Pneumocystis carinii Synthesizes Four Ubiquinone Homplogs: Inhibition by Atovaquone and Bupravaquone but not by Stigmatellin
The formation of ubiquinone (coenzyme Q, CoQ) involves two separate biosynthetic pathways. The polyprenyl chain is formed by the isoprenoid pathway and the benzoquinone ring is derived from the shikimate-chorismate pathway. The direct precursor p-hydroxybenzoic acid (PHBA), which is produced from chorismate, condenses with a polyprenyl diphosphate, then after several reactions in which the ring moiety is modified, the completed CoQ molecule is formed. We initially identified CoQ10 as the major homolog in P. carinii using high performance liquid chromatographic (HPLC) separations and UV detection; CoQ9 was also present [2]. While CoQ9 was the major homolog in the lungs of normal, untreated rats and the lungs of corticosteroid-treated, uninfected rats, CoQ10 was not detected, suggesting that at least CoQ10 was synthesized by the pathogen. In more sensitive metabolic radiolabeling experiments, we showed that P. carinii also synthesizes CoQ8 and CoQ7 [6]. Furthermore, all four CoQ homologs became radioactive when intact organisms were incubated with radiolabeled precursors of the polyprenyl chain (mevalonate) or the benzoquinone ring (shikimic acid, PHBA, or tyrosine) indicating that P. carinii has the metabolic capacity to synthesize de novo both moieties of ubiquinone [6].
Atovaquone, an analog of ubiquinone, is effective in moderate cases of Pneumocystis pneumonia (PcP). The drug binds to the mitochondrial cytochrome bc1 complex resulting in the inhibition of electron transport, cellular respiration, and ATP production in P. carinii. [I]. However, we observed that when intact organisms were treated with only 10 nM atovaquone, the biosynthesis of CoQ was also dramatically inhibited, and a complex triphasic response occurred as drug concentration was increased [3]. To better understand the action of atovaquone on P. carinii, the incorporation of PHBA into CoQ was examined using cell-free homogenates and a number of different naphthoquinone compounds and other inhibitor compounds were tested for their effects on CoQ biosynthesis.
METHODS AND MATERIALS
Pneumocystis carinii was isolated from the lungs of coriticosteroid-immunosuppressed, viral antibody-negative female Lewis rats that had been intratracheally infected with cryopreserved organisms. Inocula used to infect the experimental animals were shown to be negative for bacteria and fungi. Organisms were purified by our protocol [4] were previosuly shown to be >95% to 100% pure by several light and electron microscopic analyses and immunochemical, biochemical, and microbiological assays. Controls were the lungs from normal, untreated rats and the lungs from corticosteroid-treated, uninoculated rats. Homogenates of excised lungs were prepared following the same procedures used for P. carinii organisms.
Cell-free homogenates of freshly isolated P. carinii were prepared by disruption using a French pressure cell and a series of freeze-thaw cycles. Subcellular fractions were prepared by differential centrifugation. The whole cell homogenates and subcellular fractions were incubated in a buffered salt solution (pH 7.4) containing radiolabeled PHBA. Incubations were performed at 37 °C for 1 min, and reactions were terminated by the addition of chloroform and methanol, which also initiated lipid extraction.
Total Ubiquinones were isolated by thin-layer chromatography and individual CoQ homologs were separated by HPLC. Radioactivity in the HPLC fractions was quantified by liquid scintillation spectroscopy, and the incorporation of PHBA into total P. carinii CoQ or individual homologs were expressed in pmol/mg protein/min.
RESULTS AND DISCUSSION
No radioactivity was detected in CoQ when either control lung tissue (lungs from normal rats or corticosteroid-treated, uninoculated rats) were incubated with radiolabeled PHBA, confirming our previous report [6]. Thus, incorporation of PHBA into CoQ represented metabolic activity in P. carinii and not by potential residual enzymes from host rat lungs.
The cell-free system provided much higher rates of PHBA incorporation into CoQ because the problem of slow uptake of this compound by P. carinii was eliminated (Table 1). However, under the cell-free conditions normal regulatory mechanisms and signalling events between different cell compartments are disrupted.
| pmol/mg protein/min | ||
|---|---|---|
| CoQ homolog | Intact organisms | Cell-free homogenates |
| CoQ7 | 3.0 × 10−3 | 364 |
| CoQ8 | 3.2 × 10−3 | 381 |
| CoQ9 | 2.7 × 10−3 | 405 |
| CoQ10 | 1.2 × 10−3 | 431 |
As expected, the complex response to changes in atovaquone concentration in intact organisms was not observed using cell-free homogenates. Several other ubiquinone analogs (Fig. 1) had inhibitory activity against CoQ biosynthesis, whereas others did not. The most potent inhibitor was bupravaquone. Interestingly, Stigmatellin, a compound that has been shown to bind to the mitochondrial cytochrome bc1 complex of other organisms by similar stereochemical interactions as atovaquone, did not inhibit PHBA incorporation into P. carinii CoQ. Thus, while both drugs inhibit mitochondrial electron transport, one inhibits ubiqinone biosynthesis whereas the other does not. Further structure-activity studies comparing these two drugs on the biosynthetic machineries of the organism are needed to determine what structural features are critical for inhibition of CoQ synthesis. The mitochondrial respiration inhibitors antimycin and DCCD did not inhibit CoQ biosynthesis, indicating the inner mitochondrial proton gradient did not play a role in CoQ biosynthesis.
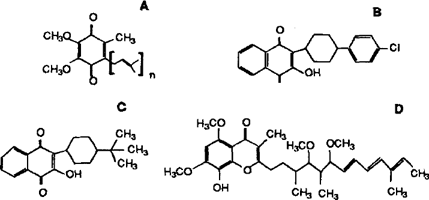
Structures of ubiquinone and some analogs tested for their effects on the incorporation of PHBA into P. carinii CoQ. A. Ubiquinone. Homolog designations are based on the number of 5-carbon isoprene units in the polyprenyl chains.B. Atovaquone. C. Bupravaquone. D. Stigmatellin.
Kinetic analysis indicated that the mechanism of atovaquone action was by competitive inhibition, but greater than 60% inhibition by this or any other compound tested was not observed in intact cells or cell-free homogcnates. These results suggested that CoQ biosynthesis was not restricted to the mitochondria, as demonstrated in other cell types, and that atovaquone may not have the same effects on components present in different cell compartments.
Preliminary results on subcellular fractions indicated that all four CoQ homologs were synthesized in two separate fractions. Drug resistance is becoming of serious concern, and there is evidence for the development of atovaquone resistance among some human pathogens, including P. jiroveci (P. carinii f. sp. hominis) [5,7]. Although heterogeneity in the nucleotide sequence of the P. jiroveci cytochrome b gene occurs among different isolates of the organism [7], patients with prior exposure to the drug have a higher percentage of mutations of the cytochrome b gene in regions coding for ubiquinone binding sites of the predicted expressed peptide [5]. In some cases, atovaquone treatment failure was not correlated with cytochrome b mutations [7].
Our findings demonstrate that atovaquone inhibits two cellular processes in P. carinii; mitochondrial electron transport and CoQ biosynthesis. Also, there are two distinct cellular compartments in which all four CoQ homologs are synthesized. These observations suggest that the development of atovaquone resistance in this organism might be more complex than only cytochrome b gene mutations.
ACKNOWLEDGMENTS
Supported in part by PHS grant RO1 AI29316.




