Pneumocystis carinii Dihydropteroate Synthase Genotypes in HIV-infected Persons Residing in San Francisco: Possible Implications for Disease Transmission
Several studies have demonstrated a significant association between the use of sulfa or sulfone drugs for pneumonia (PCP) prophylaxis in HIV-infected persons and the presence of dihydropteroate synthase (DHPS) gene mutations identified from PCP specimens [2–5,7]. In addition to the use of these drugs for prophylaxis, Huang and colleagues found that city of residence was also an independent predictor of the risk of DHPS mutation [3]. In this study, patients residing in San Francisco (odds ratio = 5.0, 95% confidence interval = 1.5–17.2, p = 0.01) and Seattle (odds ratio = 3.2, 95% confidence interval = 1.1–9.2, p = 0.03) were more likely to present with PCP that contained a mutant DHPS genotype than patients residing in Atlanta, Intriguingly, 54% (n = 14) of the 26 patients who were diagnosed with HIV infection at the time of PCP diagnosis and, who had therefore never used PCP prophylaxis, presented with Pneumocystis that contained a mutant DHPS genotype. Furthermore, the proportion of mutant DHPS genotypes among patients newly diagnosed with HIV infection within each city paralleled the overall proportion of DHPS mutants in that city. The purpose of the current study was to determine whether residence in certain San Francisco areas was associated with the presence of mutant DHPS genotypes. We also sought to determine if those patients who were diagnosed with HIV infection at the time of PCP diagnosis and who had a mutant DHPS genotype were more likely to reside in areas of San Francisco with higher proportions of DHPS mutations.
MATERIALS AND METHODS
Subjects were HIV-infected patients diagnosed with microscopically confirmed PCP at San Francisco General Hospital from May 1997 through May 2000. Study personnel collected clinical information from chart abstraction and patient interview using standardized forms.
Clinical PCP specimens were sent to the Centers for Disease Control and Prevention. DNA was extracted using the Promega Genomic DNA Purification Kit (Promega, Madison, WI), following standard kit procedures. PCR amplification was performed at the DHPS locus using the primers and conditions described previously [1,7]. Amplified DNA fragments were analyzed by direct sequencing using Big Dye Terminator chemistry (PE Applied Biosystems, Foster City, CA) on an ABI 377 automated DNA sequencer according to the manufacturers recommendations.
For the purposes of this study, the wild type P. carinii DHPS genotype was defined as the DNA sequence observed in P. carinii specimens from other mammalian species and the most common sequence observed from humans before the 1990s. A mutant P. carinii DHPS genotype was defined as any DNA sequence that differed from the wild type sequence. Samples in which more than one DHPS genotype was detected were defined as mutant since at least one DHPS genotype was mutant.
The place of patient residence at the time of PCP presentation was mapped using MapPoint (2001 Microsoft). Statistical analysis was performed using Epilnfo version 6.0. Proportions between two groups were compared using chi-square and Fisher's exact tests.
RESULTS AND DISCUSSION
One hundred and twenty-two HIV-infected patients were diagnosed with PCP and had a San Francisco residence and a DHPS genotype available. Ninety two percent of the patients were men, 48% reported being men who have sex with other men, and 25% reported injection drug use.
Thirteen percent of the patients had a previous episode of PCP at least three months before this presentation. Thirty percent of the patients reported use of PCP prophylaxis within 30 days prior to presentation. The median CD4+ T cell count was 26 cells/uL and the median HIV RNA level was greater than 190,000 copies/mL.
Overall, 84% (n = 102) of the 122 patients had a PCP specimen that contained a mutant DHPS genotype. The single most frequent genotype seen contained an amino acid substitution at positions 55 (alanine in place of a threonine) and 57 (serine in place of a proline). This genotype was observed in 58% of the specimens (n = 71). Eighty-nine percent (n = 32) of the 36 patients who reported PCP prophylaxis use had a PCP specimen that contained a mutant DHPS genotype compared to 81 % (n = 70) of the 86 patients with no prophylaxis use (p= 0.31).
Thirty-three patients (27%) were diagnosed with HIV infection at the time of PCP diagnosis. Importantly, these patients all denied prior use of PCP prophylaxis during their interview, and medical chart abstraction failed to document prescription of prophylaxis. Not surprisingly, patients diagnosed with HIV infection at the time of PCP diagnosis were less likely to present with a PCP specimen that contained a mutant DHPS genotype compared to patients who were aware of their HIV infected status at the time of PCP diagnosis (70% versus 89%, p = 0.01).
Sixty-seven percent (n = 82) of the patients resided within an approximately 2.25 × 1.75 square mile area in San Francisco (Figure 1). Patients who resided in this area were more likely to present with a PCP specimen that contained a mutant DHPS genotype compared to patients who resided outside of this area (91% versus 68%, odds ratio = 5.2, 95% confidence interval = 1.7–16.2, p < 0.001).
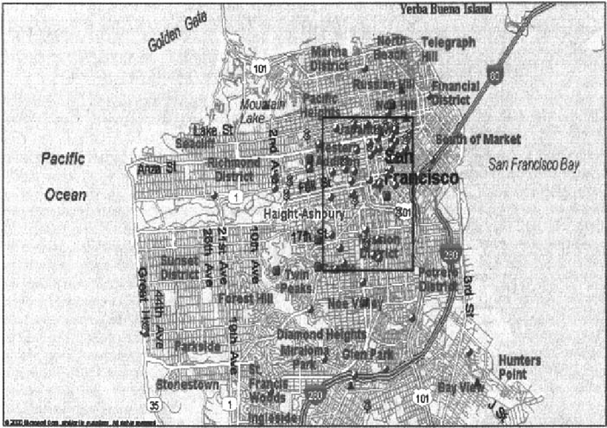
P. carinii DHPS genotypes in San Francisco. Each symbol represents the residence of a patient at the time of PCP diagnosis. Pushpins= patients who were newly diagnosed as HIV-infected who presented with a PCP specimen that contained a mutant DHPS genotype. Triangles = patients who were newly diagnosed as HIV-infected who presented with a PCP specimen that only contained the wild type DHPS genotype. Circles= patients who were aware of HIV infection who presented with a PCP specimen that contained a mutant DHPS genotype. Squares= patients who were aware of HIV infection who presented with a PCP specimen that only contained the wild type DHPS genotype.
Among the 33 patients diagnosed with HIV infection at the time of PCP diagnosis, there was a trend for patients who resided in the boxed area with the higher proportion of mutant DHPS genotypes to present with a PCP specimen that contained a mutant DHPS genotype (80% compared to patients newly diagnosed with HIV infection who resided outside of this area = 54%, p = 0.12). Unfortunately, the relatively small number of persons newly diagnosed as HIV infected limited our ability to draw any conclusions from this finding.
In San Francisco, we found that the vast majority (84%) P. carinii DHPS genotypes contain mutations that have been associated with sulfa or sulfone drug use for PCP prophylaxis [2–5,7] and, in several studies, with either PCP treatment failure [5,8] or increased mortality [2]. This high frequency was observed despite the fact that only a minority of patients reported any prophylaxis use within the preceding 30 days. Similar results were found when we used different cut-offs to define prophylaxis use (use at time of presentation, use within preceding three months). There are several potential explanations for this finding. First, it is possible that prior sulfa or sulfone prophylaxis use (beyond our three-month cut-off) is sufficient to select for P. carinii mutant DHPS genotypes. For example, a patient who used sulfa prophylaxis for years but discontinued it more than three months prior to PCP presentation would have been classified as “no prophylaxis” in our analysis. However, such an occurrence cannot explain our finding that 70% of the 33 patients diagnosed with HIV infection at the time of PCP diagnosis who had never used PCP prophylaxis presented with a PCP specimen that contained a mutant DHPS genotype. For these cases, one would have to postulate that a course of sulfa medication for reasons unrelated to PCP prophylaxis is sufficient to select for these DHPS mutations. Unfortunately, neither our patient interview nor our chart abstraction was designed to examine this possibility. Alternatively, the high frequency of DHPS mutations seen in our cohort, including those patients who had never used PCP prophylaxis, may be the result of transmission of P. carinii mutant DHPS genotypes, either from another person or from an as yet unidentified environmental source.
Whether the reservoir of human P. carinii resides solely in humans or also in the environment and whether PCP results from reactivation of a latent infection acquired early in childhood or from a more recent exposure and infection remains unclear. The current study cannot determine whether PCP in humans results from person to person transmission of P. carinii. However, our finding that patients who resided in a certain area of San Francisco were significantly more likely to present with a PCP specimen that contained a mutant DHPS genotype than patients who resided outside of this area argues for the possibility that at least some PCP cases result from a recent infection since mutant DHPS genotypes are virtually never seen in PCP specimens prior to 1990. Furthermore, the influence of geography on DHPS genotype in this and other studies [1,3] suggests that the specific factors that are involved in disease transmission might include ones found to a greater degree in certain geographic regions and in certain areas of San Francisco. Future studies should be conducted to explore explanations for the presence of mutant DHPS genotypes in persons without PCP prophylaxis, including the influence of sulfa medication for reasons unrelated to PCP prophylaxis and the possibility that the disease in humans may result from person to person transmission.
ACKNOWLEDGMENTS
The authors wish to acknowledge and thank Mr. Greg Smith for his technical assistance with MapPoint 2001 and Ms. Susan Chang for her editorial assistance.




