Putative Transmissive Form of Pneumocystis carinii f. sp. carinii
Despite the fact that Pneumocystis species have been studied since the early 1900's (3), the life cycle of this opportunistic fungal pathogen remains poorly understood. Pneumocystis can be transmitted from host-to-host by an airborne route, as initially demonstrated in rats (5, 16). Once inhaled, the transmissive agent of Pneumocystis presumably traffics to the distal alveolar spaces of the host lung, attaches to type I pneumocytes (17), and proliferates within the lung alveoli. Genetic and histological evidence indicates sexual (10) and asexual (9, 14) reproductive stages for Pneumocystis. The infectious agent(s) and its movement to/from the alveolar spaces remain undefined.
Evidence for the existence of an airborne transmissive form of Pneumocystis lies with observations of airborne transmission (5,16), as well as with reports of Pneumocystis-specific DNA collected from air samples (1, 7, 8, 11, 15). Chin et al (4) previously reported isolation of putative dormant forms of Pneumocystis from microisolator cage filters using morphological assessment, monoclonal and polyclonal antibody labeling, and fluorescent in situ hybridization.
In the present study, we sought to identify the transmissive stage of Pneumocystis by collecting air vent and oral swab samples from a rat colony by use of magnetic beads conjugated to Pneumocystis-specific antibodies, and amplification of Pneumocystis-specific DNA regions as proof of identity.
MATERIALS AND METHODS
Oral swab and air vent sample collection
Samples were collected from air vents and rat oral cavities (6) from a rat colony at the VA Medical Center Animal Facility (room 004, Cincinnati, OH) known to harbor P. carinii f. sp. carinii (Pcc). Each sample was collected by rubbing a sterile cotton-tipped applicator (Fisher, Pittsburgh, PA) over the surfaces of the air vent or rat oral cavity. A total of 10 air vent samples and 20 rat oral cavity swabs (from 20 different rats) were collected and pooled. Pooled samples were suspended in 10 ml sterile PBS, filtered through sterile gauze to remove large particulate, then centrifuged at 200rpm to further remove particulates. The supernatant for each sample was centrifuged at 20,800xg, and the supernatant discarded. The pellet was resuspended in 2ml sterile PBS/Penicillin-Streptomycin (0.125 I.U./ml, 0.125μg/ml Cellgro, Herndon, VA) and stored at 4°C for magnetic bead purification.
Magnetic bead purification
CELLection Pan Mouse IgG Kit (Dynal, Oslo, Norway) was prepared according to manufacturer protocol. Antibodies specific to Pcc-specific Major Surface Glycoproteins (MSG) (RA-C11/G10) (13) were conjugated to the magnetic beads at a concentration of 0.5μg/107 beads. Each sample was incubated with the magnetic bead/antibodies for 2 hours with gentle rotation at 4°C, and then washed in RPMI 1640/1% FCS four times. Material bound to the magnetic beads was cleaved by incubating samples in 0.02% CELLection Releasing Buffer (DNase)/RPMI 1640/1% FCS solution (pre-warmed to 37°C) for 15 minutes with rotation at 25°C, then flushing the beads through a pipette several times. The released material was then concentrated and adhered to glass microscope slides by use of a Shandon Cytospin 2 centrifuge (Pittsburgh, PA) and stored at room temperature under desiccation for analysis.
PCR analysis
Aliquots for PCR analysis were collected before the DNase treatment in the magnetic bead procedure. DNA was extracted from 250μl of each sample by an organic phase extraction method, and then amplified using Rcc (12), DHPS, and CDC5 primer sets with a GenAmp PCR System 9700 (PE Applied Biosystems, Norwalk, CT). The Rcc primers target a region of the mitochondrial large subunit rRNA specific for Pcc; the DHPS targets the dihydropteroate synthase gene of Pcc; and the CDC5 primers target a cell cycle control gene of Pcc. The DHPS (DHPS-5′: 5′-TTC CTT TGC AAT TTC GGC-3′; DHPS-3′: 5′-CCA TGA GGA ACT TTA GTA CCA ACC-3′) and CDC5 (PcCDC5-5′: 5′-TTT GGC TTG TCG TTG TAA TC-3′; PcCDC5-3′: 5′-CCC TTG ATC AAC CCA TAG G-3′) primer sets were gifts from A.G. Smulian (University of Cincinnati College of Medicine, Department of Internal Medicine, Division of Infectious Diseases, Cincinnati, OH). Each reaction used 1 X JumpStart REDTaq ReadyMix PCR Reaction Mix (Sigma, St. Louis, MO) (lOmM Tris-HC1, 2mM MgCl2, 50mM KC1, 0.001% gelatin, 0.2mM each dNTP, 0.06 unit/μl Taq DNA Polymerase, TaqStart antibody) to which 0.05ng of primer sets, 1.0 μl template DNA, and molecular grade water were added. PCR conditions were: 94°C hot start for 2 minutes, 94°C denaturing for 30 seconds, 54°C annealing for 30 seconds, 72°C extension for 60 seconds (40 cycles total), and 72°C final extension for 10 minutes. Amplified DNA products were visualized by staining with ethidium bromide in 2% agarose gels run at 90 V for 1 hour. Gel images were captured with NIH Image 1.6 software. Each sample was scored positive for Pcc, DHPS, and CDC5 by the presence of bands migrating at 137bp, 399bp, and 315bp, respectively.
Slide staining and microscopy. Each slide was heat-fixed, stained with CEV (2), and then examined by light microscopy (Olympus BH-2, Melville, NY) at 100X under oil immersion. Images were captured with a SPOT camera (Diagnostic Instruments, Inc., Sterling Heights, MI) using SPOT Basic v. 3.0.1 software (Diagnostic Instruments, Inc, Sterling Heights, MI) and stored as digital files.
RESULTS AND DISCUSSION
PCR analysis
Oral swab and air vent samples were positive for Rcc, DHPS, and CDC5 primer sets. Figure 1 shows amplicons for each of the PCR reactions, at the expected sizes.
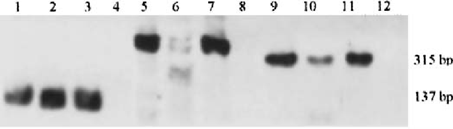
PCR amplicons for Rcc, DHPS, and CDC5 primer sets. Lanes 1–4 are amplicons from Rcc primer reactions, lanes 5–8 are from DHPS primer reactions, and Lanes 9–12 are from CDC5 primer reactions. Lanes 1, 5, and 9 are air vent samples, lanes 2, 6, and 10 are oral swab samples, lanes 3, 7, and 11 are positive controls (Pneumocystis DNA), and lanes 4, 8, and 12 are negative controls (water).
Microscopy
Air vent and oral swab samples contained entities consistent in size with forms of Pneumocystis found in the lung. Figure 2 depicts several images taken from the air vent and oral swab samples. The entity shown is ovoid in shape, ∼8–10 μm in diameter, has an intensely staining cell wall, appears to have an apical pore-like structure on one end and an internal compartment.
CEV-stained digital images of putative transmissive forms of Pneumocystis carinii collected from air vent (A-C) and rat oral swab samples (D-F). Panel B includes two magnetic beads (see arrows); panel C demonstrates the intracellular compartment (see arrow); panels D and E show apical pore-like structure (see arrows).
The data presented here provide further evidence for the existence of a transmissive form of Pneumocystis. This study showed that Pcc-specific MSG antibodies were associated with mitochondrial and nuclear DNA, suggesting that the magnetic bead assay was selecting intact organisms. Further work to define the morphology of these entities and determine their viability will be forthcoming.
ACKNOWLEDGMENT
This research was supported by NIH grant RO1 AI32436.




