Comparison of Partial (.6 mg/kg) versus Full-Dose (.9 mg/kg) Intravenous Recombinant Tissue Plasminogen Activator Followed by Endovascular Treatment for Acute Ischemic Stroke: A Meta-Analysis
J Neuroimaging 2011;21:113-120.
Abstract
ABSTRACT
BACKGROUND
In the treatment of acute ischemic stroke, intravenous (IV) recombinant tissue plasminogen (rt-PA) and intraarterial (IA) interventions are often combined. However, the optimal dose of IV rt-PA preceding endovascular treatment has not been established.
METHODS
Studies that used combined IV and IA thrombolysis were identified from a search of the MEDLINE, PubMed, and Cochrane databases. We compared the rates of angiographic recanalization, symptomatic intracerebral hemorrhage (sICH), and favorable functional outcome between patients who had been treated with .6 mg/kg IV rt-PA and those who had received .9 mg/kg rt-PA.
RESULTS
Eleven studies met our criteria. In 7 studies, .6 mg/kg IV rt-PA had been administered to 317 patients, whereas 140 patients in 4 studies had received .9 mg/kg of IV rt-PA. The weighted mean of median National Institutes of Health Stroke Scale score at presentation was 18.3 in the .6 mg/kg group (median range 9-34), and 17.3 in the .9 mg/kg group (median range 4-39). Patients in the .9 mg/kg group had higher rates of favorable outcome [odds ratio (OR) = 1.60, 95% confidence interval (CI) = (1.07-2.40), P= .022] and similar rates of sICH [OR = .86 (95% CI .41-1.83), P= .70]. Depending on the statistics used, the higher angiographic recanalization rate among patients treated with .9 mg/kg was significant (P= .03, events/trial syntax logistic regression) or borderline significant (P= .07, random effects model).
CONCLUSION
Our analysis suggests that using .9 mg/kg IV rt-PA prior to IA thrombolysis is safe and may be associated with higher recanalization rates and better functional outcome at 3 months.
Introduction
Following the results of the National Institutes of Neurological Disorders (NINDS) stroke trial,1 intravenous (IV) recombinant tissue plasminogen activator (rt-PA) has become the standard of care for the treatment of patients with acute ischemic stroke presenting within 3 hours of symptom onset. Adjunctive endovascular treatment is currently administered in most referral centers and includes pharmacological (administration of thrombolytic medication) and mechanical approaches (microwire manipulation, angioplasty, and/or stent placement) used in different combinations on a case-by-case basis (multimodal thrombolysis).
In order to not exceed the total dose of rt-PA assessed by NINDS trial (.9 mg/kg), initial studies used (“bridged”) .6 mg/kg IV rt-PA and administered the remaining dose of rt-PA or equipotent dose of a different agent via the intraarterial (IA) route.2–4 More recent data5–8 appear to suggest that endovascular treatment following full-dose IV thrombolysis may be safe. This approach offers the full benefit of IV thrombolysis and the potential added benefit of IA intervention. We performed a meta-analysis of the published data in order to compare the safety and efficacy of the 2 different doses of IV rt-PA used in the bridging approach.
Methods
Data Sources
We employed several strategies to identify studies that reported on ischemic stroke patients treated with combined IV thrombolysis and endovascular treatment. We searched the literature from 1995 to 2007 to identify individual studies, using the following computerized databases: PubMed (U.S. National Library of Medicine), Ovid (Wolters Kluwer), Cochrane Database of Systematic Reviews (Cochrane Collaboration resources), and the ClinicalTrials.gov website (U.S. National Institutes of Health). The key words used for search were “Intra-arterial therapy,”“Acute ischemic stroke,”“Combined intravenous and intra-arterial therapy,”“Bridging therapy,” and “Intra-arterial therapy for acute ischemic stroke.” Further, we searched by combining the above-mentioned keywords using PubMed's MeSH database service. Bibliographies of relevant review articles and text book chapters were reviewed to identify any pertinent studies. Web-based and manual searches of abstracts presented at scientific meetings were also performed. We contacted (when possible) authors when additional information was needed. Only studies published in English were used for this analysis.
Study Selection Criteria
Among the identified studies, we selected those that reported the use of combined IV thrombolysis and endovascular treatment for acute ischemic stroke. The studies included case series, nonrandomized controlled studies, and randomized controlled trials using either of the 2 IV t-PA dosages. We subsequently used the following inclusion criteria: (1) sample size exceeding 5 patients, (2) IV rt-PA dosing of .6 or .9 mg/kg, (3) availability of mean or median National Institutes of Health Stroke Scale (NIHSS) score at presentation, patient demographics (age, gender), and functional outcome assessed at discharge or later, (4) time interval between symptom onset and IV and endovascular treatment reported, (5) final recanalization following endovascular treatment reported, and (6) rate of symptomatic intracerebral hemorrhage (sICH) reported.
Studies were included regardless of the modality of endovascular treatment administered (pharmacological and/or mechanical). In the event of overlap of study populations, the smaller study was excluded.
Data Extraction
Using a predesigned data abstraction form, 2 reviewers (MZM and QAS) independently reviewed all manuscripts and abstracted the following information: (1) study characteristics (year of publication, design, recruitment period); (2) patient characteristics (number of patients, demographic characteristics, NIHSS score at presentation); (3) dose of IV rt-PA given and time from symptom onset to IV rt-PA administration; (4) time from symptom onset to angiography and/or IA treatment; (5) type of IA thrombolytic and mechanical devices used; (6) angiographic recanalization rates following endovascular treatment; (7) NIHSS score at 24-48 hours, when available; (8) rates of sICH; and (9) functional outcomes at discharge or later.
The primary endpoints assessed in the analysis were partial or complete recanalization, favorable functional outcome, and sICH. Studies sometimes used different definitions of those endpoints (see Table 1). We used a modified Rankin scale score of 0-1 at 1-3 months after treatment to define favorable outcome. In 2 of the 11 studies, we determined the favorable outcome rates by reviewing individual patient data presented in tables or by acquiring it directly from the authors. In 3 of the 11 studies, favorable outcome was defined by other measures acquired at discharge or last available follow-up. We repeated the analysis after excluding these 3 studies. Since we did not detect a difference in our results, we decided to include the studies and favorable outcome definitions used.
| Studies | Number of Patients | Definitions | Types of Endovascular Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Angiographic Recanalization | Symptomatic Intracranial Hemorrhage | Favorable Functional Outcome | Early Neurologic Improvement | Mechanical† | Pharmacological (Maximal Dose) | ||||
| rt-PA | RT | UK | |||||||
| .6 mg/kg IV rt-PA | |||||||||
| EMS,2 1999 | 17 | TIMI 2-3 | ≥4 p increase in NIHSSS | 3 Mo mRS ≤ 1‡ | ND | 20 mg | |||
| Ernst et al,17 2000 | 20 | ND* | ND | 2-11 Mo mRS ≤1 | ND | 24 mg or .3 mg/kg | |||
| IMS,3 2004 | 80 | TIMI 2-3 | Clinical deterioration, likely to result in disability or death | 3 Mo mRS ≤ 1 | NIHSSS ≤ 2 | 22 mg | |||
| Flaherty et al,18 2005 | 62 | ND* | ND | 3 Mo mRS ≤ 1‡ | ND | 22 mg | |||
| IMS II,19 2007 | 81 | TICI ≥ 2a* TIMI 2-3 | Clinical deterioration, likely to result in disability or death | 3 Mo mRS ≤ 1‡ | NIHSSS ≤ 2 | US | 22 mg | ||
| Wolfe et al,20 2008 | 41 | TIMI 3, TIMI 1-3 | ≥4 p increase in NIHSSS | 3 Mo mRS ≤ 2‡ | ≥4p improvement in NIHSSS | 30 mg or .3 mg/kg | |||
| Sugiura et al,21 2008 | 16 | TIMI 2-3 | Any clinical deterioration | 3 Mo mRS ≤ 1 | ND | Angioplasty | 10 mg | ||
| .9 mg/kg IV rt-PA | |||||||||
| Hill et al,5 2002 | 8 | ND* | ND | 3 Mo mRS ≤3** | ND | Angioplasty | 20 mg | ||
| Lee et al,6 2004§ | 30 | ND* | >0p increase in NIHSSS | 3 Mo mRS ≤1 | ≥4p improvement in NIHSSS | 1,000,000 U | |||
| Shaltoni et al,8 2007 | 69 | TICI ≥2a* | >2p increase in NIHSSS | d/c home or inpatient rehab | ND | Angioplasty, Stenting | 24 mg | 6 U | 750,000 U |
| Burns et al,22 2008 | 33 | Qureshi 0-1* | ≥4p increase in NIHSSS | NIHSSS 0-2** at time of d/c | ND | MERCI, Snare, Angioplasty, Stenting | 2 mg | ||
- NIHSSS = National Institutes of Health Stroke Scale Score; IV = intravenous; IA = intraarterial; rt-PA = recombinant tissue plasminogen activator; RT = reteplase; UK = Urokinase; mRS = modified Rankin scale; d/c = discharge; TIMI = thrombolysis in myocardial infarction; TICI = thrombolysis in cerebral infarction; EMS = emergency management of stroke bridging trial; IMS = Interventional Management of Stroke Study; Mo = Month; n/a = not available; rehab = rehabilitation facility; ND = not defined; p = point(s); US = ultrasound; U = units
- *By using information provided in tables or by contacting the authors we were able to obtain the number of patients who had TIMI 2-3. TICI ≥2a is equivalent to TIMI 2-3.
- **By using information provided in tables or by contacting the authors we were able to obtain the number of patients who had mRS of 0-1.
- †Beyond microcatheter manipulation.
- ‡Additional outcome measures recorded.
- §Intravenous antiplatelets used in select cases.
When possible, we used a thrombolysis in myocardial infarction (TIMI) grade of 2-3 flow post-procedure to define partial or complete recanalization. For sICH, we used the definition provided by individual studies.
Any disagreements between the 2 data abstractors were reconciled with the mediation of a third investigator (ALG).
Statistical Methods
In order to compare the .6 mg/kg with the .9 mg/kg group, we pooled the demographic and clinical data from single studies: means of means or medians weighted by the sample size, and range of minimum and maximum individual patient data. When study range was not available it was approximated by the 95% confidence interval (CI). χ2 test was not feasible to compare characteristics between the groups because individual patient data were not available and was therefore not performed.
Analysis endpoints were also pooled from individual studies. The endpoints of both groups were compared using a logistic regression model with event/trial syntax. In order to verify our results, we repeated the analysis using a more stringent random effects model based on the DerSimonian-Laird method.9,10
The random effects model assumes that the selected studies constitute a random sample, whose total variance is a composite of the individual study variance 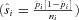 and the estimated variance
and the estimated variance 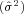 between the studies. The resulting P-value associated with the Q-statistic of between group heterogeneity was used to determine statistical significance between groups.
between the studies. The resulting P-value associated with the Q-statistic of between group heterogeneity was used to determine statistical significance between groups.
In addition, the heterogeneity across studies for the 3 endpoints was estimated using the I2 statistic.11 I2 statistic measures the percent variability of study estimates to the total variability observed. Publication bias for each dose regimens was assessed with trim and fill method.12
Angiographic recanalization and functional outcome of individual studies were also presented as forest plots. Analysis was performed using SAS 9.2 (SAS Institute Inc, Cary, NC, 2004) and R 2.6.2 (The R Foundation for Statistical Computing, 2008). The analyst (GV) was blinded to the dose used in both treatment groups. Significance was declared at P value < .05.
Results
A total of 125 studies were identified, of which 22 reported on the use of combined IV thrombolysis and endovascular treatment. A total of 11 studies were excluded (see Fig 1). Tables 1–3 present the characteristics and results of the 11 individual studies included in the analysis and Table 4 summarizes the pooled information for each group.
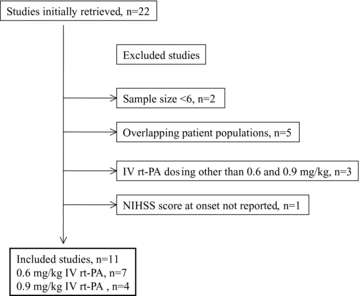
Selection diagram for the studies included in the meta-analysis.
| Studies | Occlusion Site | Time to IV in Hours | Time to IA Treatment in Hours | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior Circulation | Posterior Circulation | ||||||||||||
| Cervical | Cervical and Intracranial | Intracranial Only | |||||||||||
| ICA terminus | M1 | Distal MCA | ACA | ICVA | BA | PCA | Mean | Median | Mean | Median | |||
| .6 mg/kg IV rt-PA | |||||||||||||
| EMS2 | 1 | 6 | 2 | 3 | 2.60 | 3.3† | |||||||
| Ernst et al.17 | 1 | 6 | 4 | 8 | 1 | 2.03 | 3.5 | ||||||
| IMS3* | 3 | 21 | 7 | 18# | 15 | 2# | 5** | 2.33 | |||||
| Flaherty et al.18 | 8 | 7 | 21 | 6 | 2 | 2.15 | 3.77 | ||||||
| IMS II19§ | 2.37 | n/a | |||||||||||
| Wolfe et al.20 | 11 | 27 | 5 | 2.52 | n/a | ||||||||
| Sugiura et al.21 | 9 | 7 | 1.83 | 2.5 | |||||||||
| .9 mg/kg IV rt-PA | |||||||||||||
| Hill et al.5 | 2 | 2 | 3 | 1 | 1.70 | 5.17 | |||||||
| Lee et al.6 | 2 | 5 | 10 | 1 | 3 | 1 | 2.25 | 3.83† | |||||
| Shaltoni et al.8 | 2 | 16‡ | 31 | 19 | 1 | 2.00 | 4.75 | ||||||
| Burns et al.22§ | 1.97 | 4.00† | |||||||||||
- ICVA = intracranial vertebral artery; BA = basilar artery; ICA = internal carotid artery; M1 = M-1 segment of the middle cerebral artery; ACA = anterior cerebral artery; PCA = posterior cerebral artery; IV = intravenous; IA = intraarterial; rt-PA = recombinant tissue plasminogen activator; n/a = not available.
- †Time to angiography.
- ‡ In 11 cases, the proximal ACA and/or MCA were also involved.
- §Sites of occlusion not available.
- #Two patients had combined M1 and ACA lesions.
- *Included are 18 cases of ICA stenosis, 15 of which had distal embolization into the MCA.
- **Two cases had stenosis and not occlusion.
| Publication | Demographics | Baseline NIHSSS Median (Range) or Mean ± SD | Time to Treatment in Minutes Median (Range) or Mean ± SD | Rate of Endpoints | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age in Years Median (Range) or Mean ± SD | Men | IV rt-PA | Femoral Puncture | Microcatheter Placement | Angiographic Recanalization | Favorable Functional Outcome | sICH | Early Neurological Improvement | ||
| .6 mg/kg IV rt-PA | ||||||||||
| EMS,2 1999 | 66 ± 11 | 53% | 16 (9-21)¶ | 156 (138-168)¶ | 198 (180-228)¶ | n/a | 82% | 35% | 12%‡ | n/a |
| Ernst et al,17 2000 | 69 (36-89) | 45% | 21 (11-31) | 122 (72-250) | n/a | 210 (155-292) | 69% | 50% | 5% | n/a |
| IMS,3 2004 | 64 ± 13 | 50% | 18 ± 5 | 136 ± 30 | 183 ± 45 | 217 ± 47 | 58% | 30% | 6% | 14% |
| Flaherty et al,18 2005 | 69* (20-89) | 40% | 18 (11-34) | 129** | n/a | 226** | 59% | 35% | 8% | n/a |
| IMS II,19 2007 | 64 ± 12 | 57% | 19 ± 5 | 140 ± 31 | n/a | n/a | 58% | 33% | 10% | 19% |
| Wolfe et al,20 2008 | 67 ± 14 | 39% | 17 (11-20) | 151 ± 31 | n/a | n/a | 66% | 46% | 12% | 42% |
| Sugiura et al,21 2008 | 70 ± 9 | 69% | 19 ± 2 | 110 ± 22 | n/a | 150 ± 25 | 88% | 63% | 0% | n/a |
| .9 mg/kg IV rt-PA | ||||||||||
| Hill et al,5 2002 | 49 (41-83) | 88% | 18 (10-24) | 102 (65-129) | n/a | 310 (190-375) | 38% | 25% | 0% | 38% |
| Lee et al,6 2004 | 63 (47-82) | 60% | 18 (4-30) | 135 (60-210) | 230 (60—310) | n/a | 71% | 60% | 7% | n/a |
| Shaltoni et al,8 2007 | 60 ± 13 | 55% | 18 (6-39) | 124 ± 32 | n/a | 288 ± 57 | 73% | 55% | 6% | n/a |
| Burns et al,22 2008 | 67 ± 12 | 33% | 15 (13-19) | 118 ± 27 | n/a | 240 ± 62 | 73% | 30% | 12% | n/a |
- EMS = Emergency Management of Stroke Bridging Trial; IMS = Interventional Management of Stroke Study; SD = standard deviation; NIHSSS = National Institutes of Health Stroke Scale Score; IV = intravenous; rt-PA = recombinant tissue plasminogen activator; n/a = not available; sICH = symptomatic intracranial hemorrhage.
- *Mean age with range, **Median, no range available, ¶Median (25th-75th percentile), ‡Both hemorrhages occurred post 24 hours following treatment.
| .6 mg/kg | .9 mg/kg | |
|---|---|---|
| Number of studies (number of patients) | 7 (n= 317) | 4 (n= 140) |
| Mean age* (min–max), years | 66.5 (18-93)** | 62.1 (34-91) |
| Men (%) | 156 (49%) | 74 (53%) |
| Mean time to IV rt-PA* (min–max), minutes | 138 (66-250) | 122 (60-210) |
| Mean NIHSS score* (min–max) | 18.3 (9-34)** | 17.3 (4-39) |
| Clinical trials | 2 | 1 |
- P-values could not be assessed because individual patient data were not available.
- *Weighted (by sample size) mean of study medians/means.
- **Minimal and maximal individual patient values. If not available, the range was estimated using the 95% confidence upper and lower limits.
A dose of .6 mg/kg of IV rt-PA was administered in 7 studies that included 317 patients. Mean age was 66.5 years (range 18-93 years) and 51% were women. Mean time interval between onset of symptoms and IV rt-PA administration was 138 minutes (range 66-250 minutes). The mean (mean of median) NIHSS score at presentation was 18.3 (range 9-34) in the pooled data.
A dose of .9 mg/kg of IV rt-PA was administered in 4 studies that included 140 patients. Mean age was 62 years (range 34-91 years) and 47% were women. Mean time interval between onset of symptoms and administration of IV rt-PA was 122 minutes (range 60-210 minutes). The mean (mean of median) NIHSS score at presentation was 17.3 (range 4-39) in the pooled data.
sICH was seen in 26 (8%) patients in the .6 mg/kg group compared with 10 (7%) of patients in the .9 mg/kg group, odds ratio (OR) .86, 95% CI .41-1.83, P= .70) using a logistic regression model with events/trial syntax. This result was confirmed using a random effects model. No heterogeneity in rates of sICH was seen between series.
Favorable functional outcome was observed in 118 (37%) of patients in the .6 mg/kg group compared with 68 (49%) of patients in the .9 mg/kg group, OR 1.6 (95% CI 1.07-2.40, P= .022, events trial/syntax). The random effects model confirmed the presence of a significant difference between the 2 dose regimens, P= .023. The proportion of favorable functional outcome across studies were heterogeneous, I2: 60%, 95% CI: 22-80%. Rates of good functional outcome at study level are presented as a Forest plot in Fig 2. The direction of association did not change after excluding the 2 studies (one from each group) where the proportion of patients with mRS of 0 or 1 at last available follow-up was not provided. The magnitude of association decreased from 1.6 to 1.4 and significance could not be detected because of the small sample size. Assessment for publication bias for favorable outcome revealed no publication bias for .9 mg/kg and suggested 2 missing studies for .6 mg/kg yielding an estimate of 35%.
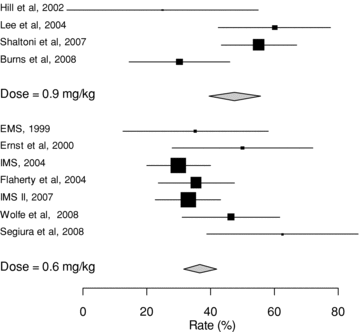
Forest plot of rates (%) of good functional outcome.
Partial or complete recanalization was observed in 179 (56%) of patients in the .6 mg/kg group compared with 94 (67%) of patients in the .9 mg/kg group, OR 1.57 (95% CI 1.03-2.37, P= .03). There was only borderline significance in the difference of the rates between the 2 treatment groups using the random effects model (P= .07). Heterogeneity across studies regarding angiographic recanalization rates was high I2: 72% (50-84%). Rates of angiographic recanalization in the studies included in the analysis are shown as a Forest plot in Fig 3. Clinical and angiographic outcomes are summarized in Table 5. Assessment for publication bias for partial or complete recanalization revealed no publication bias.
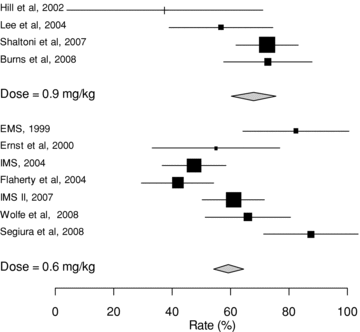
Forest plot of rates (%) of angiographic recanalization.
| .6 mg/kg | .9 mg/kg | OR (95% CI) | P-Value | |
|---|---|---|---|---|
| I. Using Logistic Regression with Events/Trial Syntax | ||||
| Angiographic recanalization | 179 (56%) | 94 (67%) | 1.57 (1.03-2.37) | 0.03 |
| Symptomatic ICH | 26 (8%) | 10 (7%) | 0.86 (.41-1.83) | 0.70 |
| Favorable functional outcome at last available follow-up | 118 (37%) | 68 (49%) | 1.60 (1.07-2.40) | 0.02 |
| .6 mg/kg | .9 mg/kg | Diff | P-Value | |
| II. Using Random Effects Model | ||||
| Angiographic recanalization | 59.3% (54.2- 64.5%) | 67.9% (60.3-75.4%) | 8.5%I2: 75.1% (55-86%) | 0.07 |
| Favorable functional outcome at last available follow-up | 36.8% (31.5-42.0%) | 47.5% (39.5-55.5%) | 10.8%I2:60% (22-80%) | 0.03 |
| Symptomatic ICH | 7.5% (4.6-10.4%) | 6.9% (2.7-11.1%) | .62%I2: 0% (0-5.7%) | 0.81 |
- I2 is a measure of heterogeneity (ranges from 0 to 1) and is the percent variation between study estimate and total variation.
- rt-PA = recombinant tissue plasminogen activator; OR = odds ratio; CI = confidence interval; ICH = intracerebral hemorrhage.
Discussion
We found no significant difference in sICH rates between the .6 mg/kg (8%) and the .9 mg/kg (7%) groups. In the .9 mg/kg group, rates of angiographic recanalization and favorable functional outcome appeared to be higher (OR 1.60, 95% CI 1.07-2.40 and OR 1.57, CI 1.03-2.37, respectively) when compared using a logistic regression model with events/trial syntax. Using the more stringent random effects model, the results were similar with the exception of recanalization, which achieved only borderline significance.
The .9 mg/kg dose for IV rt-PA was established following the 2 NINDS dose-finding studies.13,14 Escalating rt-PA doses were administered to patients, within 90 minutes from stroke onset in Part I13 and between 91 and 180 minutes from onset in Part II.14 No sICH was noted in the 58 patients who received .85 mg/kg of IV rt-PA or less in Part I versus 3/26 patients who had received a dose of .95 mg/kg or greater. Higher doses of rt-PA were significantly related to the risk of developing sICH (P= .045). There was no clear correlation between early neurological improvement and rt-PA dose administered. Based on these findings, an intermediate dose between .85 and .95 mg/kg was selected for the NINDS efficacy trial.1 Subsequent studies combining IV thrombolysis and endovascular treatment were designed to avoid exceeding a total dose of .9 mg/kg rt-PA by administering a partial IV dose (.6 mg/kg) followed by IA administration of up to .3 mg/kg.
Our findings suggest that .9 mg/kg IV rt-PA prior to endovascular treatment is a safe alternative to .6 mg/kg. In our analysis, sICH rates were not higher in patients who were treated with full-dose IV rt-PA than in those treated with partial dose prior to endovascular therapy. The lack of difference in sICH rates could be related to the short half-life of IV rt-PA (3-5 minutes),15 which means that the thrombolytic effect would wane prior to the endovascular intervention. Moreover, in patients who have received full-dose IV rt-PA, smaller doses of IA thrombolytics are administered with greater emphasis on mechanical thrombectomy. It is also possible that the occurrence of sICH depends more on factors such as magnitude of ischemic injury and blood–brain barrier disruption rather than the dose of thrombolytics.16
Although we observed that the patients treated with .9 mg/kg IV rt-PA had a significantly higher rate of favorable outcomes, we believe that prospective studies ensuring randomization with uniform outcome ascertainment are required in order to confirm this finding.
Future studies should also address the optimal dosing of thrombolytic medications in IA procedures following .9 mg/kg IV rt-PA. The implication of our findings for current clinical practice is that patients who have been treated with full-dose IV rt-PA can be considered for endovascular treatment under well-defined protocols.
Our study has several limitations that must be considered prior to interpretation of the results. This was a meta-analysis combining the data of several case series with variable methodologies. The methodology and ascertainment of outcomes may have been more rigorous in the .6 mg/kg group (ascertainment bias) where 2 case series were derived from studies that were conducted as clinical trials. Outcome estimates were heterogeneous across studies that used the same treatment regimen. This was likely due to discrepancies in the definition of outcome measures and in the time points of data ascertainment. Furthermore, the variations in endovascular techniques and doses of IA thrombolytics induce additional heterogeneity in angiographic and clinical outcomes. While the numbers of patients in the studies were too small to perform subgroup analysis, we provided descriptive statistics in Tables 1 and 2 to facilitate interpretation. Because individual patient data were not available, we were unable to control for important prognostic factors such as patient age, time to treatment, NIHSS score at presentation, site of arterial occlusion, and technique of endovascular treatment (confounding bias). Meta-analyses are prone to publication bias. We therefore applied the trim and fill method which did not support the presence of substantial bias.
Conclusion
Our analysis suggests that using .9 mg/kg as opposed to .6 mg/kg of IV rt-PA prior to endovascular treatment is safe and associated with higher recanalization rates and functional outcome. Further controlled studies are needed to compare the angiographic and clinical outcomes associated with each of the 2 regimens of rt-PA used in the bridging approach.




