Urokinase Plasminogen Activator and TGF-β production in Immunosuppressed Patients with and without Pneumocystis carinii
Pneumocystis carinii infection is the most frequent complication in AIDS patients and alveolar macrophages (AM) play a significant role in the clearance of P. carinii from the lung by binding, phagocytizing and degrading the offending organism [5]. Several studies indicate that urokinase plasminogen activator (uPA) and transforming growth factor β (TGF-β) are important factors in host defense against pulmonary pathogens. Urokinase PA is a serine protease that converts plasminogen to the active enzyme plasmin, and appears to play a role in the normal functioning of the immune response. In fact, using transgenic mice lacking the uPA gene it has been demonstrated that uPA is required for the pulmonary inflammatory response to P. carinii [4]. TGF-β is secreted by different cell types as an inactive complex and then activated by proteolysis with release of active TGF-β from the complex. Activated mouse peritoneal macrophages are capable of activating endogeneous latent TGF-β when stimulated with LPS: this process depends upon the presence of uPA and plasmin. Active TGF-β, in turn, reduces uPA production and induces PAI production [7]. Several evidences suggest a role for TGF-β in HIV replication [8] and P. carinii clearance [9]. Therefore the aim of this study was to assess the production of uPA and TGF-β in HIV+ and immunodepressed patients with and without P. carinii.
MATERIALS AND METHODS
Thirty-one subjects were divided into 5 groups. Ten healthy volunteers with no past history of lung disease or symptoms suggesting viral infections in the last three months, as controls. Ten HIV+ patients, 7 without (H+PC) and 3 with (HIV+PC+) P. carinii pneumonia (PCP). Eleven immunosuppressed patients, 8 without (IS PC−) and three with (IS PC+) PCP. Peripheral blood monocytes (PBM) obtained from defibrinated venous blood of patients were isolated by centrifugation with Ficoll-Paque and cultured at a density of 2.5 × 106 monocytes/ml in DMEM. Cells were allowed to adhere to the dish in the presence of 5% PCS and, 24 h later, non-adherent cells were washed off with medium. After further 20 h of culture, conditioned medium was collected and cells lysed in 0.5% Triton X-100. PA activity in conditioned media and cell lysates was assayed by a chromogenic substrate assay [2]. For phase separation in Triton X-l 14, cells were solubilised in 3% Triton X-l14 and after 3 min at 37°C, they were centrifuged for 15 min at 12,000 xg. Aliquots of total cell lysate before phase separation (Total), of the upper (Aqueous) and the lower (Detergent) phases were analysed by SDS-PAGE and zymography [6]. Active TGF-β was assayed by adding PBM conditioned medium to sub-confluent MLECs transfected with a PA inhibitor-1 promoter luciferase construct [1]. In order to measure total TGF-β latent TGF-β was activated by treating the medium at 85°C for 5 min. After 16 h of culture MLECs were lysed in a lysis buffer and the luciferase activity measured with an assay kit obtained from Promega.
RESULTS AND DISCUSSION
PA production by PBM obtained from the different groups of patients was determined by chromogenic substrate assay in cell lysates and conditioned media. Similarly to what was shown with alveolar macrophages (AMs) [3], uPA activity was found mainly in cell lysates. The highest levels of PA activity were found in healthy subjects. In contrast, all HIV+ and IS patients showed significant decreases in PA activity independent from the presence of P. carinii pulmonary infection (Table 1). However, it is interesting to note that, although all HIV+ and IS patients showed decreased production of uPA, in patients with PCP, uPA activity was still below normal but higher than in other patients. This increase appears to be related to the presence of P. carinii infection. Indeed, in a previous work [3] we observed a significant increase in uPA production by PBMs challenged in vitro with P. carinii.
| Group | uPA activity | active TGF-β |
|---|---|---|
| Healthy | 35.1 2.8 | 102 4.5 |
| H+PC− | 12.1 3.0* | 47 7.2* |
| H+PC+ | 20.6 3.7* | 35 3.0* |
| IS PC | 7.4 1.6** | 59 2.9* |
| IS PC+ | 17.9 4.6* | 44 1.9* |
- *P<0.05 and **P<0.01 vs. healthy subjects.
These results are in accord with our previous data showing impairment in uPA production in AMs from IS and HIV+ patients [3]. The decreased production of uPA can lead to several macrophage disfunctions. In fact, among other effects, uPA and plasmin regulate the release and the activation of several cytokines involved in inflammatory reaction such as TGF-β [7]. Moreover, it has been demonstrated that latent TGF-β activation is promoted by the surface localization of uPA by its receptor [7]. This observation prompted us to investigate the cellular distribution of uPA activity in PBM. Enzyme localization was analyzed by detergent phase partitioning, based on the heat-induced separation of Triton X-l14 solution into two phases: aqueous and detergent phase. Both phases were analyzed by SDS-PAGE and zymography. Approximately 70% of uPA partitioned into the detergent phase, which is typical of membrane-associated proteins (Fig. 1). This suggests that the uPA molecules are present mainly bound to their receptors, where they can activate latent TGF-β.
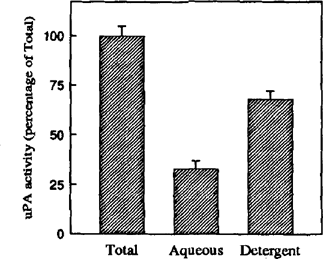
Phase separation of PA activity in PBM obtained from healthy subjects. Aliquots of the complete sample before phase separation (Total), and the upper (Aqueous) and lower (Detergent) phases were analyzed by zymography. Urokinase PA activity was by densitomctric scanning of zymography. Data represent the mean SEM of three independent experiments.
To verify whether a decrease in uPA activity could affect latent TGF-β activation, we measured the levels of active and total TGF-β in PBM conditioned media. Active TGF-β was higher in control subjects and decreased in HIV+ and IS patients (Table 1). Also, a further decrease was observed in patients affected by PCP. In contrast, no differences were observed among the different groups when total TGF-β was assayed (data not shown). This suggests that the decrease in TGF-β activity is not a consequence of decresed production, but of decreased activation of the latent form. The decrease in uPA found in HIV+ and IS patients may negatively affect TGF-β activation. The decrease in active TGF-β may in turn influence virus replication since it has been demonstrated that TGF-β can suppress human HIV expression in monocyte/macrophages [8]. Further studies will help to elucidate whether the decrease of uPA and of active TGF-β might play a role in the impairment of the host defense to P. carinii infection.




