Characteristic Neuroimaging in Patients with Tumefactive Demyelinating Lesions Exceeding 30 mm
J Neuroimaging 2011;21:e69-e77.
Abstract
ABSTRACT
BACKGROUND AND PURPOSE
Features of tumefactive demyelinating lesion (TDL) on magnetic resonance imaging (MRI) can facilitate the differential diagnosis of TDL and neoplastic lesions, but vary considerably among patients. The larger TDL grows, the more difficult it becomes to differentiate TDL from neoplastic lesions. The purpose of this study was to elucidate typical MRI features in 12 patients with large TDL (>30 mm in diameter).
METHODS
We identified 12 patients with large TDL (six men, six women; age range 17-64 years, median age 27 years) and studied the clinical histories and the results of laboratory and various radiological studies in these patients. All cases of clinically definite multiple sclerosis were diagnosed in accordance with McDonald's revised criteria.
RESULTS
Common MRI features of large TDLs included variable degrees of mass effect (71%) and edema (100%), a T2 hypointense rim (79%), venular enhancement (57%), and peripheral restriction on diffusion-weighted images (50%). Ring enhancement (38%), open-ring enhancement (31%), or decreased N-acetylaspartate ratios on magnetic resonance spectroscopy (22%) were less frequently observed. Brain angiography demonstrated venous dilatations on and around the TDL.
CONCLUSIONS
The diagnosis of large TDL is challenging. Our findings suggest that multiple venous dilatations on and around TDLs on angiography can facilitate diagnosis.
Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system (CNS), characterized by persistent demyelination, inflammation, and axonal injury with recurrent attacks. MS is commonly associated with multiple small ovoid lesions lacking a mass effect. Such lesions are usually localized and periventricular, oriented perpendicularly to the lateral ventricle. MS rarely occurs as a large lesion, referred to as a tumefactive demyelinating lesion (TDL),1 which can be misdiagnosed as a neoplastic lesion. The diagnosis of TDL is thus challenging and often requires a brain biopsy. On magnetic resonance imaging (MRI), TDLs are characterized by large white-matter lesions with variable degrees of mass effect or edema, absence of cortical involvement, complete or open-ring enhancement, vessel-like structures running through the lesion center, a T2 hypointense rim, and peripheral restriction around the lesion on diffusion-weighted images (DWI).2–6 Magnetic resonance spectroscopy (MRS) generally shows a decreased N-acetylaspartate (NAA)/creatine (Cr) ratio, an increased choline (Cho)/Cr ratio, and the presence of glutamate/glutamine or lactate peaks.3,5,7,8 These MRI features are useful for differentiating TDL from neoplastic lesions, but their prevalence is quite variable; enhancement patterns on imaging studies are especially diverse.2 The larger TDL grows, the more difficult it becomes to differentiate large TDL from neoplastic lesions. The purpose of this study was to elucidate typical MRI features in 12 patients with large TDL.
Materials and Methods
TDL was defined as a circumscribed hyperintense lesion 30 mm or more in diameter on fluid-attenuated inversion recovery (FLAIR) and T2-weighted images (T2W), associated with neurological signs and symptoms. A diameter of 30 mm or greater was used to define TDL because the larger a brain lesion grows, the more difficult it becomes to differentially diagnose and treat. This value was reached by consensus among the authors. There are no universally accepted criteria defining the size of lesions able to be treated surgically. In Japan, lesions less than 30 mm in diameter are considered candidates for noninvasive gamma-knife radiotherapy. MS lesions were required to be more than 3 mm in maximum diameter. All cases of clinically definite MS were diagnosed in accordance with McDonald's revised criteria.9 Clinical information was obtained from detailed medical records. Exclusion criteria included metabolic etiology, vascular disorders, CNS infections, and systemic immunological disorders, as confirmed by serological and radiological investigations and cerebrospinal fluid (CSF) cultures. Patients strongly suspected to have viral encephalitis, such as those in whom MRI abnormalities were restricted to the limbic system, were also excluded. We studied the clinical histories and the results of laboratory and radiological studies in patients with TDL.
Clinical Data
Clinical features and outcomes were recorded in detail, including sex, age at onset, neurologic symptoms at presentation, treatment regimens, and functional outcomes, assessed according to the Expanded Disability Status Scale (EDSS).10 The clinical course was classified as monophasic, relapsing-remitting, secondary progressive, and primary progressive.
Biological Analysis
Laboratory analysis included routine serum examinations, routine CSF examinations, and oligoclonal band analysis in all 12 patients, and IgG index in 10. Aquaporin-4 antibodies and visual evoked potentials were evaluated in 6 patients.
MRI Imaging
Patients were examined with a 1.5-T magnetic resonance imager (Magnetom Sonata A.G., Siemens, Erlangen, Germany). Because some patients were studied retrospectively, various techniques were used for imaging, but the scan slice thickness and interslice gap were similar in all patients. In 10 patients with TDL, conventional imaging techniques were used as follows: turbo spin-echo sequences for T2W(TR 4,400 ms; TE 110 ms; 5-mm slice thickness, with a 1-mm interslice gap), FLAIR images (TR 9,000 ms; TE 120 ms; TI 2,200 ms; 5-mm slice thickness, with a 1-mm interslice gap), and T1-weighted images (T1W) (TR 450 ms; TE 9 ms; 5-mm slice thickness, with a 1-mm interslice gap). Contrast T1W was obtained with the use of a bolus of gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA 0.1 mmol/kg). DWI and MRS were performed in 12 and 9 patients with TDL, respectively. DWIs (TR/TE = 3,400/96, b = 1,000 sec/mm2, matrix 128 × 128, field of view (FOV) 230 mm) were created from signals obtained from images with two b values (b = 0 and 1,000). MRS data were obtained using chemical shift imaging based on spin-echo single-sequence (TR/TE = 1,500/135). Water suppression was accomplished by three chemical-shift-selective radio-frequency pulses preceding the section-selective radio-frequency pulses. Two-dimensional spectra were created using 16 × 16 phase encoding steps (FOV = 160 × 160, volume of interest [VOI]= 80 × 80, slice thickness = 15 mm, voxel size = 10 × 10 × 15, average number of signals = 4, and acquisition time 7.8 minutes). Brain angiography was also performed in 3 patients.
Imaging Analysis
The radiographic features of MS lesions, including location, number, and size range of T2W margin, were evaluated. The size of TDL was defined as the largest diameter of margin-to-margin signal abnormalities on T2W images, including surrounding edema. When it was possible to differentiate discrete lesion borders from the surrounding edema, the largest diameter of a discernible TDL was also calculated. The following variables were recorded for TDL: presence and degree of mass effect (mild, sulcal effacement; moderate, pressure on ventricle; or marked, midline shift, uncal hernia, or subfalcine hernia); presence and degree of edema (mild, <1 cm from the lesion; moderate, 1-3 cm from the lesion; or marked, >3 cm from the lesion); presence of a T2W hypointense rim, defined as a discernible, smooth, complete thin border of T2W hypointensity; presence of venular enhancement, defined as described previously,3 presence of gray matter (cortical, subcortical, or deep gray matter) involvement; presence of corpus callosum involvement; and presence of hemorrhage. Enhanced patterns were evaluated as homogeneous, heterogeneous, open-ring-like, or closed-ring-like, as described previously.2 DWI was visually assessed as heterogeneous (variable and complex pattern and distribution of high signals), diffuse and patchy (uniform and solid high signals throughout the lesion), or ringed (complete circular border or incomplete ring). MRS data were analyzed on the basis of NAA/Cr and Cho/Cr ratios relative to those of normal white matter obtained from five healthy control subjects with NAA/Cr ratios (1.10-1.72 [mean − 2SD to mean + 2SD]) and Cho/Cr ratios (0.86-1.56) within normal ranges. The size of the T2W margin of TDL at the last MRI examination (n= 10) was evaluated relative to that of the TDL at initial presentation (unchanged, reduced, or resolved). Additional MS lesions on T1W and T2W and enhanced lesions were recorded at MRI of the TDL at initial presentation and at the last MRI examination. The presence of spinal cord lesions was evaluated on MRI. All images were assessed by visual inspection, performed independently by two experienced reviewers (T.K. and H.K.). In the case of disagreement, a decision was reached by consensus.
Results
Clinical Characteristics
We identified 12 patients with TDL (six men, six women; age range 17-64 years, median age 27 years) among 102 patients with MS diagnosed according to McDonald's revised criteria from January 1993 to June 2009. In 1 patient (Patient 4) TDL developed after multiple recurrences of brain, spinal cord, or optic nerve involvement over the course of two decades and aquaporin-4 antibodies were positive, but these demyelinating lesions were not restricted to the spinal cord and optic nerve, which is infrequent in Devic's neuromyelitis. Table 1 summarizes the clinical course, relapsing times, and clinical features of presenting TDLs. Among the 12 patients, patients 1 and 2 had two TDLs. Relatively, frequent clinical features were motor impairment (75%) and visual impairment (58%). Disturbed consciousness (33%), headache (33%), and aphagia (25%) were also noted. Four patients had seizures (generalized in three and partial in one), which rarely occur in patients with MS.11 Eleven patients (92%) received steroids. Other treatments included infusion of intravenous immunoglobulin (17%), immunosuppressive agents (17%), and interferon (8%). The EDSS score at the time of TDL presentation (median 3.5, range 1-9.5) was decreased in 8 patients (67%) at the last follow-up (median 60 weeks, range 2.6-169 weeks).
| Patient | Age/Sex | Age at Symptom Onset* | Number of TDLs | Brain Biopsy | Clinical Course | Clinical Relapsing Time | Disease Duration from Symptom Onset to MRI* (Weeks) | Clinical Features* | EDSS* | CSF WBC [/mm3] | CSF Protein [mg/dL] | OCB | IgG Index | AQP4 | VEP | Intracranial Multiple Lesions on MRI | Spinal Cord Lesions on MRI | Treatments | Last Follow-Up EDSS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63/M | 63 | 2 | + | Monophasic | 0 | 4 | Decreased consciousness, tetraplegia | 9.5 | 4 | 29 | - | 0.88 | - | NE | + | + | Steroids | 9.0 |
| 2 | 33/M | 30 | 2 | + | Relapsing-remitting | 1 | 8 | Headache, hemianopsia, hemiplegia | 3.0 | 4 | 59 | – | 0.62 | – | NE | + | – | Steroids | 3.0 |
| 3 | 30/F | 24 | 1 | + | Relapsing-remitting | 4 | 69 | Hemiplegia, visual loss | 3.5 | 7 | 35 | – | 0.53 | – | + | + | – | Steroids | 2.0 |
| 4 | 38/F | 20 | 1 | – | Secondary progressive | ** | 56 | Headache, unconsiousness, visual loss, tetraplegia, seizures, sensory impairment | 9.0 | 91 | 180 | – | 0.82 | + | + | + | + | Steroids, interferon, methotrexate, ciclosporin | 10 |
| 5 | 21/M | 19 | 1 | – | Relapsing-remitting | 2 | 36 | Visual loss, hemiplegia, sensory impairment | 7.0 | 3 | 58 | – | 0.80 | – | + | + | + | Steroids | 3.5 |
| 6 | 64/F | 64 | 1 | – | Monophasic | 0 | 12 | Visual loss, aphagia | 3.5 | 1 | 35 | – | 0.19 | NE | + | + | – | None | 3.5 |
| 7 | 20/M | 17 | 1 | – | Relapsing-remitting | 1 | 20 | Headache, double vision, facial paresis | 1.0 | 113 | 168 | – | 0.87 | NE | – | + | – | Steroids | 0 |
| 8 | 31/M | 23 | 1 | – | Relapsing-remitting | 5 | 72 | Hemiplegia, seizures, sensory impairment | 3.5 | 4 | 25 | – | 0.29 | NE | – | + | – | Steroids | 1.5 |
| 9 | 57/M | 56 | 1 | – | Relapsing-remitting | 1 | 4 | Hemiplegia | 2.0 | 17 | 46 | – | NE | – | – | + | – | Steroids, IVIG | 1.0 |
| 10 | 53/F | 46 | 1 | – | Monophasic | 0 | 4 | Decreased consciousness, tetraplegia | 9.0 | 3 | 27 | – | 0.43 | NE | + | + | – | Steroids, IVIG, methotrexate | 2.5 |
| 11 | 41/F | 20 | 1 | + | Secondary progressive | ** | 552 | Hemiplegia, myoclonus, aphagia, sensory impairment | 7.0 | 5 | 32 | – | 0.53 | NE | + | + | + | Steroids | 9.0 |
| 12 | 64/F | 58 | 1 | – | Relapsing-remitting | 4 | 512 | Visual loss, headache, hemiplegia, aphagia | 3.5 | 2 | 104 | – | NE | NE | – | + | + | Steroids | 2.0 |
- *At presention of tumefactive multiple sclerosis (TDL), **Over 10 times. M = male; F = female; EDSS = Expanded Disability Status Scale; VEP = visual evoked potential; CSF = cerebrospinal fluid; WBC = white blood cells; OCB = oligoclonal bands; IVIG: intravenous immunoglobulin; AQP4 = aquaporin4; MRI = magnetic resonance imaging; VEP = visual evoked potential; NE = not examined.
Inflammatory reactions and increased protein levels in CSF were evident in 3 and 6 patients, respectively. Oligoclonal bands were absent in all patients. This low prevalence of oligoclonal bands has been reported to a unique feature of MS in Japanese patients as compared with Western patients12 or might have been related to the use of a technique (agarose gel electrophoresis) with low sensitivity.13 The CSF-IgG index was increased in 4 of 10 patients. Aquaporin-4 antibodies were detected in 1 of 6 patients, and abnormal visual evoked potentials were present in 6 of 10 patients. All patients had more than two intracranial MS lesions, and spinal cord lesions were evident in 5 patients.
Conventional Neuroimaging of TDL
A total of 14 TDLs were located in the frontal (43%), parietal (14%), occipital (14%), and temporal regions (29%), as shown in Table 2. These sizes of the T2-T2 margins varied widely (median 41.3 mm, range 30-101 mm) and were more than 20 mm for 27 MS lesions. The surface of 13 TDLs was well defined. Discernible margins were present in 12 TDLs (median width 21.2 mm). Mass effect was present in 10 TDLs (mild in five, moderate in one, and marked in four). Edema was evident in all 14 TDLs (mild in three, moderate in seven, and marked in four). Eleven TDLs (79%) had a hypointense rim on T2W (Fig 1A), and all TDLs showed hypointensity on T1W (Fig 1B). The enhancement pattern was heterogeneous for 13 TDLs (62%; Fig 1C), open-ring-like for 4 (31%), and closed-ring-like for 1 (7%). Venular enhancement was present in 8 of 13 TDLs (57%) (Fig 1C). The cortical gray matter was involved in 6 (79%) of 11 TDLs, the subcortical gray matter in 10, and the deep gray matter in 4. Corpus callosum involvement was evident in 3 (21%) of 14 TDLs. One TDL (patient 5) had hemorrhage (Fig 2). On DWI, 6 of 12 TDLs were diffuse and patchy, and 6 of 12 were ringed (Fig 1F); no TDL showed a heterogeneous pattern. The size of 12 TDLs at the last follow-up MRI examination (median 60 weeks, range 2.6-169 weeks) was unchanged in two, reduced in eight, and resolved in two. All except 1 patient (patient 6) with reduced or resolved TDL had received pulse therapy with intravenous methylprednisolone. Some patients received oral prednisolone with taper after pulse therapy or intravenous immunoglobulins.
| Radiologic Features of Large Tumefactive Demyelinating Lesions (≥30 mm) | %[n= 14] |
|---|---|
| Location | |
| Frontal | 43 [6] |
| Parietal | 14 [2] |
| Occipital | 14 [2] |
| Temporal | 29 [4] |
| Margins | |
| Well defined | 93 [13] |
| Diffuse | 7 [1] |
| Size: T2-T2 margins | |
| ≥30 mm | Median 41.3 (range 30-101) |
| 20-29 mm (n) | 13 |
| 3-19 mm (n) | 134 |
| Size: discernible margins (mm) | Median 21.1 (range 14.5-42) |
| Present | 86 [12] |
| No discernible margins | 14 [2] |
| Mass effect | |
| None | 29 [4] |
| Mild | 35 [5] |
| Moderate | 7 [1] |
| Marked | 29 [4] |
| Edema | |
| None | 0 |
| Mild | 21 [3] |
| Moderate | 50 [7] |
| Marked | 29 [4] |
| T2W hypointense rim present | 79 [11] |
| T1W hypointense | 100 [14] |
| Enhanced pattern [n= 13] | |
| Heterogeneous | 62 [8] |
| Open ring | 31 [4] |
| Closed ring | 7 [1] |
| Venular enhancement present [n= 13] | 57 [8] |
| Gray matter (GM) involvement | 79 [11] |
| Cortical GM | 43 [6] |
| Subcortical GM | 71 [10] |
| Deep GM | 29 [4] |
| Corpus callosum involvement | 21 [3] |
| Hemorrhage | 7 [1] |
| DWI [n= 12] | |
| Diffuse and patchy | 50 [6] |
| Ringed | 50 [6] |
| MRS [n= 9] | |
| NAA reduction | 22 [2] |
| NAA/Cr ratio | Median 1.76 (range 1.0-4.53) |
| Evelated Cho peak | 78 [7] |
| Elevated Cho/Cr ratio (n= 7) | Median 1.49 (range 1.23-2.83) |
| Follow-up [n= 12] | |
| Intervals* (days) | Median 240 (range 18-1186) |
| Size of T2-T2 margins | |
| unchanged | 17 [2] |
| reduced | 66 [8] |
| resolved | 17 [2] |
- DWI = diffusion-weighted imaging; MRS = MR spectroscopy; NAA = N-acetylaspartate; Cho = choline; Cr = creatine. *Between MRI with TDL and last MRI examination.
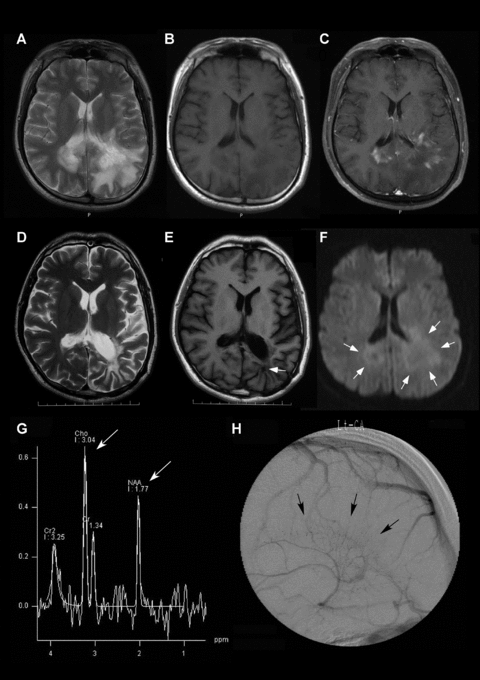
Neuroimaging findings of large TDL in patient 2. A T2-weighted image (A), showing two TDLs exceeding 30 mm in diameter, with a T2-weighted hypointense rim and massive edema. A T1-weighted image (B), showing hypointensity. A contrast T1-weighted image (C), showing heterogeneous and venular enhancement. A diffusion weighted image (F), showing hypointensity in the center with peripheral restricted hyperintensity, appearing as a ring-like border (white arrows). Decreased N-acetylaspartate and increased choline ratios were evident on MR spectroscopy (G, white arrows). During the venous phase on angiography, stasis of venous blood was evident around the TDL, suggesting venous dilatations (black arrows). The last follow-up MRI showed decreased hyperintensity in the TDL on a T2-weighted image (D), and a T1-weighted image (E) showed a biopsy defect within the TDL (white arrow).
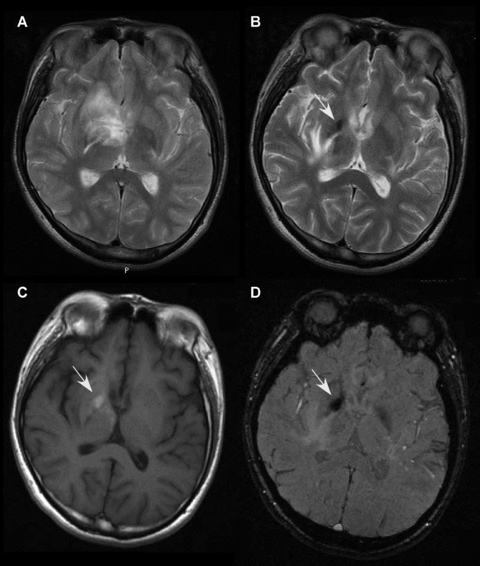
MRI images showing hemorrhage within a large tumefactive demyelinating lesion (TDL) in patient 5. A follow-up T2-weighted image (B) showing shrinkage of a large TDL as compared with TDL at the onset (A). On and around the TDL, follow-up T2-weighted (B) and susceptibility-weighted imaging (D) showed hypointensity, and T1-weighted imaging (C) demonstrated hyperintensity, suggesting subacute hemorrhage. The hemorrhage was located within the hyperintense region of the TDL at its onset (A).
Nonconventional Neuroimaging of TDL
MRS data in 9 patients showed a reduced NAA ratio in 2 patients (22%) and an elevated Cho peak in 7 (78%) (Fig 1G). Brain angiography in patient 2 demonstrated venous dilatations on and around the TDL (Fig 1H).
Pathological Findings of TDLs
Four patients with TDL underwent brain biopsies. In all 4 patients, biopsy demonstrated characteristic features of inflammatory demyelinating disease associated with hypercellular lesions with myelin loss, as shown in Figure 3. Cell infiltration of the brain parenchyma, perivascular lesions, and reactive gliosis were evident. Myelinic macrophages were also present (Fig 3D). Hypercellular pathological findings in 2 patients (patient 1 and 2) were Pattern I according to Lassmann's classification.14 Inflammatory demyelination was not restricted to perivenular lesions, which can be pathologically distinguished from acute disseminated encephalomyelitis.15 Immunohistochemistry for the simian vacuolating virus 40 in patient 1 and 2 or the major capsid protein VP1 of JC virus in patient 1 was negative.
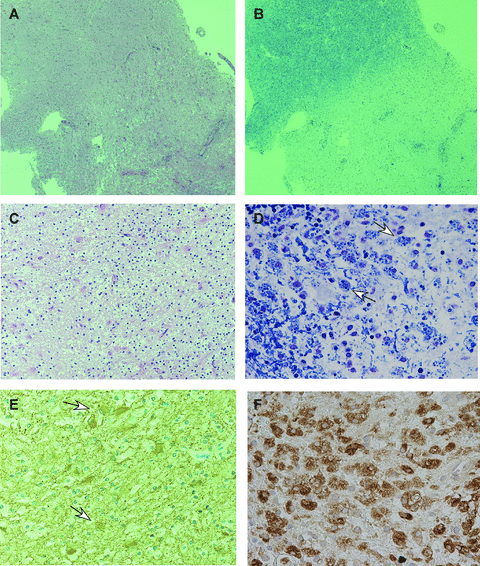
Pathological findings of a large tumefactive demyelinating lesion in patient 2. Hematoxylin-eosin staining (A) showed cell infiltrations in the brain parenchyma and perivascular lesions, with reactive gliosis. Luxol-fast blue (LFB) staining (B) showed myelin loss. In the demyelinating lesions, macrophages and lymphocytes (C, hematoxylin-eosin stain) and foamy macrophages consisting of LFB-stain-positive granulations (D, LFB stain) were evident. Gemistocytes on glial fibrillary acidic protein (GFAP) staining (E) and macrophages on KP-1 (CD68) staining (F) were also evident. Materials employed in this study, ie, 20% formalin-fixed and paraffin-embedded specimens, were retrieved from the surgical pathology files of the Pathology Section of Nara Medical University Hospital, Kashihara City, Nara, Japan.
Discussion
Consistent with previously published series,2–6 common MRI features of large TDLs included variable degrees of mass effect (71%) and edema (100%), a T2 hypointense rim (79%), venular enhancement (57%), and peripheral restriction on DWI (50%). Ring enhancement (38%) was less frequently observed in large TDLs. TDLs exceeding 20 mm showed ring enhancement most frequently.2 The pattern of ring enhancement associated with demyelination is most often “open,” with the incomplete portion abutting the cortical gray matter or basal ganglia.16 Open-ring enhancement has been considered a characteristic finding of demyelinating lesions,17 but was not so frequent (31%) in our series. This discrepancy may have important diagnostic implications because it might lead to difficulty in differentiating TDLs from neoplastic lesions that show variable enhancement patterns. Also in contrast to previous studies,4 most large TDLs (79%) had gray-matter involvement, which is uncommon in MS.18 Demyelinating lesions have been found in gray matter in a pathological study19 and are often detected by unconventional imaging techniques, such as double inversion recovery imaging.20 Edema surrounding large TDLs may involve the gray matter, but inflammatory demyelination, which we confirmed pathologically, might also involve the gray matter. Gray-matter involvement or, less frequently, ring enhancement and open-ring enhancement seem to be a key cause of difficulty in differentiating large TDLs from neoplastic lesions.
Decreased NAA/Cr and increased Cho/Cr ratios on MRS have been considered useful for the diagnosis of MS.21 However, decreased NAA is observed in various disease conditions, including neoplasia,22 and a relatively low proportion of large TDLs (22%) showed decreased NAA/Cr ratios in our series. One study reported that acute MS lesions, including TDL, were associated with normal or minimally reduced NAA levels, indicating minimal neuronal loss.23 Increased Cho levels are also observed in neoplastic lesions such as gliomas.22 The presence of glutamate/glutamine7 or lactate peaks8 has been considered a useful diagnostic marker of TDL, but glutamate/glutamine peaks and lactate peaks in our series were present in only 4 patients and 1 patient, respectively. MRS findings alone thus seem not to provide an adequate basis for differentiating demyelinating lesions from neoplasms. These results suggest that complementary information obtained by different MRI techniques is crucial to improving the accuracy of diagnosing large TDL.
Other interesting imaging features in large TDL were multiple venous dilatations on and around TDLs on angiography; signs of hemorrhage were also evident. These findings may be associated with focal vascular structural abnormalities related to demyelination in patients with MS.24 Marked signs of microvascular involvement in MS lesions, with abnormal signals on and around the venous wall on 7-T MRI provide direct evidence of the vascular pathogenesis in MS.25 Transcranial and extracranial Color-Doppler sonography studies have demonstrated that cerebral venous return anomalies were more evident in patients with MS than in control subjects, suggesting that cerebral venous drainage is impaired in MS.26 Venular enhancement, which was frequently observed in TDL, is attributed to dilated veins draining toward distended subependymal veins,3 as shown in Figure 1. Acute lesions of MS include perivascular inflammation as well as reactive astrogliosis and demyelination, leading to cell membrane or venous structure breakdown,27 which can cause hemorrhage in TDL. Some authors speculate that CNS-demyelinating disease progresses along the venous vasculature by perivenular demyelination or dense perivascular inflammatory infiltration, thereby contributing to demyelination and axonal damage.24,27 Some large TDLs might have venous structural abnormalities.
Acute episodes of TDL are usually isolated and rarely progress to typical MS.28 In contrast, most patients with TDL develop definite (70%) or probable MS (9%).2 Nine patients with large TDL in our study had episodes of relapse, including three (patients 2, 5, and 7) who had the first episode of demyelinating relapse after the onset of TDL. Many patients with repeated relapse and remission had large TDL as compared with patients who showed other clinical courses. There was no radiographic risk factor in TDL associated with an increased risk of a second MS attack.2 Lesion size, mass effect, edema, and enhancement pattern in TDL did not correlate with clinical course.2 A weak correlation of TDL size with the EDSS score at the onset of TDL has been reported,2 but whether the course of TDL affects clinical outcomes in patients with MS remains unclear. Among our 10 patients with large TDL, nine showed shrinkage or resolution of TDL on the last follow-up MRI. However, the EDSS score at the last follow-up examination did not decrease in 4 patients with TDL. The number of T1W or T2W lesions in the 10 patients increased (Fig 4). MS patients with TDL have a relatively benign course,28 but lesions exceeding 50 mm in diameter are associated with a higher EDSS score at follow-up.2 Our findings suggest that clinical outcomes in patients with large TDL are likely to be associated primarily with MS lesion loads, rather than with the characteristics of the TDL itself. However, persistent T1 hypointense lesions,29 found in six of our patients, might contribute to clinical outcomes.

Total numbers of MS lesions in 10 patients with large tumefactive demyelinating lesion (TDL). Total numbers of MS lesions that exceeded 3 mm on T1 hypointense (T1W), T2 hyperintense (T2W), and gadolinium-enhanced (Gd-T1W) imaging and periventricular lesions on T2-weighted imaging (periventricular disease: PVD). The numbers of T1W, T2W, and PVD lesions at the onset of (TDLs) (white bar) had increased on the last follow-up MRI examination (gray bar). The number of Gd-T1W had decreased on the last follow-up MRI examination.
Six patients in our study had large TDLs as their first demyelinating event, and three of these patients had second MS attacks. The reason why TDLs presented as the first MS attack is uncertain, but the rate of TDLs presenting as a first demyelinating event was reported to be high (53%).2 A previous study reported that the frequency of TDLs with a monophasic course was also relatively high (24%),2 consistent with our findings (25%). In that study, the median time from the first TDL presentation to the second attack was prolonged (4.8 years).2 Therefore, our 3 patients with a monophasic course may have second MS attacks several years later.
Median EDSS at the time patients presented with large TDLs in our study was generally similar to that associated with TDLs exceeding 20 mm in diameter in previous large series (3.5, range 3.0-4.5).2 Of interest to clinical neurologists, the neurologic disability in each patient varied, despite the presence of large TDLs. In particular, Patient 7 had minimal neurologic disability, despite a large TDL in the right temporal lobe. Prognostic MRI features of clinically silent or benign MS associated with a low level of locomotor disability several years after clinical onset have been reported to include minimal tissue damage within and outside MS lesions, the relative sparing of clinically eloquent regions, and the presence of effective compensatory mechanisms, although TDLs were not mentioned.30 Furthermore, it is apparently not possible to establish which MRI characteristics can be used to distinguish benign MS from other disease phenotypes.30 The clinical implications of large TDLs with respect to neurologic disability remain unclear, but lesions that do not involve clinically eloquent regions, as seen in Patient 7, might contribute to a low EDSS score. Clinicians should be aware of the existence of patients with large TDLs who present with minimal disease severity.
The prevalence of large TDLs in our study was higher than that in a previous study, which estimated that TDLs occur in 1-2 per 1,000 cases of MS.31 Our high prevalence of large TDLs might be related to the fact that our department is a key center for the diagnosis and treatment of neurologic diseases in Nara and has a high-introduction rate, especially for differential diagnosis. However, the incidence of large TDLs remains speculative and must await the results of the multiple sclerosis Lesion Project.2
Ethnic differences in the frequency of MS or MS phenotype have long been recognized. Asians have a lower prevalence of MS or a higher frequency of optic and spinal involvement as compared with whites, although the Asian phenotype has recently shifted to classic type MS, presumably because of environmental factors related to “Westernization.”32 Whether our findings of TDLs can serve as the basis for identifying neuroimaging or biologic markers specific to Asians remains uncertain. Confirmation must await further large studies of TDLs in Asia.
In conclusion, our findings suggest that a low frequency of ring enhancement, open-ring enhancement, or NAA reduction on MRS and a high frequency of gray-matter involvement might create difficulty in differentiating large TDLs from neoplastic lesions. In addition to vessel-like structures on TDLs, multiple venous dilatations around TDLs on angiography can be useful for the diagnosis of large TDLs. Although the diagnosis of TDLs, especially large lesions, remains challenging, these findings may facilitate diagnosis.




