Forensic Discrimination of Dyed Hair Color: I. UV-Visible Microspectrophotometry* †
Presented in part at the 61st Annual Meeting of the American Academy of Forensic Sciences, February 16–21, 2009, in Denver, CO.
Funded by the Forensic Sciences Foundation Jan S. Bashinski Criminalistics Graduate Thesis Grant.
Abstract
Abstract: Current protocols for examining hair do not attempt to differentiate hair color using instrumental analysis. In this study, hair samples treated with 55 different red hair dyes were analyzed using UV-visible microspectrophotometry between 200 and 700 nm. Using air as a background reference gave the best results, although mounting media such as glycerin could also be used. The contribution of the hair substrate is predominantly observed in the range of 300–400 nm while the dye peak is evident in the range of 425–550 nm. It was found that the presence of hair dye reduces the overall intrasample variability of the hair color. In addition, visual inspection and spectral interpretation showed that dyed hair exhibits distinct and discernable shades. The color of all samples was stable during storage and while all hair dyes fade with washing, significant fading of the color was only evident after daily washing for 3 weeks.
Although cosmetic modifications such as hair bleaching and/or dyeing occur with significant frequency, insufficient research has been performed to distinguish hair samples based on their color. Hence, this research is intended to assess the ability of UV-visible microspectrophotometry (MSP) to differentiate the color of dyed hair in the context of forensic hair comparisons. Microscopic examination of hair does not provide a definitive link between an unknown specimen and the suspected source, and the proposed research will not claim to do so; however, extending the current hair analysis protocol to include forensic characterization of dyed hair color may ultimately provide a higher degree of association between the sample and the source, as well as reducing false positive conclusions by revealing additional data which has been previously overlooked.
Microscopic hair examination provides valuable evidence and insight into civil and criminal investigations. For example, the morphology and microscopic features of human hair provide a wealth of information such as species of origin, area of the body from which the hair originated, ethnicity, removal from the body, either forcible or naturally shed, in addition to disease states, thermal damage, and cosmetic modifications (1,2). In cases where the root of the hair is absent, and thus nucleated DNA (nDNA) is unavailable, mitochondrial DNA (mtDNA) analysis can be utilized, if available. Microscopic hair examination is particularly critical in circumstances where either insufficient DNA is present in the sample to carry out the mtDNA analysis or the suspects are maternally related (1,2). These cases support the necessity of exploiting all potential data present in each hair sample; thus, it is important to explore alternative analytical techniques.
Hair dyes can be classified as either nonoxidative or oxidative based on the reactivity of the dyes (3). Nonoxidation dyes only physically coat the surface of the hair and contain a single component. Oxidative dyes represent approximately 80% of the hair color market and are favored for providing a more consistent overall appearance of the hair as well as lasting for longer periods of time (3). All of the dyes presented in this study are oxidative dyes; thus, only the chemical reactivity of this type of dye will be discussed in detail. Two component mixtures, referred to as the base colorant and developer oxidant, are characteristic of oxidative dyes and are stored separately until the dyeing process is initiated. Generally, the developer is a lotion or emulsion, while the base colorant is either a gel or a cream (4). Oxidative dyes are generally comprised of an oxidant, alkalizer, primary intermediates, couplers, solvents, thickeners, surfactants, buffers, a chelating agent, as well as an antioxidant. Primary intermediates, also called precursors, and couplers diffuse into the cuticle independently as low molecular weight species and react with one another to create larger dye molecules within the hair. Demi-permanent, or long-lasting semi-permanent, and permanent dyes are two types of oxidative dyes. Although demi-permanent dyes are oxidative in nature, the mild compositions of alkalizing and oxidizing agents do not lighten the natural hair color, but only deposit the color. Permanent colors both lighten the natural hair color and effectively deposit color in the cortex of the hair (4). The final shade of color produced for hair dyes relies primarily on three factors: the natural or preexisting color of the hair substrate, the interaction of the dye components with the hair, and the resulting color molecules deposited during the reaction process. The intensity of the natural hair color is indicated numerically, on a scale of 1–10, with level one representing black and level ten signifying the lightest shade of blonde hair. Lightening of the natural hair color is directly proportional to the concentration of hydrogen peroxide in the developer along with the pH of the base colorant (5–7).
Hair dyes in solution are commonly analyzed with high-performance liquid chromatography or gas chromatography/mass spectrometry (GC/MS), with emphasis on the primary intermediates and couplers involved in dye formation. The majority of the existing literature focuses on analyzing the initial dye components in the base colorant. There is little in the literature on the analysis of the components following the dyeing procedure either directly on the hair substrate or postextraction. Tanada et al. performed GC/MS analysis of five components commonly found in oxidative hair dyes. The general protocol involved an alkaline digestion of the dyed hair followed by extraction and derivatization, which successfully detected the target hair dye components (8). The complete digestion of the hair, and thus destructive nature of this procedure, is a substantial drawback. A more straightforward approach would be favorable for integration into casework.
A microspectrophotometer is an instrument that combines the powerful techniques of microscopy and spectrophotometry, providing an objective assessment of the absorption of light for small objects (9). The analysis of fibers by microspectrophotometry is well established, and of particular interest for this study, as it will provide insight into potential applications and strategies applicable for analyzing dyed hair with this method (10,11). Although the human eye is sensitive to color, microspectrophotometers have a higher sensitivity and broader wavelength range for assessment.
While MSP data cannot be used to unambiguously identify the chromophores responsible for sample absorption, it does provide an objective view of the absorption characteristics of the sample and thus its exact color. An MSP is also able to detect small variations of wavelengths and intensities between samples (12). Analysis via MSP is nondestructive and requires minimal sample preparation, thus making this technique highly amenable to implementation within the majority of trace evidence applications. This is particularly true with the examination of dyed hair, where it is not yet an established examination (2).
The protocol for hair examination follows a forensic taxonomy, whereby the questioned object is initially identified as a hair and described as either human or nonhuman. Upon the determination that the hair is human, further examination ensues to deduce the somatic origin, approximate ancestry, growth phase and nature of removal, tip condition, along with environmental, biological, thermal, or chemical modifications of the hair (1,13). Class characteristics are evaluated both macroscopically and microscopically. If substantial similarities are observed for individual microscopic hairs, then suitable exemplars are selected and compared directly with the questioned sample. The results of direct comparison may yield a dissimilar, similar, or inconclusive determination (1,13). A microscopic evaluation that indicates two samples are consistent with one another does not positively identify the source, as coincidental matches potentially exist (1). Appropriate hairs are selected for DNA analysis during the microscopic examination of the hair, when the identity of the source of the hair is vital (13). Some analysts bolster all positive associations between hairs from their morphology comparisons with mtDNA analysis.
In assessing the natural color of the hair, bleached or dyed hair may exhibit a distinct demarcation between the untreated and treated portions near the root. Based on the distance between the demarcation and the root of the hair, an estimated time interval between the treatment and loss of the hair can be estimated using the approximate growth rate of hair, 0.5 inches per month (14–16). Staining of the cuticle, uniform color distribution in the cortex, damage of the cuticle, separation of cortical cells, as well as atypical coloring are additional indicators of artificially colored or bleached hair (1,14).
The discriminating potential of UV-vis MSP, with respect to dyed hair samples, will be evaluated for consideration as a practical analytical technique for inclusion in the protocol for the examination of hair in forensic laboratories. Differentiation of class evidence using instrumental methods also lends itself to several advanced statistical techniques that add an element of objectivity to the comparison of spectral features. Hence, extensive statistical evaluations of the data including multivariate statistical analysis and validation studies will be presented in a subsequent article.
Materials and Methods
Hair Samples and Dye Products
This research was approved by IUPUI Clarian Institutional Review Board (IRB) for research, not subject to common rule or FDA definitions of human subject research. Natural hair specimens were donated by Aveda Fredric’s Institute and Honors Beauty College in Indianapolis, Indiana. Twenty-five suitable specimens, listed in Table 1, were collected and assigned alphanumeric identifiers according to the level designation of the natural hair color as well as the chronological order of collection. The array of natural hair colors ranged from level 2, dark brown, to level 8, light blonde, where a maximum of five samples from a single level designation were analyzed (6). The length of each of the hair specimens was at least 4 inches. The underlined sample, L602, was utilized for discriminating dyes on the same substrate. It was selected as the best candidate for testing as it was the closest to having an intermediate initial hair color, and a sufficient amount was available to conduct extensive research. Initially, 3 cm from the tip of the tress were discarded as unrepresentative (17).
| Level | Description | Sample ID |
|---|---|---|
| 2 | Dark brown | L201 |
| 3 | Medium brown | L301, L302, L303 |
| 4 | Light brown | L401, L402, L403 |
| 5 | Lightest brown | L501, L502, L503, L504 |
| 6 | Dark blonde | L601, L602, L603, L604 |
| 7 | Medium blonde | L701, L702, L703, L704, L705 |
| 8 | Light blonde | L801, L802, L803, L804, L805 |
Fourteen professional-grade red hair dyes are listed in Table 2A by the manufacturer. The selection of red-colored hair dyes was based on reported success of UV-vis MSP analysis of wool fibers, relative to those dyed black (10). Salon Care® Volume 20 Developer (Brentwood Beauty Labs International Inc., Dallas, TX) was used for all professional dyes to provide comparable lift for comparison purposes. This volume of developer was selected because it is most frequently used in salons. As a result of the elevated levels of hydrogen peroxide present in this formulation, each of the professional dyes may be classified as permanent. Alphanumeric identifiers were assigned to each of these red dyes based on the manufacturer, brand, and product code. The nominal description of each dye is also provided. Forty-one commercially available red hair dyes, listed in Table 2B by the manufacturer, were purchased from CVS, Kroger, and Walgreens. The naming nomenclature and tabular information are consistent with Table 2A. Semi-permanent dyes typically survive 6–12 washes, demi-permanent dyes last for approximately 28 washes, while permanent dyes will persist for the lifetime of the hair (5). It is common for permanent dyes to fade and for the natural hair color to become evident near the root.
| (A) | ||||||
|---|---|---|---|---|---|---|
| Product ID | Brand | # Code | Description | Base Colorant | Base:Developer | Developing Time (min) |
| Clairol Professional (Division of Procter & Gamble, Stamford, CT) | ||||||
| CPC6R | Complements | 6R | Dark red blonde | Cream | 1:1 | 15 |
| CPHIR6RR | Hi-intensity reds | 6RR | Dark reddest blonde | Cream | 1:1 | 30 |
| CPJ40 | Jazzing | 40 | Red hot | Gel | 1:1 | 30 |
| CPMC206RR | Miss Clairol-real reds | 206RR | Dark reddest blonde | Gel | 1:1 | 30 |
| CPR6R | Radiance-colorgloss | 6R | Dark red blonde | Gel | 1:1 | 15 |
| CPT660RV | Torrids | 660RV | Audacious red | Gel | 1:1 | 30 |
| Color Brilliance (Omaha, NE) | ||||||
| CBI7RR | Ion | 7RR | Intense red | Gel | 1:1 | 30 |
| L'Oréal Technique (Clark, NJ) | ||||||
| LCSPEH8 | Excellence- hicolor | H8 | Red fire | Cream | 2:3 | 30 |
| LCSPF36 | Féria | 6.66 | Double intensity auburn red | Gel | 1:1 | 25 |
| LCSPPMR6 | Preference-mega reds | MR6 | Dark intense auburn red | Gel | 1:1 | 25 |
| LTCG5.6 | Technique-color gems | 5.6 | Dark red | Cream | 1:1 | 20 |
| LTP7.43 | Technique-preference | 7.43 | Red penny | Gel | 1:1 | 25 |
| Wella (Division of Procter & Gamble, Stamford, CT) | ||||||
| WCC6RV | Color charm | 6RV | Fiery red | Cream | 1:2 | 20 |
| WCC810 | Color charm | 810 (7R) | Red-red | Cream | 1:2 | 30 |
| (B) | |||||||
|---|---|---|---|---|---|---|---|
| Product ID | Brand | # Code | Description | Classification | Base Colorant | Base:developer | Developing Time (min) |
| Clairol (Division of Procter & Gamble, Stamford, CT) | |||||||
| CB613 | Balsam | 613 | Dark auburn | Permanent | Cream | 1:1 | 25 |
| CHE35 | Herbal Essences | 35 | Auburn | Permanent | Gel | 1:1 | 25 |
| CHE44 | Herbal Essences | 44 | Deep red | Permanent | Gel | 1:1 | 25 |
| CHE48 | Herbal Essences | 48 | Radiant burgundy | Permanent | Gel | 1:1 | 25 |
| CHY32 | Hydrience | 32 | Dark red | Permanent | Cream | 1:1 | 25 |
| CHY33 | Hydrience | 33 | Dark auburn | Permanent | Cream | 1:1 | 25 |
| CHY34 | Hydrience | 34 | Burgundy | Permanent | Cream | 1:1 | 25 |
| CLC80 | Loving care | 80 | Auburn | Semi-Permanent | Cream | All-in-one | 20 |
| CNI16 | Natural Instincts | 16 | Light auburn | Demi-Permanent | Gel | 1:1 | 10 |
| CNI22 | Natural Instincts | 22 | Medium auburn brown | Demi-Permanent | Gel | 1:1 | 10 |
| CNI30 | Natural Instincts | 30 | Dark auburn brown | Demi-Permanent | Gel | 1:1 | 10 |
| CNNE108 | Nice ‘n Easy | 108 | Natural reddish blonde | Permanent | Gel | 1:1 | 25 |
| CNNE110 | Nice ‘n Easy | 110 | Natural light auburn | Permanent | Gel | 1:1 | 25 |
| CNNE112 | Nice ‘n Easy | 112 | Natural dark auburn | Permanent | Gel | 1:1 | 25 |
| Garnier (L'Oréal, Clark, NJ) | |||||||
| G100562 | 100% color | 562 | Bright auburn brown | Permanent | Gel-cream | 2:3 | 30 |
| G100660 | 100% color | 660 | Intense auburn | Permanent | Gel-cream | 2:3 | 30 |
| G100764 | 100% color | 764 | Bright auburn blonde | Permanent | Gel-cream | 2:3 | 30 |
| GN42 | Nutrisse | 42 | Deep burgundy | Permanent | Cream | 2:3 | 25 |
| GN56 | Nutrisse | 56 | Medium reddish brown | Permanent | Cream | 2:3 | 25 |
| GN66 | Nutrisse | 66 | True red | Permanent | Cream | 2:3 | 25 |
| GN69 | Nutrisse | 69 | Intense auburn | Permanent | Cream | 2:3 | 25 |
| GN76 | Nutrisse | 76 | Rich auburn blonde | Permanent | Cream | 2:3 | 25 |
| GN535 | Nutrisse | 535 | Medium golden mahogany brown | Permanent | Cream | 2:3 | 25 |
| GN452 | Nutrisse | 452 | Dark reddish brown | Permanent | Cream | 2:3 | 25 |
| L'Oréal (Clark, NJ) | |||||||
| LF36 | Féria | 36 | Deep burgundy brown | Permanent | Gel | 1:1 | 25 |
| LF41 | Féria | 41 | Rich mahogany | Permanent | Gel | 1:1 | 25 |
| LF56 | Féria | 56 | Auburn brown | Permanent | Gel | 1:1 | 25 |
| LF66 | Féria | 66 | Very rich auburn | Permanent | Gel | 1:1 | 25 |
| LF67 | Féria | 67 | Rich auburn | Permanent | Gel | 1:1 | 25 |
| LF74 | Féria | 74 | Deep copper | Permanent | Gel | 1:1 | 25 |
| LF77 | Féria | 77 | Bright auburn | Permanent | Gel | 1:1 | 25 |
| LP4R | Preference | 4R | Dark auburn | Permanent | Gel | 1:1 | 25 |
| LP5MB | Preference | 5MB | Medium auburn | Permanent | Gel | 1:1 | 25 |
| LP6R | Preference | 6R | Light auburn | Permanent | Gel | 1:1 | 25 |
| LPRR04 | Preference | RR04 | Intense dark red | Permanent | Gel | 1:1 | 25 |
| LPRR07 | Preference | RR07 | Intense red copper | Permanent | Gel | 1:1 | 25 |
| Revlon (Oxford, NC) | |||||||
| RCS42 | Colorsilk | 42 | Medium auburn (4R) | Permanent | Gel | 1:1 | 25 |
| RCS45 | Colorsilk | 45 | Bright auburn (4BR) | Permanent | Gel | 1:1 | 25 |
| RCS48 | Colorsilk | 48 | Burgundy (4B) | Permanent | Gel | 1:1 | 25 |
| RCS49 | Colorsilk | 49 | Auburn brown (4RR) | Permanent | Gel | 1:1 | 25 |
| RCS72 | Colorsilk | 72 | Strawberry blonde (7R) | Permanent | Gel | 1:1 | 25 |
To enhance the interpretation of the spectral data, it is important to have a fundamental knowledge of the chemical composition of the dyes analyzed. The composition of the professional hair dyes from Table 2A is summarized in Table 3A and the composition of the commercial hair dyes from Table 2B is summarized in Table 3B. Five common precursors were accounted for in the aforementioned composition summaries, including p-phenylenediamine (PPD), toluene-2,5-diamine (T25DA), N,N-Bis(2-hydroxyethyl)-p-phenylenediamine (N,N Bis 2HEPPD), o-aminophenol (OAP), and p-aminophenol (PAP). Although comparable chemical mechanisms transpire for these primary intermediates, PAP derivatives tend to have less intense shades than those of PPD (5–7). Nitro derivatives of PPD found in some professional hair dyes serve to brighten the intensity. Direct dyes, which are not activated by separate components and typically only stain the cuticle, are sometimes included in formulations for acquiring the desired final shade. Six frequently observed dye couplers were tracked in the previously described composition summaries, including resorcinol (R), 1-naphthol (1N), 4-amino-2-hydroxytoluene (4A2HT), 2-methylresorcinol (2MR), m-aminophenol (MAP), and 2-methyl-5-hydroxyethylaminophenol (2M5HEAP). For those products with a traditional listing of components from greatest abundance to least, the corresponding order is depicted numerically. Relative positions with respect to the overall formulation are also included. Other products are listed either alphabetically or are ambiguous in defining the presence of specific components; consequently, checkmarks were utilized for these entries.
| (A) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Intermediates | Couplers | Color Retarder | Other | ||||||||||||
| PPD | T25DA | N,N Bis 2HEPPD | OAP | PAP | R | 1N | 4A2HT | 2MR | MAP | 2M5HEAP | PhMPyr | PMAP | Nitro | Direct | |
| CPC6R | |||||||||||||||
| CPHIR6RR | 3 | 2 | 1 | √ | |||||||||||
| CPJ40 | √ | ||||||||||||||
| CPMC206RR | 1 | 7 | 5 | 4 | 8 | 2 | 6 | 3 | √ | ||||||
| CPR6R (alphabetical, all or some) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| CPT660RO | 5 | 1 | 6 | 4 | 2 | 3 | 7 | 8 | √ | ||||||
| CBI7RR | |||||||||||||||
| LCSPEH8 (alphabetical) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| LCSPF36 (alphabetical) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| LCSPPMR6 (may contain) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| LTCG5.6 | 3 | 2 | 5 | 1 | 6 | 4 | |||||||||
| LTP7.43 (may contain) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| WCC6RV | 2 | 3 | 1 | √ | |||||||||||
| WCC810 (one or more) | √ (definite) | √ | √ | √ | √ | √ | √ | ||||||||
| (B) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Intermediates | Couplers | Color Retarder | Comments | |||||||||||
| PPD | T25DA | N,N Bis 2HEPPD | OAP | PAP | R | 1N | 4A2HT | 2MR | MAP | 2M5HEAP | PhMPyr | PMAP | ||
| CB613 | 2 | 4 | 1 | 6 | 3 | 5 | – | |||||||
| CHE35 | 4 | 6 | 1 | 3 | 5 | 2 | √ | – | ||||||
| CHE44 | 4 | 2 | 3 | 1 | 1 > >2,3,4 | |||||||||
| CHE48 | 2 | 1 | Early | |||||||||||
| CHY32 | 2 | 3 | 1 | – | ||||||||||
| CHY33 | 2 | 4 | 1 | 6 | 3 | 5 | – | |||||||
| CHY34 | 2 | 4 | 3 | 1 | – | |||||||||
| CLC80 | Direct | |||||||||||||
| CNI16 | 4 | 2 | 3 | 6 | 1 | 5 | 1,2 > >3,4,5,6 | |||||||
| CNI22 | 2 | 5 | 3 | 4 | 1 | 1,2,3 > >4,5 | ||||||||
| CNI30 | 3 | 6 | 1 | 4 | 5 | 2 | 1,2,3,4 > >5,6 | |||||||
| CNNE108 | 4 | 5 | 2 | 3 | 1 | √ | Late | |||||||
| CNNE110 | 4 | 6 | 1 | 3 | 5 | 2 | √ | Late | ||||||
| CNNE112 | 2 | 5 | 1 | 4 | 3 | 6 | 7 | √ | Late | |||||
| G100562 | 2 | 3 | 5 | 1 | 4 | Spans range | ||||||||
| G100660 | 2 | 3 | 5 | 1 | 4 | 1,2,3 > >4,5 | ||||||||
| G100764 | 3 | 1 | 2 | 1 > 2>3 | ||||||||||
| GN42 | 2 | 3 | 1 | 1 > 2>3 | ||||||||||
| GN56 | 2 | 3 | 5 | 4 | 1 | 1,2,3,4 > 5 | ||||||||
| GN66 | 3 | 4 | 5 | 1 | 2 | 1,2 > 3,4 > 5 | ||||||||
| GN69 | 3 | 1 | 2 | 1 > 2>3 | ||||||||||
| GN76 | 3 | 1 | 2 | 1 > 2>3 | ||||||||||
| GN452 | 1 | 3 | 4 | 2 | 5 | Central | ||||||||
| GN535 | 2 | 1 | 6 | 3 | 4 | 5 | √ | 1 > 2,3 > 4>5 > 6 | ||||||
| LF36 | 2 | 5 | 4 | 3 | 1 | √ | 1,2 > 3>4 > 5 | |||||||
| LF41 | 1 | 2 | 3 | √ | 1 > 2,3 | |||||||||
| LF56 | 3 | 4 | 5 | 2 | 1 | √ | √ | 1,2,3 > 4,5 | ||||||
| LF66 | 3 | 2 | 5 | 1 | 4 | √ | 1 > 2,3 > 4,5 | |||||||
| LF67 | 2 | 3 | 5 | 1 | 4 | √ | 1 > 2,3,4,5 | |||||||
| LF74 | 1 | 4 | 3 | 2 | √ | 1,2 > 3,4 | ||||||||
| LF77 | 2 | 3 | 5 | 1 | 4 | √ | 1 > 2,3 > 4,5 | |||||||
| LP4R | 2 | 1 | 5 | 4 | 6 | 3 | √ | 1 > 2,3,4 > 5,6 | ||||||
| LP5MB | 1 | 2 | 5 | 4 | 6 | 3 | √ | 1,2 > 3,4,5,6 | ||||||
| LP6R | 4 | 1 | 6 | 3 | 5 | 2 | √ | 1,2,3 > 4,5 | ||||||
| LPRR04 | 3 | 4 | 2 | 1 | √ | √ | – | |||||||
| LPRR07 | 4 | 3 | 5 | 2 | 1 | √ | 1 > 2>3 > 4>5 | |||||||
| RCS42 | 1 | 3 | 4 | 5 | 2 | √ | Late | |||||||
| RCS45 | 1 | 3 | 4 | 2 | 5 | √ | Late | |||||||
| RCS48 | 1 | 2 | 3 | √ | Late | |||||||||
| RCS49 | 1 | 2 | 3 | 4 | 5 | Late | ||||||||
| RCS72 | 1 | 2 | 3 | 4 | √ | Late | ||||||||
- PPD, p-phenylenediamine; T25DA, toluene-2,5-diamine; N,N Bis 2HEPPD, N,N-Bis(2-hydroxyethyl)-p-phenylenediamine; OAP, o-aminophenol; PAP, p-aminophenol; R, resorcinol; 1N, 1-naphthol; 4A2HT, 4-amino-2-hydroxytoluene; 2MR, 2-methylresorcinol; MAP, m-aminophenol; 2M5HEAP, 2-methyl-5-hydroxyethylaminophenol; PhMPyr, phenyl methyl pyrazolone; PMAP, p-methylaminophenol.
Washing and Dyeing Procedure
Standardized bundles were assembled with lengths of 6 ± 1 cm (8,17) and a target mass of 0.50 ± 0.05 g (17). The hair was then washed with a shampoo solution consisting of 0.25 ± 0.05 g of CVS Baby Shampoo® in 500 mL of de-ionized water. Each bundle was then rinsed by immersing it in a series of four de-ionized water stations. The hair was then dried using a hair dryer. Washed sample bundles were stored independently in appropriately labeled and sized evidence bags. After the bundles of hair were washed and rinsed, each was submitted to the dyeing process. The dyes were prepared as per the manufacturer’s instructions, where the ratio of base to developer along with corresponding developing times are provided in Table 2 as well as the state of the base colorant. Manufacturer’s instructions call for the hair to be saturated with the activated dye. The appropriate volumes of each base and developer were tabulated, where the total volume of combined activated dye is 10 mL, with the exception of the Wella professional dyes, where the total amount of dye was 9 mL. Each sample was activated by administering the base from the syringe into the glass vial with corresponding developer. The vial was capped and agitated for 30 sec to appropriately combine the colorant and activator. The hair sample was then introduced to the dye mixture and completely immersed. The vial was capped and set aside for the designated developing time. This process was repeated for each of the samples. After the samples were dyed, the samples were submitted to a series of rinsing stations consisting of warm tap water. Following the initial stages of rinsing, both the water and dyed hair samples were visually examined for persistent residual dye. The samples were continually rinsed as needed. Rinsing stations were disposed of and regenerated using clean, warm tap water for each subsequent sample in the series. The hair was then dried and stored as per the procedure described earlier.
Instrumental Methods
A CRAIC QDI 2000© microspectrophotometer was used to obtain absorbance spectra for each of the samples. The instrument was allowed to equilibrate for a minimum of 30 min prior to performing Köhler illumination, wavelength calibration, and photometric calibration. A standard ink slide was utilized to set the instrumental optics, while NIST traceable standards were utilized for the wavelength and photometric calibrations.
Five dyed hairs were randomly selected from the bundle for further analysis (18). Hair-by-hair analysis was investigated as an attempt to reduce variation observed from different hairs on the same individual. Each hair was mounted on a tri-holed metal slide with tape and pulled taut with tweezers. After focusing the microscope on the hair sample, the aperture was moved off of the sample, and the instrument was automatically optimized for integration time. A dark scan and a reference scan were then taken. Next, the aperture was placed in the center of the width of the dyed hair sample, avoiding the medulla regions. In total, five scans from each of five hairs representing a particular sample were analyzed. The experimental parameters listed in Table 4 were used to obtain all absorbance spectra.
| Parameter | Description |
|---|---|
| Wavelength range | 200–700 nm |
| Overall magnification | 150× |
| Number of scans | 25 total per sample (5 scans × 5 hairs) |
| Scan average | 10 |
| Sampling time | ≈100–300 ms (optimized for each hair individually) |
| Resolution factor | 2 |
Data Analysis
The spectra files were imported into Microsoft Excel®, where the absorbance values observed at each wavelength were subjected to a pretreatment procedure consisting of baseline correction, normalization, and average tabulation. The baseline was corrected by subtracting the average absorbance observed over the first 10 nm of each spectrum from each absorbance value. The region from 200–210 nm is a universally flat region for the spectra in this data set and was ideal for a reference baseline. A “sum of squares” approach was taken for the normalization of the baseline corrected data. The summation of the squares of all peak areas was calculated, and the square root of this sum was then applied. The normalized value was derived as the quotient of each peak area divided by the square root of the sum of squares (19). Finally, the average of all 25 scans was calculated to represent each dye sample, while the average of five scans was also tabulated to represent each hair individually. Unless otherwise noted, all demonstrative spectra are baseline corrected, normalized, and depict the average of 25 scans.
Effect of Substrate
A study was performed to evaluate the dependence of the spectra on the initial substrate, evaluated against the discriminating ability of the technique for different dyes on the same individual’s hair. CHE44 and RCS42 dyes were selected for analysis on all 25 hair samples. Unique hair samples were systematically bundled, washed, dried, and dyed as per the manufacturer’s instructions of the two aforementioned dyes.
Extraction Procedure—Effect of Background Reference
The selection of an appropriate background reference is vital when collecting absorbance spectra. Each of the hairs selected for this study were cut in half. Half of the hair was preserved for additional analysis by taping the ends on a labeled index card and sealing the sample in an evidence bag. The other half of the dyed hair was submitted to an extraction procedure. The extraction procedure involved inserting the selected half of the hair into the tip of a Pasteur pipet, then annealing the tip with a Bunsen burner. After the pipet was cooled, the pipet was then filled with 1:1 pyridine:distilled water, placed in a beaker of water and heated in an oven at 70°C for 30 min (11). The extracted hair was vented overnight, then removed and stored with the original hair sample on a labeled index card within a sample bag. The resulting individual hairs were submitted to instrumental testing. Extracted and dyed hair samples were mounted side-by-side on the metal plate, and the extracted hair sample was scanned as the reference.
An investigation of mounting media was also performed where five hairs dyed with CHE48 were placed on a quartz slide. On the leftmost side of the slide, Permount® mounting media was administered to the hair samples, leaving an empty space in the center of the slide, and glycerin was applied to the other side of the slide. Two quartz cover slips were placed side-by-side over the samples. Each type of medium served as the background reference for the three aforementioned sampling regions along the slide. Five scans were collected for each of the five mounted hairs; however, the scans were in a closer proximity to one another, relative to the single hair evaluated for the comprehensive dye set.
Time Study
To illustrate the temporal stability of the dyed hair samples, a longitudinal study was conducted. Five hairs from the undyed L602 sample were selected as a reference, while dyed samples of L602 (using LCSPF36, LF36, and LP6R) were selected as the representatives of the various tones of dye. These samples were freshly prepared by bundling, washing, drying, and dyeing protocols described earlier. The hairs from this study were individually taped to a labeled index card and exposed to artificial light and air. Each sample was analyzed every week over 5 weeks.
Fading of Dyes with Successive Washing
A wash study was conducted to monitor the stability of the samples after repeated washing and drying applications. Samples of L602 that were undyed and samples of L602 dyed with LCSPF36, LF36, and LP6R were also selected for this study, with the same logic as described for the time study. New representative samples were created using the preparative protocol previously described. Each of the bundles was submitted to a wash protocol involving three stages. The washing station was comprised of a shampoo solution consisting of 0.25 ± 0.05 g of CVS Baby Shampoo® in 500 mL of warm tap water. The dyed hair bundle was moved back and forth in this shampoo solution for 1 min and allowed to soak for 4 min in another lukewarm water station. Finally, the bundle was rinsed until the sample was visually free of residual shampoo. The bundles of hair were dried for 3 min using the drying procedure described earlier. Five random hairs were removed initially and after wash cycle 1, 2, 3, 4, 5, 6, 7, 14, 21, 28, 35, and 42. This procedure was intended to simulate the effects of daily washing for the first week and weekly for 6 weeks. After all of the samples had been collected, approximately 5% of the total mass of the bundle was submitted for analysis.
Results and Discussion
The Effect of Original Hair Color
The original substrate is known to contribute to the overall color achieved for a dyed hair; thus, it is important to understand how this dependence affects the classification process (8). Two commercial dyes representing the breadth of the hair dyes investigated in the compilation set were utilized for empirically describing this phenomenon. Both RCS42 and CHE44 dyes were applied to each of the 25 natural hair samples. Normalized spectra representing 25 scans collected for both substrate series were reviewed in conjunction with the macroscopic visual comparison. Three undyed substrates representing the range of natural tones, including L303, L602, and L802 are compared to the same samples dyed with RCS42 and CHE44 dyes in Fig. 1A–C. As predicted, the substrate influence was more substantial for RCS42, which contributes the smallest amount of dye absorbance to the spectrum. For the CHE44 trial, the lighter hair samples produced vibrant shades with well-defined dye absorbance peaks versus the shouldering observed for the majority of the darker substrates. The reduction of the variability between hairs exhibiting different pigmentation is one of the desirable characteristics of dye products, which draw consumers to this cosmetic application, primarily to conceal or blend gray hair. Both data sets did still exhibit some variability as a function of substrate, with higher variability observed within the RCS42 data set, as depicted in Fig. 2. However, the contribution of the dye to the spectra reduces the intrasample variability originating from the natural substrate palette, which is inherently problematic for spectral analysis of undyed hair samples (20,21).
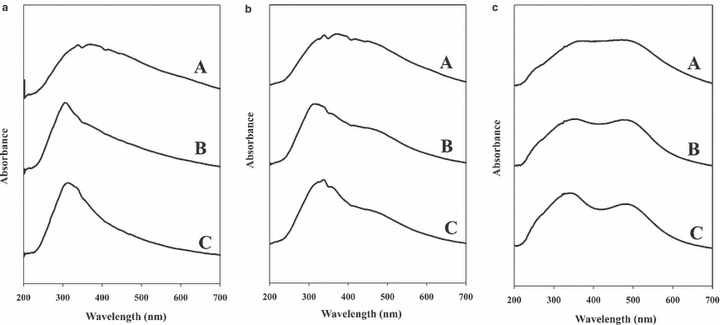
—(a) UV-vis microspectrophotometry (MSP) spectra of undyed hair samples spanning the range of natural tones of hair color. (A) L303—Medium Brown, (B) L602—Dark Blonde, and (C) L802—Light Blonde. (b) UV-vis MSP spectra of hair samples dyed with RCS42. (A) L303, (B) L602, and (C) L802. (c) UV-vis MSP spectra of hair samples dyed with CHE44. (A) L303, (B) L602, and (C) L802.
—Intra- versus intersample variability exhibited for L602 hairs dyed with RCS42 (left) and CHE44 (right). UV-vis microspectrophotometry spectra for each of the five hairs analyzed are presented for each representative dye.
Discrimination of Dyed Hair Color
Spectra for all 55 red hair dyes on the L602 substrate were collected and reviewed. The contribution of the hair substrate is predominantly observed in the range of 300–400 nm, with a maximum near 330 nm; while the dye peak is evident in the range of 425–550 nm. Differences in tones were readily apparent upon visual assessment of the macroscopic dye bundles and spectral analysis. To aid in the visualization of both the natural substrate, L602, and an array of dyed hair samples, three dyes from the same brand and manufacturer, Revlon Colorsilk®, are exhibited in Fig. 3. The three dyes selected represent different tones of dyes, as RCS42 possesses the least red character as opposed to RCS49 with the most. One advantage of including dyes from the same brand and manufacturer is that the formulations and application procedures are comparable. Both RCS48 and RCS49 more closely resemble one another; however, the dye region relative to the hair peak is larger for RCS49, thus indicating a more vibrant red tone.
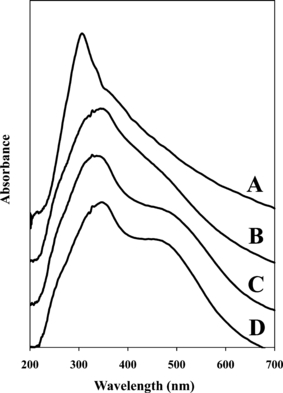
—UV-vis microspectrophotometry spectra representing multiple shades of dyes from the same brand and manufacturer relative to the natural substrate. (A) L602 undyed hair sample and (B) RCS42, (C) RCS48, (D) RCS49 dyed hair samples.
Overall, a large gradient of spectra and colors was observed for the comprehensive set of red hair dyes, and deducing trends between visual and spectral appearance is relatively straightforward. However, correlating MSP data with the dye formulation is not possible because of the lack of molecular information present in a UV-visible spectrum and the number of factors contributing to the final shade achieved.
Effect of Background Reference
The UV-vis MSP spectrum of dyed hair includes a substantial contribution of the hair substrate relative to that of the dye, and in some cases the substrate can dominate the spectrum. This portion of the study explored substituting the air reference background with hair substrate that had been extracted to remove the dye. This approach would ideally substantially reduce or eliminate the contribution of the hair substrate to the final spectrum, thereby targeting the color of the hair dye. UV-vis MSP spectra corresponding to the same RCS42 sample dyed on L602 hair are illustrated in Fig. 4. The spectrum collected with air as the background has a much higher absorbance relative to the sample collected with the extracted hair as a reference. For the spectra, the peaks of the hair and dye peaks generally fall in the ranges of 300–400 and 425–550 nm, respectively. The wavelength of maximum absorbance for the hair is approximately 330 nm. The extraction procedure was specifically investigated for the analysis of hairs without roots or an evident line of demarcation. Extraction would not be necessary if even a minimal region of the natural hair was available for reference. The spectrum of the dyed hair with the extracted hair as a reference has a much lower signal and higher propensity for noise relative to the spectrum with air as the reference. The shouldering observed on the front of the dye peak indicates that this method does not completely negate the contribution of the hair, although the absorption of the dye is effectively highlighted with this adjusted reference. This approach was explored further with 14 professional hair dyes applied to L602 hair and the commercial dye, RCS42, to all 25 natural hair samples. However, results were not satisfactory and all subsequent analysis was performed with air as the reference, as extract reference data were deemed unreliable.
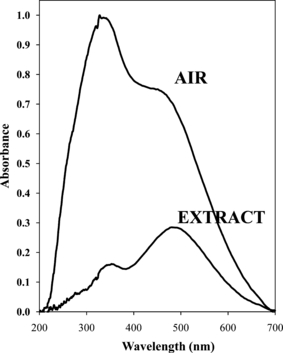
—Baseline corrected (but not normalized) UV-vis microspectrophotometry spectra representing air as a background reference relative to the extracted portion of the L602 hair dyed with LCSPPMR6.
Microscopic analysis of hair samples commonly involves mounting the exemplar hairs on microscope slides for enhanced visibility (1). Two common types of mounting media commonly used for this application include glycerin and Permount®. The analysis of dyed hairs would be performed following microscopic evaluation, where only the significant samples would be analyzed with this technique; thus, it is important to consider the optimum conditions for simultaneous evaluation. For the analysis of samples within the UV region, samples must be mounted on quartz slides, as glass is known to absorb in the UV region. A preliminary analysis of the initial air technique with the tri-holed metal plate compared to glycerin and Permount® media with quartz slides was conducted. The average UV-vis MSP spectra are illustrated in Fig. 5. The quartz slide control did not exhibit peak shifting, thus verifying that spectral differences may be attributed to the mounting media. The spectrum with glycerin mounting medium is very similar to that of the CHE48 spectrum collected with air. In contrast, the Permount® media absorbs in the UV region up to approximately 350 nm, thus masking the sample absorbance in this region. Consequently, the analysis of dyed hair is not appropriate for samples mounted with Permount®. These results indicate, however, that this application would be feasible with the use of quartz microscope slides and glycerin mounting media. Further experimentation would need to be performed to evaluate the effects on the discrimination of hair dyes with UV-vis MSP using glycerin among other potential mounting media; additional media should be considered as well that will not degrade the sample, nor absorb in the UV region, and will have an approximate refractive index between 1.5 and 1.6 (1,13).
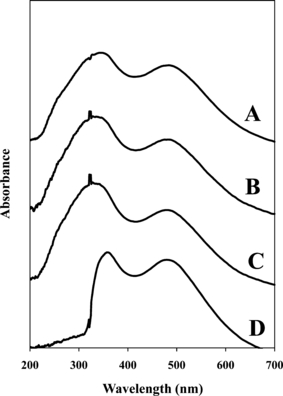
—UV-vis microspectrophotometry spectra representing the effect of mounting media used as background references relative to air and quartz slide control samples: (A) air, (B) quartz microscope slide, as well as (C) glycerin and (D) Permount® mounting media.
Time Study
Understanding the stability of samples over time is an important factor to take into consideration. In many cases, hair evidence may be collected and then stored for long periods prior to comparison with known exemplars from a suspect. Hence, this study was conducted to empirically demonstrate that MSP data is constant with time in a laboratory setting. The spectra were nearly identical throughout the first 5 weeks; thus, the study was halted and the samples were considered stable in the presence of artificial light and air. Longer stability studies spanning months or years may provide additional clarity on the longevity of this type of sample. Other environmental conditions, including exposure to ultraviolet light and humidity may be interesting to explore in both a controlled laboratory setting, and an all-encompassing longitudinal study with individual volunteers.
Fading of Dyes with Successive Washing
It is well established that hair dyes are known to fade over time; however, for the purposes of this application, it is critical to have a better gauge on the extent and rate of color loss. Although casework samples are collected as expeditiously as possible, varying intervals of time between the collection of a questioned sample at the scene of a crime and collection of known source specimens is inevitable (13). Although many environmental conditions contribute to fading of hair dyes, this study specifically examines the effect of successive washing on hair. With the approximation that individuals wash their hair daily, this study is intended to model the behavior of different hair dyes on hair over the course of 6 weeks, where the manufacturers recommend reapplication between 4 and 6 weeks.
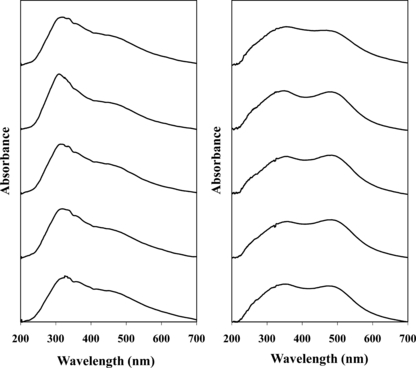
Average spectra representing the initial samples and samples simulating weekly collection are presented in Fig. 6A–D. For the L602 negative control, the spectra were only intended to represent the intrasample variation present within the natural substrate. The spectra exhibit the variability in the UV region, representing the natural variability of undyed hair, which has been discussed previously. While these inconsistencies are also apparent in the dyed hair samples, the presence of a dye tends to reduce this intrasample variation. As for the LCSPF36 spectra, little change is observed for the washing associated with the first week. A noticeable decline in the absorbance of the red dye is observed between the second and third weeks. By the end of the sixth week of washing, the contribution of the dye to the spectrum is essentially nullified. The LF36 series also suggests minimal color loss within the first week, accompanied by a substantial loss of color between the third and fourth weeks. In contrast to the LCSPF36 sample, however, absorbance within the dye region remains apparent through the end of the sixth week. Finally, the spectra from week one of the LP6R trial are relatively consistent in the dye region. Fading associated with similar tones of dyes may be more difficult to recognize, as some dyed hair samples closely resemble the natural color of the L602 hair. By the end of the second week, the presence of the dye peak is slightly weaker than initially observed; however, the extent of the color loss is difficult to pinpoint as a much smaller difference is possible with this particular class of dyes. These spectral observations correlate with the visual observations accrued during the washing process. Gradual fading was apparent visually; however, only macroscopically evident following 20–30 washes. Visual inspection of the bundles submitted to all washes indicated that the final tone of the LFCSPF36 sample was considerably closer to the natural L602 control hair sample, relative to the freshly dyed sample; whereas the final LF36 sample remained considerably darker than the control.
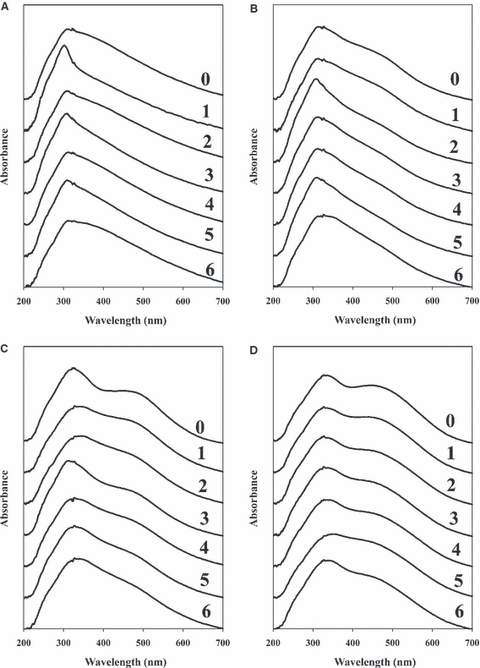
—(A) UV-vis microspectrophotometry (MSP) spectra for the L602 control sample of the wash study. (0) initial sample and (1–6) simulated number of weeks elapsed based on daily washing. (B) UV-vis MSP spectra for the LP6R dyed sample of the wash study. (0) initial sample and (1–6) simulated number of weeks elapsed based on daily washing. (C) UV-vis MSP spectra for the LCSPF36 dyed sample of the wash study. (0) initial sample and (1–6) simulated number of weeks elapsed based on daily washing. (D) UV-vis MSP spectra for the LF36 dyed sample of the wash study. (0) initial sample and (1–6) simulated number of weeks elapsed based on daily washing.
Based on this study, reasonably reliable sample classifications are achieved only within the first 3 weeks following the initial application of the dye. Chemically, red hair dyes are associated with reduced longevity, as the coupled dye molecules tend to be smaller relative to other colors. As these molecules are assembled and retained within the cuticle, smaller molecules are more susceptible to removal by interaction with anionic shampoos and humidity (22). It is hypothesized that brown or black hair dyes would be retained for greater lengths of time relative to the red hair dyes examined in this study. These results are preliminary and intended to provide a foundation for future longitudinal experimentation, as well as an empirical basis for a recommended interval of time associated with the utility of this method.
Conclusions
Given the ability of MSP to discriminate subtle differences in hue, it was found that the original hair color had a large effect on the overall spectra. However, the presence of the dye peak in dyed hair samples reduced intrasample variability and provided another spectral feature for differentiating samples.
Subsequent analysis of a comprehensive set of 55 red hair dyes showed that visual inspection and spectral interpretation allow for discrimination of dyed hair samples, as distinct and discernable shades were observed. Spectral comparisons of replicate hair samples representing each dye indicate that outlying samples can occur. Consequently, consistent with protocols for natural and synthetic fibers, it is recommended that a set of spectra from individual hair samples be evaluated as opposed to a global average for a particular dye.
An attempt to selectively analyze the contribution of the dye to eliminate intraindividual variability was conducted by utilizing an extracted hair as a background reference. This technique was unsuccessful as peak shifting and elevated noise were apparent. Glycerin and Permount® were also evaluated as mounting media for the analysis of dyed hair on quartz slides. Permount® media is not compatible with this technique, but glycerin produced comparable results with those obtained with air as a reference.
To provide insight into the interval of time acceptable for this method, the sample was exposed to artificial light and air for 5 weeks and independently washed successively for 42 times. The spectra of hair samples exposed to normal laboratory conditions were stable for the entire span of the temporal stability study. Significant fading associated with successive washing was observed after 21 washings (simulated 3 weeks).
Contingent on the determination that an exemplar hair is dyed and the selection of an appropriate mounting medium, it is proposed that UV-vis MSP could be used following comparison microscopy but prior to DNA analysis, as depicted in Fig. 7. Overall, the results obtained from preliminary spectral analysis of UV-vis MSP data of red hair dyes supports future research focused on validating the method and optimizing it for implementation in routine analysis of dyed hair samples in forensic crime laboratories.

—Proposed implementation of UV-vis MSP analysis of dyed hairs.
Acknowledgments
Without the generosity of the following organizations, this research would not have been possible. Hair samples were donated by Aveda Fredric’s Institute and Honors Beauty College in Indianapolis, Indiana.




