Phylogenetic Position of the Adeleorinid Coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) Inferred Using 18S rDNA Sequences
Abstract
Investigating the evolutionary relationships of the major groups of Apicomplexa remains an important area of study. Morphological features and host-parasite relationships continue to be important in the systematics of the adeleorinid coccidia (suborder Adeleorina), but the systematics of these parasites have not been well-supported or have been constrained by data that were lacking or difficult to interpret. Previous phylogenetic studies of the Adeleorina have been based on morphological and developmental characters of several well-described species or based on nuclear 18S ribosomal DNA (rDNA) sequences from taxa of limited taxonomic diversity. Twelve new 18S rDNA sequences from adeleorinid coccidia were combined with published sequences to study the molecular phylogeny of taxa within the Adeleorina and to investigate the evolutionary relationships of adeleorinid parasites within the Apicomplexa. Three phylogenetic methods supported strongly that the suborder Adeleorina formed a monophyletic clade within the Apicomplexa. Most widely recognized families within the Adeleorina were hypothesized to be monophyletic in all analyses, although the single Hemolivia species included in the analyses was the sister taxon to a Hepatozoon sp. within a larger clade that contained all other Hepatozoon spp. making the family Hepatozoidae paraphyletic. There was an apparent relationship between the various clades generated by the analyses and the definitive (invertebrate) host parasitized and, to lesser extent, the type of intermediate (vertebrate) host exploited by the adeleorinid parasites. We conclude that additional taxon sampling and use of other genetic markers apart from 18S rDNA will be required to better resolve relationships among these parasites.
APICOMPLEXAN protists are exclusively parasitic in hosts that range from marine invertebrates to terrestrial vertebrates. Ultrastructural features synapomorphic for the phylum, namely the conoid, polar ring, apical rings, and rhoptries, comprise an apical complex that functions in invasion of host cells and in movement (Adl et al. 2005; Siddall 1995). Many species feature a complex multistage life cycle of alternating sexual and asexual reproduction in one or more hosts (Adl et al. 2005).
In addition to the recently recognized apicomplexan affinities of the colpodellid protists (Kuvardina et al. 2002; Siddall et al. 2001), historically recognized “groups” of apicomplexan parasites include the gregarines, piroplasms, haemosporinids, cryptosporidia, and coccidia. Coccidia are usually considered to include the tissue coccidia (i.e. Eimeriorina: Sarcocystidae), the enteric coccidia (i.e. Eimeriorina: Eimeriidae), the adeleorinid coccidia (i.e. Adeleorina: Adeleidae), and the haemogregarines (i.e. Adeleorina: various families). Biology and taxonomic affinities of taxa in the suborder Adeleorina Leger, 1911 remain poorly understood (Barta 1989, 2000). Monoxenous adeleorinid coccidia infect a wide range of invertebrates (Levine 1988). As noted by many authors (e.g. Siddall 1995; Smith et al. 2000), most heteroxenous haemogregarines have been described only from gamonts found within the erythrocytes or leukocytes of their intermediate vertebrate hosts. Haemogregarines utilize a wide variety of invertebrate definitive hosts, such as leeches, acarines, and insects (Ball and Oda 1971).
Adeleorinid coccidia are characterized by a complex life cycle involving one or more asexual cycles of merogony, followed by gametogony, syngamy, and sporogony. Many haemogregarines appear to have morphological variation in types of meronts and merozoites that either initiate further rounds of merogonic replication or differentiate into gamonts (Barta 2000). Gamete formation occurs via syzygy, which is the association of gamonts prior to final gamete maturation and fertilization, within the definitive host. Major groups within the Adeleorina include monoxenous parasites belonging to the genera Adelina and Klossia in the Family Adeleidae, Legerella in the Family Legerellidae, and Klossiella in the Family Klossiellidae, and heteroxenous parasites in the genera Dactylosoma and Babesiosoma in the Family Dactylosomatidae, Haemogregarina, Desseria, and Cyrilia in the Family Hemogregarinidae, Hepatozoon in the Family Hepatozoidae, and Hemolivia and Karyolosus in the Family Karyolysidae (see Barta 2000).
Although limited molecular phylogenetic analyses of the Adeleorina have been attempted, morphological features, cytopathologic changes to infected host cells, and host associations have been the mainstay of their taxonomic classification (Boulianne et al. 2007; Siddall 1995; Smith 1996; Smith and Desser 1997; Smith et al. 2000; Vilcins et al. 2009). Classification based on biology and morphology of these parasites is fraught with homoplasy and differences in opinion with regard to the weight to give individual characters. This has led to revisions of various taxa and groupings that have compounded the problem rather than solved it as noted by Siddall (1995). The choice of 18S rRNA gene sequences in the present study is a pragmatic one: the gene has been shown to be useful for inferring genus and species level relationships within the Apicomplexa and there are more sequences available for comparison with newly obtained sequences than any other molecular target (Carreno and Barta 1999; Criado-Fornelio et al. 2006; Kopecna et al. 2006; Rubini et al. 2006). The objectives of this study were to elucidate the phylogenetic position of adeleorinid coccidia in relation to other parasites within the phylum Apicomplexa using newly obtained 18S rDNA sequences to test the monophyly of the Adeleorina and, if monophyletic, to evaluate the morphological and life history characters in the context of this molecular phylogeny.
Materials and Methods
Parasite material
Klossia helicina (Schneider 1875)
Oocysts of Klossia helicina were obtained by dissecting infected grove snails Cepaea nemoralis Linn. collected at Lochnagar Crater in Picardy, France (50°00′56′′N, 2°41′50′′E). Oocysts were cleaned of debris manually using a De Fonbrune micromanipulator (A. S. Aloe Co., St. Louis, MO) and then suspended in 5.25% (w/v) sodium hypochlorite for 10 min to destroy any contaminating host DNA. After washing in phosphate-buffered saline (PBS), oocysts were broken by five freeze-thaw cycles by transferring the samples repeatedly between liquid nitrogen and a water bath at 37 °C.
Blood parasites of various poikilotherms
Gamonts of Hepatozoon magna (Grassi & Feletti 1891) Labbé 1899 and Dactylosoma ranarum (Lankester 1882) Wenyon 1926 were obtained from naturally infected edible frogs Pelophylax kl esculentus (Linn.) (syn. Rana esculenta Linn.) collected by hand from the Fium'Orbo River, Corsica, France (42°0′49″N, 9°24′21″E). Babesiosoma stableri Schmittner & McGhee 1961 and Hepatozoon catesbianae (Stebbins 1903) Desser, Hong & Martin 1995 were obtained from mink frogs, Rana septentrionalis Baird 1854, and bullfrogs, Rana catesbeiana Shaw 1802, respectively, collected by hand from Lake Sasajewun, Algonquin Provincial Park, Ontario, Canada (45°35′17′′N, 78°31′45′′W). Also collected at Lake Sasajewun were blood samples containing gamonts of Haemogregarina balli Paterson and Desser 1976 from the common snapping turtle, Chelydra serpentina serpentina Linn. Hepatozoon sipedon Smith, Desser & Martin 1994 was obtained from northern water snakes, Nerodia sipedon sipedon Linn., collected by hand near Lake Opinicon, Ontario, Canada (44°35′N, 76°15′W). Finally, two samples of Hepatozoon clamatae (Stebbins 1905) Smith 1996 were collected from the blood of green frogs, Rana clamitans (Latreille 1801), collected by hand at Birge Mills, Ontario, Canada (43°39′59′′N, 80°15′30′′W) and the Speed River, Guelph, Ontario, Canada (43°32′51′′N, 80°11′52′′W). For all blood samples, duplicate thin blood films were made from each host animal; one was stained with a modified Wright stain using a HEMA-TEK automated slide stainer (Siemens Diagnostics, Burlington ON, Canada), and the other was maintained as an unstained specimen for DNA extraction.
In addition to parasites that were collected by the authors, samples containing gamonts of Hemolivia mariae (Smallridge and Paperna 1997) were kindly provided by Dr. C. Smallridge as dried blood films of experimentally infected shingleback skinks, Tiliqua rugosa Gray 1825, infected with gamonts. The parasite was originally obtained from naturally infected T. rugosa collected near Mt. Mary, South Australia (near 33°55′S, 139°20′E). Uninfected lizard blood was also provided as a suitable PCR amplification control.
DNA extraction and rDNA PCR amplification
For all samples of haematozoa, air-dried blood smears containing parasites were scraped into 100 μl of PBS using sterile scalpel blades. DNA extraction of blood or freeze-thawed oocysts of K. helicina was achieved using a guanidine thiocyanate-based, rapid DNA isolation kit according to the manufacturer's instructions (IsoQuick Nucleic Acid Extraction Kit; Orca Research Inc., Bothell, Washington).
For amplification of K. helicina DNA, standard PCR reactions with eukaryote-specific forward and reverse primers (Medlin et al. 1988) were used to amplify full-length 18S ribosomal DNA (rDNA) using Platinum® Taq DNA polymerase (Invitrogen Corp., Carlsbad, CA). The resulting PCR amplicons (~ 1.8 kb in length) were gel-purified by separation and excision from a 1.5% agarose gel prior to cloning into a pCR2.1 plasmid vector using the TOPO TA Cloning® kit (Invitrogen Corp.). Plasmid isolation was performed using GenElute Plasmid Mini-Prep kit (Sigma, St. Louis, MO). Complete sequences were obtained using M13 forward and reverse primers as well as a number of internal sequencing primers including: 366F—5′-AGGGTTCGATTCCGGAG-3′, 555F—5′-GTGCCAGCRGCCGCGG-3′, 571R—5′-ACCGCGGCKGCTGGC-3′, 1125F—5′-GAAACTTAAAKGAATTG-3′, and 1139R—5′-ATTCCTTTRAGTTC-3′ (see Elwood et al. 1985) or NS2—5′-GGCTGCTGGCACCAGACTTGC-3′ and NS3—5′-GCAAGTCTGGTGCCAGCAGCC-3′ (see White et al. 1990; equivalent to 4558 of Mathew et al. 2000). All sequencing reactions were performed on ABI Prism 3730 and 3100 DNA sequencers by the Molecular Biology Section, Laboratory Services Division, University of Guelph. All clones were sequenced completely in both directions and chromatogram-based contigs were generated using Geneious 5.4 (Drummond et al. 2010) to provide high quality sequences for further analyses.
Amplification of the nuclear 18S rDNA of the parasites in blood samples from various poikilotherms was not possible using universal eukaryotic primers because of the large surplus of contaminating host DNA that would be amplified preferentially. Instead, two sets of primers, each combining a eukaryote-specific primer with an apicomplexan-specific primer, were used to generate partial, but substantively overlapping, rDNA fragments. The “A fragment,” consisting of the first ~ 1.35 kb of the nuclear 18S rDNA, was obtained using a “universal” forward primer (Medlin et al. 1988) with a parasite-specific reverse primer 18AP1488.R 5′-CGGAATTAACCAGACAAATC-3′ (Wozniak et al. 1994). The “B fragment,” consisting of the last ~ 1.65 kb of the nuclear 18S rDNA, was amplified using a parasite-specific forward primer (NS3 of White et al. 1990; see above) with a “universal” reverse primer (Medlin et al. 1988). The resulting PCR products were separated using agarose electrophoresis, purified after excision from the gel, cloned, and sequenced as described above for K. helicina. Complete rDNA sequences of the blood parasites were obtained by combining the largely overlapping cloned A and B sequences to provide a contiguous, complete rDNA sequence. In most cases, fragments A and B provided unambiguous complete nuclear 18S rDNA sequences but, inevitably, clone to clone sequence variation was present in some parasite rDNA sequences. In these cases, unique sequences were treated as paralogs and analyzed separately (see below). All sequences have been submitted to GenBank.
Phylogenetic analyses
In addition to the new sequences obtained in the present study, sequences from a large number of apicomplexan taxa were included in the phylogenetic analyses (Table 1). New and existing sequences were aligned initially using CLUSTAL W (Larkin et al. 1972) implemented from within the Geneious (Ver. 5.4) bioinformatics software package. This primary alignment was then optimized by eye using a staggered alignment method (Barta 1997; Chenna et al. 2003). The resulting alignment was then used to test phylogenetic hypotheses. For initial testing of monophyly of the adeleorinid taxa among other apicomplexan parasites, all sequences in Table 1 were used. After this global analysis, six sequences from monoxenous parasites in the family Adeleidae were used as a functional outgroup to root 73 sequences from heteroxenous adeleorinid taxa in the families Hemogregarinidae, Karyolysidae, Dactylosomatidae, and Hepatozoidae for a total of 79 sequences representing 22 named or unnamed haemogregarine taxa. A complete realignment of all sequences was completed using MUSCLE (Edgar 2004) implemented from within the Geneious (Ver. 5.4) bioinformatics software package. Both alignments have been submitted to TreeBASE (http://www.treebase.org).
| Species | GenBank accession | Taxonomic positiona | Definitive hostb | Intermediate hostc |
|---|---|---|---|---|
| Prorocentrum minimum | AY421791 | Dinophyceae; Prorocentrales; Prorocentraceae | n/a | n/a |
| Lessardia elongata | AF521100 | Dinophyceae; Peridiniales; Podolampaceae | n/a | n/a |
| Akashiwo sanguinea | EF492486 | Dinophyceae; Gymnodiniales; Gymnodiniaceae | n/a | n/a |
| Scrippsiella nutricula | SNU52357 | Dinophyceae; Perinidales; Scrippsiella | n/a | n/a |
| Chromera velia | DQ174731 | Chromerida | n/a | n/a |
| Chromerida sp. RM11 | DQ174732 | n/a | n/a | |
| Colpodella tetrahymenae | AF330214 | Apicomplexa; Colpodellida | n/a | n/a |
| Colpodella edax | AY234843 | n/a | n/a | |
| Voromonas pontica | AF280076 | Apicomplexa; Voromonadida | n/a | n/a |
| Voromonas pontica | AY078092 | |||
| Hepatocystis sp. | EU400394 | Apicomplexa; Aconoidasida; Haemospororida | Culicine mosquitoes: Culicoides fulvithorax, C. adersi | Primates: Cercopithecus sp., Papio sp., Hylobates sp. |
| Plasmodium falciparum | M19172 | Anopheline mosquitoes | Humans | |
| Babesia equi | Z15105 | Apicomplexa; Aconoidasida; Piroplasmorida | Ticks: Dermacentor sp., Rhipicecphalus sp., Hyalomma sp. | Equines |
| Babesia caballi | Z15104 | Equines | ||
| Babesia rodhaini | DQ641423 | Ticks | House mouse (Mus musculus) | |
| Babesia felis | AF244912 | Felines | ||
| Babesia leo | AF244911 | |||
| Theileria sp. | L19081 | Ruminants | ||
| Theileria annulata | DQ287944 | Ticks: Hyalomma sp. | Cattle, water buffalo | |
| Hepatozoon sp. Curupira 2 | AY461377 | Apicomplexa; Conoidasida; Coccidiasina; Eucoccidiorida; Adeleorina; Hepatozoidae | Ticks | Mammals |
| Hepatozoon canis Venezuela 1 | DQ439543 | Ticks: Rhipicephalus sanguineus | Canines | |
| Hepatozoon canis Venezuela 2 | DQ439540 | Ticks: Rhipicephalus sanguineus | Canines | |
| Hepatozoon felis | AY628681 | Ticks: Rhipicephalus sanguineus | Felines | |
| Hepatozoon felis | AY620232 | Ticks: Rhipicephalus sanguineus, Ixodes tasmani? | Felines | |
| Hepatozoon sp. Boiga | AF297085 | Unknown | Brown tree snake (Boiga irregularis) | |
| Hepatozoon ayorgbor | EF157822 | Mosquitoes: Culex quinquefasciatus (experimental) | Royal python (Python regius) | |
| Hepatozoon sipedon QUBS-1994 | JN181157 | Mosquitoes: various Culex spp. | Frogs (Rana spp.) and then Northern Water snake (Nerodia sipedon sipedon) | |
| Hepatozoon sp. HepBiCM001 | AB181504 | Ticks: Haemaphysalis bandicota? | Bandicoot rats (Bandicota indica) | |
| Hepatozoon sp. BV2 | AY600625 | Ticks: Ixodes ricinus? | Bank vole (Clethrionomys glareolus) | |
| Hepatozoon sp. BV1 | AY600626 | |||
| Hepatozoon magna 16A7_6 | HQ224960 | Unknown, presumed to be a culicine mosquito | Edible frog (Pelophylax kl. esculentus) | |
| Hepatozoon catesbianae | AF130361 | Culex territans and other Culex spp. | Bullfrog (Rana catesbeiana) | |
| Hepatozoon cf catesbianae A_4 | HQ224954 | |||
| Hepatozoon cf clamatae clone B1 | HQ224962 | Green frog (Rana clamitans) | ||
| Hepatozoon cf clamatae clone B2 | HQ224963 | |||
| Haemogregarina balli SAS_1 | HQ224959 | Apicomplexa; Conoidasida; Coccidiasina; Eucoccidiorida; Adeleorina; Haemogregarinidae | Glossiphoniid leeches: Placobdella parasitica, P. ornata | Common snapping turtle (Chelydra serpentina) |
| Babesiosoma stableri Hem_B_7 | HQ224961 | Apicomplexa; Conoidasida; Coccidiasina; Eucoccidiorida; Adeleorina; Dactylosomatidae | Glossiphoniid leech: Desserobdella picta | Ranid Frogs: Rana septentrionalis, Rana clamitans, Rana catesbeiana |
| Dactylosoma ranarum 1A2_2 | HQ224957 | Glossiphoniid leech: Desserobdella picta (experimental) | Edible frog (Pelophylax kl. esculentus) | |
| Dactylosoma ranarum 1B1_6 | HQ224958 | |||
| Hemolivia mariae Flinders1 | JN211118 | Eucoccidiorida; Adeleorina; Karyolysidae | Ticks (Amblyomma limbatum) | Blue-tongued skink (Tiliqua rugosa) |
| Adelina bambarooniae Ba3:4 | AF494058 | Apicomplexa; Conoidasida; Coccidiasina; Eucoccidiorida; Adeleorina; Adeleidae. | Greyback cane grub (Dermolepida albohirtum) | n/a |
| Adelina bambarooniae Ba3:5 | AF494059 | n/a | ||
| Adelina grylli | DQ096836 | Crickets: Gryllus bimaculatus | n/a | |
| Adelina dimidiata | DQ096835 | Centipedes: Scolopendra cingulata | n/a | |
| Klossia helicina Clone 2_6 | HQ224955 | Land snails: Cepaea nemoralis | n/a | |
| Klossia helicina Clone 4_3 | HQ224956 | n/a | ||
| Cryptosporidium wrairi | AF115378 | Apicomplexa; Conoidasida; Coccidiasina; Cryptosporidiidae | Guinea pig (Cavia porcellus) | n/a |
| Cryptosporidium meleagridis | AF112574 | Birds, (Turkeys) | ||
| Cryptosporidium felis | AF108862 | Felines (Cat) | ||
| Cryptosporidium parvum | AF112571 | Mammals (Mouse) | ||
| Cryptosporidium parvum | AF108864 | Mammals (Cattle) | ||
| Cryptosporidium parvum | AF108865 | Homo sapiens | ||
| Cryptosporidium sp. K1 | AF108860 | Koala Phascolarctos cinereus | ||
| Cryptosporidium sp. EGK3 | AF513227 | Kangaroo Macropus giganteus | ||
| Aggregata octopiana | DQ096837 | Apicomplexa; Conoidasida; Coccidiasina; Aggregatidae | Octopi: Octopus vulgaris | Prawns: Palaemon elegans |
| Aggregata eberthi | DQ096838 | Cutllefish: Sepia officinalis | Prawns: Portunus depurator | |
| Goussia janae | AY043206 | Apicomplexa; Conoidasida; Coccidiasina; Eimeriidae | Common Dace (Leuciscus leuciscus) | n/a |
| Goussia metchnikovi | FJ009244 | Gudgeons: Gobio gobio | n/a | |
| Goussia noelleri | FJ009241 | Agile frog (Rana dalmatina) | n/a | |
| Goussia neglecta | FJ009242 | Edible frog (Pelophylax kl. esculentus) | n/a | |
| Goussia sp. Bufo | FJ009243 | Common toad (Bufo bufo) | n/a | |
| Choleoeimeria sp. | AY043207 | Diadem snake (Spalerosophis diadema) | n/a | |
| Isospora robini | AF080612 | American robin (Turdus migratorius) | n/a | |
| Eimeria falciformis | AF080614 | Mice | n/a | |
| Eimeria nieschulzi | ENU40263 | Mice | n/a | |
| Eimeria acervulina | U67115 | Chickens | n/a | |
| Eimeria tenella | U67121 | Chickens | n/a | |
| Cyclospora sp. | U40261 | Humans | n/a | |
| Cystoisospora belli | DQ060674 | Apicomplexa; Conoidasida; Eucoccidiorida; Eimeriorina; Sarcocystidae | Humans | n/a |
| Cystoisospora ohioensis | AF029303 | Canines | n/a | |
| Cystoisospora suis | U97523 | Pigs | n/a | |
| Frenkelia glareoli | AF009245 | Voles: Clethrionomys glareolus | n/a | |
| Frenkelia microti | AF009244 | Voles: Microtus arvalis | n/a | |
| Toxoplasma gondii | EF472967 | Felines | Rodents | |
| Neospora sp. BPA1 | U17345 | Canines | Cattle | |
| Neospora caninum | AJ271345 | |||
| Sarcocystis mucosa | AF109679 | Carnivorous marsupials | Marsupials | |
| S. lacertae | AY01511 | Smooth snake (Coronella austriaca) | Wall lizard (Podacris muralis) | |
| S. scandinavica | EU282032 | Canines | Moose (Alces alces) | |
| S. rangi | EF056011 | Foxes: Vulpes sp. | Reindeer (Rangifer tarandus) | |
| S. grueneri | EF056010 | Unknown | ||
| S. rangiferi | EF056016 | Unknown | ||
| Sarcocystis sp. (strain HM050622) | AB257157 | Unknown | Sika deer (Cervus nippon) | |
| Syncystis mirabilis | DQ176427 | Apicomplexa; Conoidasida; Gregarinasina; Neogregarinorida; Syncystidae | Water scorpions: Nepa cinerea | n/a |
| Mattesia sp. SV–2003 | AY334569 | Apicomplexa; Conoidasida; Gregarinasina; Neogregarinorida; Lipotrophidae | Fire ants: Solenopsis invicta | |
| Mattesia geminata | AY334568 | Fire ants: Solenopsis geminata | ||
| Ascogregarina taiwanensis | DQ462454 | Apicomplexa; Conoidasida; Gregarinasina; Eugregarinida; Lecudinidae | Culicine mosquitoes | |
| Ascogregarina armigerei | DQ462459 | |||
| Ascogregarina culicis | DQ462457 |
- a For ranks above orders, taxonomic positions are based on Adl et al. 2005;. Lower level taxonomic ranks are based on Levine (1988).
- b For parasitic species, the definitive host is that species in which gametes are formed and syngamy occurs.
- c For parasitic species, the intermediate host is a required participant in the life cycle of the parasitic species, but in which only asexual development and/or replication occurs.
PAUP Ver. 4.10b (Swofford 2001) was used to analyze the dataset using both maximum likelihood (ML) and maximum parsimony (MP) criteria. Bayesian analysis was performed using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) on the same dataset. Nucleotide substitution models used as a priori assumptions for the ML and Bayesian analyses were generated using MrModeltest 2.3 (Nylander 2004). The model and parameters generated using MrModeltest for each dataset were used for all ML and Bayesian analyses performed on each dataset. All Bayesian analyses used the following parameters: number of generations = 2 × 106; number of runs = 2; sample frequency = 1,000; burnin value = 100,000; number of chains = 4; and temp = 0.20.
For all MP analyses, characters were considered unordered and changes were given equal weighting. During all MP analyses, bootstrap analyses of 100 replicates were performed using the heuristic search algorithm within PAUP. Strict consensus trees generated from all analyses were visualized using Treeview (Page 1996).
Results
Parasite sequences
Twelve complete or partial 18S rDNA sequences from adeleorinid parasites were obtained as follows (length in bp; GenBank accession number): Haemogregarina balli SAS_1 [1,817 bp; HQ224959], Babesiosoma stableri [1,649 bp; HQ224961], Dactylosoma ranarum Clone 1A2_2 [1,810 bp; HQ224957], Dactylosoma ranarum Clone 1B1_6 [1,810 bp; HQ224958], Hepatozoon magna 16A7_6 [1,817 bp; HQ224960], Hepatozoon cf catesbianae A_4 [1,810 bp; HQ224954], Hepatozoon sipedon QUBS-1994 [1,830 bp; JN181157]; Hemolivia mariae Flinders1 [884 bp; JN211118] Hepatozoon cf clamatae Clone B_1 [1,655 bp; HQ224962], Hepatozoon cf clamatae Clone B_2 [1,655 bp; HQ224963], Klossia helicina Clone 2_6 [1,799 bp; HQ224955], and Klossia helicina Clone 4_3 [1,799 bp; HQ224956]. The two K. helicina clones varied at nine positions, the two D. ranarum clones varied at five positions and the two H. cf. clamatae clones varied at three positions.
Phylogenetic analyses
Initial analyses included a wide range of taxa to establish the monophyly of the adeleorinid taxa among the 85 apicomplexan eukaryotes included in the analysis (see Table 1). For this dataset containing near full-length 18S rDNA sequences of ~ 1,800 bp from a wide range of apicomplexan taxa, likelihood settings from best-fit model was General Time Reversible plus Gamma variation plus Invariant Proportion (GTR+I+G) selected by hLRT in MrModeltest. The alignment totalled 3,906 bp in length, including spaces to permit staggered alignment (see Barta 1997). Of these 3,906 characters, all of which had equal weight, 1,941 characters were invariant, 817 characters were parsimony uninformative, and 1,148 characters were parsimony informative. Both ML and MP gave similar tree topologies with only minor variation in the arrangements of closely related terminal taxa. The MP consensus tree length was 5,170 with a consistency index (CI) of 0.61, a CI excluding uninformative characters of 0.506, and a retention index of 0.805 (tree not shown). Bayesian analysis was performed using the following parameters: Lset Base = (0.2626 0.1770 0.2174), Nst = 6, Rmat = (1.1395 3.6900 1.4327 0.9773 4.0754), Rates = gamma, Shape = 0.3682, Pinvar = 0.0842. In all analyses, monophyly of the 25 adeleorinid taxa included in the analysis was strongly supported with bootstrap support of 94% and 100% for MP and ML, respectively, and a posterior probability of 1.0 (Fig. 1).
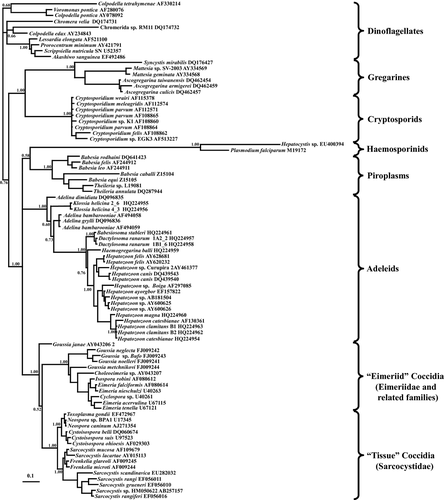
For more detailed analysis of the adeleorinid ingroup, 79 haemogregarine sequences were rooted by six sequences from monoxenous adeleorinid parasites (i.e. species of Adelina and Klossia in the Family Adeleidae) that acted as a functional outgroup. MrModeltest suggested the general time reversible with gamma rate of distribution model as best for the data using the following parameters: Lset Base = (0.3054 0.1697 0.2217), Nst = 6, Rmat = (1.1072 4.6577 1.6225 0.7601 4.0870), Rates = gamma, Shape = 0.7973, Pinvar = 0.3680. Of the 1,859 characters of equal weights in the dataset, 1,272 characters were invariant, 215 characters were variable, but parsimony uninformative, and 372 characters were parsimony informative. All three phylogenetic analysis methods (ML, MP, and Bayesian analyses) provided identical tree topologies. The MP tree length was 1,075 with a CI of 0.70, a CI excluding uninformative characters of 0.60, and a retention index of 0.89 (tree not shown). MrBayes settings for the best-fit model (GTR+I+G) were selected by hLRT in MrModeltest. The tree generated using Bayesian analysis is shown (Fig. 2).
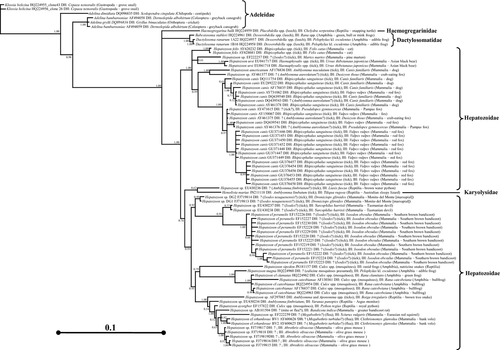
The haemogregarine taxa included in the ingroup analysis together formed a monophyletic group with strong branch support for a trichotomy that consisted of: (i) Haemogregarina balli; (ii) three sequences from the family Dactylosomatidae (D. ranarum and B. stableri); and (iii) a large clade consisting of all Hepatozoon sp. sequences (i.e. family Hepatozoidae) as well as the single Hemolivia mariae sequence in the family Karyolysidae. The genus Hepatozoon was found to be paraphyletic if the genus Hemolivia is considered valid.
Within the strongly supported clade of Hepatozoon species and the single Hemolivia sp. described above, there was a large basal clade with high posterior probability (1.00) that included a number of Hepatozoon species that infect carnivores and that use ixodid ticks as definitive hosts, including Hepatozoon canis, Hepatozoon americanum, Hepatozoon ursi, Hepatozoon felis, and an unnamed Hepatozoon sp. (EF222257) from the pine marten, Martes martes. The next clade, with 0.62 posterior probability, was an unnamed Hepatozoon sp. (EU430234) from a brown water python, Liasis fuscus, that was a sister taxon to Hemolivia mariae; this monophyletic clade was supported in all analyses. The next clade, with 0.92 posterior probability, included parasites from marsupials from Australia and the Americas; the suspected, but not demonstrated, definitive hosts for all these Hepatozoon spp. are ixodid ticks. The next clade, with 0.79 posterior probability, contained Hepatozoon species infecting culicine mosquitoes and either the blood of frogs, such as Hepatozoon clamatae and Hepatozoon catesbianae, or the viscera of frogs as the first intermediate host and the blood of snakes as the second intermediate host such as H. sipedon. The final large clade of Hepatozoon spp., with 1.00 posterior probability, included Hepatozoon spp. that infect squamates or rodents and that are transmitted by a wide range of arthropod definitive hosts (Fig. 2 and Table 1).
Discussion
Classical systematics of the Apicomplexa has been problematic because of the variability to which morphological details are subjected and due to the lack of extensive molecular phylogenetic studies of individual groups within the phylum. In the present study, using 18S rDNA sequences and a variety of phylogenetic reconstruction methods, we were able to demonstrate that the monoxenous adeleorinid coccidia (Adeleidae) and heteroxenous haemogregarines (i.e. taxa in the families Hepatozoidae, Karyolysidae, Haemogregarinidae, and Dactylosomatidae) form a well-supported, monophyletic clade of apicomplexan parasites. All of the parasites within the suborder Adeleorina are united biologically by demonstrating syzygy during their life cycle (Barta 2000). The gregarines (Gregarinia) and piroplasms (Piroplasmida such as Babesia and Theileria species, likewise share this biologic feature suggesting that syzygy may be an ancestral feature of apicomplexan protists. Biologically, syzygy may be a means of ensuring availability and proximity of mature male and female gametes during infections that are not otherwise synchronized (e.g. gregarine development in the digestive tracts of invertebrates).
The high posterior probability (1.0) of the sister group relationship between the gregarines and cryptosporidia (bootstrap support of 57% and 93% by MP and ML, respectively) was higher than in some previous analyses that found no bootstrap support for the Cryptosporidium and gregarine node using ML (Perkins and Keller 2001) or lower support for this node (e.g. 64% bootstrap support using MP) obtained by Carreno et al. (1999). Having confirmed monophyly of the adeleorinid coccidia among a variety of apicomplexan taxa, the monoxenous adeleorinid sequences were used as a functional outgroup for a more detailed analysis of the heteroxenous adeleorinid parasites, the haemogregarines.
The ingroup analysis, that included all available sequences from adeleorinid parasites greater than 799 bp in length, demonstrated that the current taxonomic groups found within the blood-dwelling haemogregarines were reasonably well-supported. The haemogregarines include at least 400 described species belonging to four principal genera (Jakes et al. 2003): Haemogregarina Danilewsky, 1885, Karyolysus Labbé, 1894, Hepatozoon Miller 1908; and Cyrilia Lainson, 1981. A fifth haemogregarine genus, Hemolivia, was described by Petit et al. (1990) to acknowledge the unique stellate oocyst formed by the type species Hemolivia stellata, and the sixth genus, Desseria, was erected by Siddall (1995) to accommodate piscine haemogregarines that lacked intraerythrocytic merogony. Although originally thought to be most closely related to the piroplasms but now conclusively demonstrated to be small haemogregarines with intraerthrocytic merogony, species of Dactylosoma Labbé, 1894 and Babesiosoma Jakowski and Nigrelli, 1956 in the family Dactylosomatidae infect, where known, glossiphoniid leeches as definitive hosts and are transmitted during blood feeding to various aquatic intermediate hosts, such as amphibia and fish (Barta 1991). Until recently, many haemogregarine species, in particular members of the families Haemogregarinidae, Karyolysidae or Hepatozoidae, have been misclassified taxonomically because of a necessary reliance on morphology of gamonts in the blood of their vertebrate intermediate hosts, and their associations with these hosts, as principal taxonomic criteria (Siddall 1995; Zhu et al. 2009). In all cases, assignment of any haemogregarine to the correct genus ultimately requires knowledge of the nature of the sexual reproduction of the parasite in its definitive host (a blood-feeding invertebrate, where known).
The present study suggests that 18S rDNA sequences may be useful adjuncts for identifying generic affiliation of a particular parasite without prior knowledge of the sporogonic development of these parasites. Hemolivia and Hepatozoon species, united by transmission that occurs via the ingestion of the definitive host, together formed a monophyletic group in the ingroup analysis, indicating that 18S rDNA sequences also support this as a taxonomic grouping. To our knowledge, the sporogonic development of the Hepatozoon sp. isolated from the brown water python Liasis fuscus that formed a monophyletic clade with Hemolivia mariae has not been observed. If the sister relationship of this Hepatozoon sp. is accurate, this water python parasite may actually belong to the genus Hemolivia. Hemolivia mariae is transmitted by ticks of the genus Amblyomma, and ticks of the same genus are probably definitive hosts for at least one “Hepatozoon” sp. that apparently infects both the water python, Liasis fuscus, and the Argus monitor lizard, Varanus panoptes, in northern Australia based on 18S rDNA sequence data from the reptiles and the ticks (Ujvari et al. 2004; Vilcins et al. 2009).
The formation of relatively large polysporocystic oocysts by Hepatozoon, Klossia, and Adelina species appears to be a symplesiomorphic character. Haemogregarines that undergo sporogonic development within cells lining the digestive tract of leeches without the formation of a resistant oocyst structure, such as Haemogregarina, Dactylosoma, and Babesiosoma species, were shown to cluster together in the present ingroup analysis as two arms of a trichotomy with the large clade of Hepatozoon and Hemolivia species. Only a single member of the family Haemogregarinidae, Haemogregarina balli, was available for study and consequently insufficient taxon sampling probably contributed to our inability to determine the branching order among the members of this trichotomy. If formation of large polysporocystic oocysts is truly symplesiomorphic, the large clade of Hepatozoon and Hemolivia species would be expected to branch basally to the remaining haemogregarines in the analysis.
The ingroup analysis suggests a possible evolutionary history for the group: the ancestral form of the adeleorinid parasites was probably similar to the monoxenous Adelina and Klossia species, with the formation of large, resistant, polysporocystic oocysts. These parasites probably infected initially the digestive tract and later exploited deeper tissues in their definitive hosts. The heteroxenous Hepatozoon spp. retained these resistant, polysporocystic oocysts and depended on the ingestion of their hosts for transmission. Hemolivia and Karyolysus species, the latter which were not sampled in the present work, do not form resistant oocysts; instead, sporozoites within oocysts of Hemolivia and Karyolysus further exploited the tissues of their definitive hosts by initiating a round of merogonic development. However, these species still depend on ingestion of their definitive host, as infective sporozoites or merozoites have not invaded the salivary glands to permit transmission during blood feeding. Ultimately, as parasites in the families Haemogregarinidae and Dactylosomatidae evolved to be transmitted via the bite of their definitive hosts and vectors, such resistant oocysts became unnecessary. In these latter families, sporozoites initiate a round of merogony within the tissues of their leech definitive host; subsequently, merozoites move into the tissues of the definitive host, ultimately infect the salivary glands, and are transmitted to the next susceptible vertebrate host during blood feeding. Additional taxon sampling may provide support for this proposed evolutionary scenario.
Within the large clade of Hepatozoon and Hemolivia species, there was a relatively high degree of host-parasite association of various species with their definitive hosts, a pattern that is observed among most groups of Apicomplexa (see Barta 1989). Species of three clades at the base of this large group use ixodid ticks as definitive hosts. Species in the first of these clades, containing H. canis, H. americanum, H. ursi, H. felis, and an unnamed Hepatozoon sp. from the pine marten, utilize species of various genera of ixodid ticks as definitive hosts, whereas species in the second of these clades, consisting of an unnamed Hepatozoon species from the brown water python and Hemolivia mariae from the Australia sleepy lizard, strictly use Amblyomma species, and species in the third of these clades, comprised of species infecting marsupial mammals, appear only to use Ixodes species for this purpose. Of the two more highly derived clades of Hepatozoon species, species of the first, which infect the blood or viscera of frogs, are transmitted among vertebrate hosts by culicine mosquitoes, whereas species of the second, which infect snakes or rodents, are transmitted by a wide range of arthropods, including ixodid ticks, mites, fleas, and mosquitoes. In a phylogenetic analysis of adeleorins based on morphological and developmental characters (Smith et al. 2000), all eight Hepatozoon species that use mosquitoes as definitive hosts grouped as a clade. This group included H. sipedon from frogs and snakes and H. catesbianae from frogs, species that are analyzed in the current study, along with six species infecting the blood of snakes and lizards (i.e. Hepatozoon gracilis, Hepatozoon aegypti, Hepatozoon domerguei, Hepatozoon rarefaciens, Hepatozoon moccassini and Hepatozoon breinli), for which 18S rDNA data are still lacking. Thus, it is not clear to which of the two clades of Hepatozoon species containing species infecting mosquitoes these species belong: the one containing H. sipedon, H. catesbianae, and H. clamatae, or the one containing H. ayorgbor, which also infects the blood of snakes. Strict coevolution of definitive hosts and parasite was therefore not observed in this group, but this lack of resolution may be addressed through obtaining DNA sequence data from more species of these parasites and, perhaps, using a more quickly evolving alternative gene target, such as cytochrome c oxidase subunit I (COI). COI, a mitochondrial protein-coding gene, was recently shown to be equal to and perhaps better than 18S rDNA sequences for defining clades among closely related coccidian parasites (Ogedengbe et al. 2011).
The results of the present study reinforce the idea suggested by Siddall (1995) and Smith et al. (2000) that the genus Hepatozoon is paraphyletic, and that these parasites may be more properly assigned to a number of distinct genera, each of which would be more uniform biologically. In a phylogeny of adeleorins based on phenotypic features (Smith et al. 2000), Hepatozoon and Hemolivia species comprised three separate and successive branches of a monophyletic group containing haemogregarines, with the fourth, most derived, lineage containing those haemogregarines transmitted by the saliva of leeches. Species in each of the three branches of Hepatozoon and Hemolivia species differed with regard to location of sporogonic development and the presence or absence of merogony in the arthropod definitive host. In the current study, Hemolivia species, along with the incompletely described haemogregarine from the brown water python, form a well-supported clade well within the larger clade of Hepatozoon species. This suggests that sporogonic development in the arthropod gut, rather than the hemocoel, and a further round of asexual development following sporogony, both characteristics of Hemolivia species, may in fact have evolved independently within a lineage of Hepatozoon species rather than representing an intermediate mode of development between Hepatozoon species and the more derived, saliva-transmitted haemogregarines. According to the present analysis, the genus Hepatozoon remains paraphyletic, with any attempt to assign a new genus name to species of the basal clade (i.e. those infecting carnivores) or the three most derived clades (i.e. those that infect marsupials, frogs, and rodents and snakes, respectively) confounded by the fact that DNA sequence data are lacking for H. muris, the type species of the genus described by Miller (1908).
Life cycles of Hepatozoon species are the most complex of all the haemogregarines, and the current phylogenetic analysis allows some speculation on their evolution. In transmission experiments of H. domerguei (see Landau et al. 2007) and H. sipedon (see Smith et al. 1994), mosquitoes containing oocysts were fed to lizards or frogs, respectively, in which tissue cysts were observed, and these first intermediate hosts were then fed to snakes, in which bloodstream gamonts were observed. The life cycle of H. ayorgbor, which infects the blood of pythons and the haemocoel of culicine mosquitoes, is probably as complex, although the first intermediate host in which cystic development occurs has not yet been found (Sloboda et al. 2007). Complete life cycle data and additional DNA sequences from more species of Hepatozoon are required to more precisely hypothesize the evolution of life cycles for these species. Such information would enable determination, for example, if the three-host life cycle of H. sipedon in mosquitoes, viscera of frogs, and blood of snakes has evolved from the simple two-host life cycle of H. catesbianae or H. clamatae in mosquitoes and the blood of frogs. If the reverse were found to be true, Hepatozoon species may evolve toward simpler life cycles in the same manner as many lineages of trematodes (Poulin and Cribb 2002).
Acknowledgments
This work was supported by a Discovery Grant to JRB from the Natural Sciences and Engineering Research Council of Canada. Susan Kopko is thanked for her assistance with cloning some of the genes reported in this article.




