young, and repeated this process 50 times to generate a distribution of fitness differentials. We repeated this procedure but using only males who bred successfully (i.e., produced ≥ 1 social young; 121 males who sired extra-pair young and 38 who did not). We contrasted the mean standardized fitness differentials when total nest failure was included (all breeding males) to that when it was excluded (successful males only) using a t-test.
Results
EFFECTS OF EPP ON SEXUAL SELECTION
Male reproductive success showed considerable variation within each year (Fig. 1). We found little difference between the standardized variance in apparent male reproductive success (number of social young produced, Ta) and the standardized variance in actual male reproductive success (number of young sired, T): the standardized variance in T exceeded that for Ta in only two of six years, and averaged across years these two measures were virtually identical (Table 1).
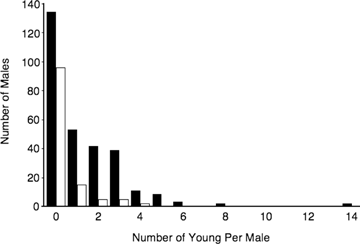
The distribution of annual reproductive success (number of young sired per year) for breeding males (dark bars, N= 294 male-years) and auxiliaries (white bars, N= 102 male-years).
| Annual reproductive success | Total reproductive success across years (N= 204) | |||
|---|---|---|---|---|
| All males (N= 204) | Breeding males (N= 160) | Successful males (N= 105) | ||
| var(TA) | 1.47 | 0.93 | 0.14 | 1.78 |
| var(T) | 1.42 | 1.20 | 0.55 | 1.98 |
| var(W) | 0.83 (58.8) | 0.83 (69.2) | 0.35 (63.8) | 0.84 (42.7) |
| var(E) | 0.60 (42.0) | 0.38 (31.8) | 0.17 (31.6) | 0.79 (39.7) |
| cov(W, E) | −0.01 (−0.8) | −0.01 (−1.0) | 0.03 (4.9) | 0.35 (17.5) |
| var(MW) | 0.16 (11.3) | 0.00 (0.0) | 0.00 (0.0) | |
| var(ME) | 0.60 (42.4) | 0.37 (31.1) | 0.17 (30.8) | |
| var(PW) | 0.18 (12.7) | 0.21 (18.0) | 0.28 (50.8) | |
| var(T)/var(TA) | 0.966 | 1.290 | 3.929 | 1.112 |
Several results demonstrate that reproductive promiscuity contributed strongly to the opportunity for sexual selection in this population. First, EPP contributed substantially to the annual reproductive success of some males (Fig. 1), including several “nonbreeding” auxiliary males who sired young (see Webster et al. 2004 for additional details on auxiliaries siring offspring). For example, one male sired 14 offspring in a single year, 10 of which were extra-pair. Second, partitioning the total opportunity for selection into its component parts revealed that variance in extra-pair success (var(E)) was relatively large, contributing 42% (Table 1) to the total variance in male reproductive success (range across years: 16% to 59%). The covariance between within-pair and extra-pair success varied considerably across years (accounting for −17% to 20% of the total variance in each year), but on average was negligible (Table 1). Variance in number of extra-pair mates (var(ME)) across all males was relatively large, exceeding the standardized variance in number of social mates by 375% (Table 1). Finally, males also varied in the extent to which they were cuckolded (PW, Table 1); combining the variance generated by male gains from EPP (var(E)) and male losses to EPP through cuckoldry (var(PW)), EPP contributed approximately 55% of the total opportunity for selection.
The importance of EPP to sexual selection can be visualized with the Bateman gradient, which shows that male reproductive success was closely tied to number of mates (Fig. 2A); because this species is socially monogamous, males can obtain multiple mates only by mating with extra-pair females. As a consequence, the average annual reproductive success of males who sired extra-pair young (EPY) was approximately three times that of males who did not (2.74 vs. 0.87 offspring/male; N= 226, U= 12,695.5, P < 0.0001).

Bateman gradients showing the relationship between number of mates and reproductive success, calculated using (A) the average reproductive success per year for all males (log-transformed data: F1, 203= 437.7, R2= 0.682, P < 0.0001), (B) the total reproductive success across years for all males (log-transformed data: F1, 203= 594.7, R2= 0.744, P < 0.0001), and (C) average annual reproductive success of breeding males alone (log-transformed data: F1, 159= 261.7, R2= 0.618, P < 0.0001). Regression lines shown are from regressions with untransformed data.
The effects of EPP on sexual selection might be magnified or reduced by variation across years. In our study population, 49.0% of all adult males spent at least one year as an auxiliary (range = 0–4 years, mean = 0.71, N= 204), but only 18.1% of adult males never attained a breeding position. Males spent up to six years as a breeder (mean = 1.75, N= 204), and male lifetime reproductive success ranged from 0 to 22 offspring, with a mean of 2.09 ± 2.94 (SD). Partitioning total lifetime reproductive success into component parts revealed that EPP contributed strongly to the total opportunity for selection (Table 1): variance in extra-pair success accounted for 39.7% of the standardized variance in lifetime reproductive success (Table 1), and male lifetime reproductive success was strongly related to total number of mates (Fig. 2B). Not surprisingly, lifetime reproductive success also was strongly associated with number of years as a breeder (log-transformed data: F1,203= 92.2, R2= 0.310, P < 0.0001), and breeding life span was correlated with lifetime number of mates (r= 0.564, Z= 9.07, P < 0.0001). A multiple regression model that included both factors (log-transformed data: F2,201= 111.5, R2= 0.521, P < 0.0001) revealed lifetime reproductive success was independently associated with both breeding life span (t= 2.39, P= 0.018) and number of mates (t= 9.50, P < 0.0001).
EPP had a strong effect on male reproductive success in part because 22.2% of unsuccessful breeders (i.e., those males who lost all social young to predation) sired extra-pair offspring (unsuccessful and successful breeders did not differ with respect to whether they sired EPY; N= 135, 159, χ2= 0.116, P= 0.734). When unsuccessful males were removed from the analysis, successful males who sired EPY produced approximately twice as many offspring as those who did not sire EPY (3.24 vs. 1.63 offspring/male; N= 38, 121, U= 3493.5, P < 0.0001), but this difference is less than when all males were included in the analysis (above). Thus, the standardized fitness differential between males who did and did not sire EPY was significantly greater when the effects of predation were included than when effects of predation were excluded (Fig. 3, t= 25.85, df = 71, P < 0.0001).

The effects of predation on standardized fitness differentials (i.e., the difference in mean fitness between males who did and did not sire extra-pair young (EPY), standardized to the mean male fitness. Fitness differentials calculated using field data from successful breeding males (effects of predation excluded) and from all breeding males (effects of predation included). Error bars show 95% confidence intervals around the mean as determined by bootstrapping.
OTHER POSSIBLE SOURCES OF SEXUAL SELECTION
Sexual selection could also operate through variance in social pairing success: in this study an average of 27.5% of the focal males each year were “nonbreeding” auxiliaries who did not obtain a social mate (range: 17.0–42.4% per year), and the average adult sex ratio (males/females) across years was 1.36 (range: 1.12–1.46; see Webster et al. 2004). Despite this biased sex ratio, social pairing success (var(Mw)) contributed little (11.3%) to the variance in annual reproduction across all males (Table 1). Further, restricting our analyses to breeding males by excluding data on auxiliaries had a relatively small effect on the opportunity for selection: the standardized variance in actual reproductive success of breeding males was only 15% less than that for all males (Table 1). Among breeding males, standardized variance of reproductive success was modestly (29%) larger than that of apparent reproductive success (Table 1), and the relationship between reproductive success and number of mates remained strong (Fig. 2C). Thus, despite a biased adult sex ratio and significant variance in within-pair reproductive success (Table 1), the effect of social pairing success per se on the strength of sexual selection was minor relative to that of EPP.
A third possible mechanism for sexual selection in this monogamous species is variation in female quality. We found substantial variation in both within-pair reproductive success (var(W), Table 1) and the reproductive success of males who obtained only a single mate (Fig. 2A). This variation might partially reflect female quality, but in our study population females varied little in the number of eggs per clutch (92% of all clutches contained three eggs, N= 103 full clutches) and most cases of nest failure were caused by predation (see Methods). Fairy-wrens are small (8–9 g) and likely unable to drive away most nest predators. Thus, variation in number of young per social mate likely reflects the stochastic effects of predation rather than female quality. Indeed, restricting analyses to breeding males who successfully produced social offspring within a year indicated a strong effect of nest failure: the standardized variance in the reproductive success of successful males was 61% lower than that for all males (Table 1), and var(T) greatly exceeded var(Ta), by an average of 393% across years (var(T) exceeded var(Ta) in every year except 1992, the year with smallest sample size). After removing the effects of predation, some variation in within-pair success remained (var(W), Table 1), but most (79%) of this was explained by variation in cuckoldry rates across males (var(Pw), Table 1). Thus, variation in social mate quality contributed little to sexual selection.
Discussion
A number of recent studies have found little difference between the opportunity for selection (standardized variance in male reproductive success) calculated from apparent male reproductive success (number of social young produced, Ta) or from actual male reproductive success (number of young sired, T; see Webster et al. 2001; Freeman-Gallant et al. 2005; Whittingham and Dunn 2005). Because standardized variance in reproductive success measures the opportunity for selection (Arnold and Wade 1984), similarity of variances for Ta and T is typically interpreted to mean that EPP does not increase the strength of sexual selection (e.g., references above).
Simple comparisons of apparent to actual reproductive success, however, can be misleading because the former is not based on actual parentage, and in this study several results demonstrate that reproductive promiscuity contributed strongly to the opportunity for sexual selection. In particular, partitioning the opportunity for selection into component parts demonstrated that much of the variation in male mating success was due to variation in the ability to sire EPY (var(E)) and/or avoid being cuckolded (var(Pw)); both of these sources of variance arise from reproductive promiscuity.
The effects of EPP are also evident in the strongly positive Bateman gradients (Fig. 2), which demonstrate that male reproductive success was closely tied to number of extra-pair mates. Consequently, any male trait closely associated with EPP success should be strongly favored by selection in this population. We did not identify any male traits associated with EPP success (see Tarvin et al. 2005), in part because we were unable to properly quantify the iridescent blue coloration of males for selection gradient analyses. Moreover, because the opportunity for selection gives the maximum strength of selection that can act on a trait (Arnold and Wade 1984), the actual strength of selection acting on specific traits may be weaker than implied here. Nevertheless, some studies of other birds have identified traits, including plumage ornamentation (e.g., Thusius et al. 2001; Kleven et al. 2006), that are associated with a male's ability to sire EPY. Indeed, in the closely related superb fairy-wren (M. cyaneus), male EPP success is closely associated with prenuptial molt date (Mulder and Magrath 1994; Dunn and Cockburn 1999), which is a trait we were unable to measure in our study.
In contrast to the effects of EPP, the other mechanisms of sexual selection proposed by Darwin seem to be relatively unimportant in this population of fairy-wrens. First, despite a biased adult sex ratio and the presence of many adult males without social mates, social pairing success contributed relatively little to the total opportunity for selection. Moreover, several auxiliary males without social mates were able to sire young through EPP (Fig. 1), which acts to reduce the fitness variance produced by pairing success. Variation in female quality also seems to be relatively unimportant in this study population; females varied little in fecundity, and the effects of nest predation swamped any differences in female parenting ability. Thus, although the total contribution of within-pair reproductive success to male fitness was approximately equal to that of extra-pair reproductive success (Table 1), this did not appear to be due to variation in male pairing success or female quality, and instead likely reflects the stochastic effects of nest predation.
Intriguingly, nest predation seemed to affect the strength of sexual selection acting through EPP—the fitness advantage of males who sired EPY was highest when the effects of nest predation were included, but lower when they were excluded (Fig. 3). One possible explanation for this result is that EPP buffers males against the negative effects of nest predation by reducing the likelihood of total reproductive failure. Similar “bet-hedging” against predation has been suggested as a selective benefit of intraspecific brood parasitism to females (Rubenstein 1982; Pöysä 2007), but this hypothesis is controversial as theoretical studies suggest that bet-hedging effects will be weak (Bulmer 1984). Moreover, in this study we were unable to sample young for parentage analysis until after most nest predation had occurred, and therefore some males may have been misclassified. Further theoretical and empirical work is necessary before it is clear how nest predation might shape the strength of sexual selection acting on males.
In summary, these results indicate that genetic promiscuity, acting through EPP, underlies the opportunity for sexual selection in this socially monogamous species. Other potential mechanisms for sexual selection—biased breeding sex ratios and variable mate quality—might also be operating in this system, but their effects are weak relative to that of reproductive promiscuity. Thus, although the total opportunity for selection may be less than is seen in socially polygynous systems (e.g., Trail 1985; Pruett-Jones and Pruett-Jones 1990), the opportunity that does occur appears to be due to EPP. Although EPP may be unimportant in some monogamous systems (e.g., Dearborn et al. 2001), few other studies have partitioned the sources of variance in male reproductive success or calculated Bateman gradients (but see Freeman-Gallant et al. 2005; Kleven et al. 2006; Albrecht et al. 2007). Indeed, most studies have relied on simple comparisons of variance in apparent reproductive success to variance in actual reproductive success (see table 1 in Freeman-Gallant et al. 2005), and this comparison does a relatively poor job of quantifying the effects of EPP (see above). Thus, additional detailed studies of this sort are needed before we can know whether EPP resolves the paradox of sexual selection in socially monogamous birds.
Associate Editor: Kimberly Hughes
ACKNOWLEDGMENTS
We thank the great number of field and lab assistants who provided critical assistance during this work. The South Australian government provided permission to carry out this research. Logistical assistance and advice were provided by S. Williams, J. Stelman, the Brookfield Conservation Park Friends Group, M. Ashley, J. Eadie, R. Gomulkiewicz and S. Sillett. K. Hughes and anonymous referees provided valuable comments on a previous version. This research was conducted with proper authorization and using protocols approved by the University of Chicago IACUC. Financial support was provided by Chicago Zoological Society, M. A. Ingram Trust, Norman Wettenhall Foundation, and grants from the National Science Foundation (USA) to SP-J and MSW.




