Inactivation of Cryptosporidium parvum Oocysts by Bacterial Strains
Since the 1970s, the protozoan parasite Cryptosporidium parvum has been recognized as an important pathogen of humans and many other mammals [8]. It has been well documented that contamination of a drinking or recreational water source entails a risk of waterborne outbreaks of cryptosporidiosis and contamination of food vehicles with oocysts may lead to foodborne cryptosporidiosis. In addition to the extremely low infectious dose of C. parvum, much of this is attributed to the exceptional ability of oocysts to persist in the environment. Oocysts, with thick, doublelayered walls, can survive for long periods in the environment under appropriate conditions [1,10]. On the other hand, disinfectants used for drinking water treatment, with the possible exception of ozone, are not effective against C, parvum, nor are many commercial disinfectants.
Survival of oocysts in association with microorganisms and the role of biological antagonism have been studied only recently. It was found that in vitro survival of oocysts in natural waters was influenced by the levels of heterotrophic bacteria present in waters [9] and that oocysts survived better in membrane-filtered (through 0.2 μm-pore diameter) river water than in unfiltered river water [2]. Another study demonstrated the adverse effect of co-incubation with Serratia marcescens, a bacterium with strong chitinolytic activity, on oocyst survival [11]. These studies demonstrated that microbial antagonism, either from one microbial species or a group of organisms, may be responsible for decreased oocyst viability. The present study was undertaken to test the potential anticryptosporidial activities of certain strains of environmental bacteria and to characterize the mode of oocyst inactivation.
MATERIALS AND METHODS
Bacterial strains
A total of 10 bacterial strains were used in this study: #7: Bacillus cereus; #10: Enterococcus faecium; #12: Bacillus brevis', #13, Bacillus brevis', #14: Bacillus spp. #18: Bacillus subtilis', #22: Bacillus subtilis; #25: Pseudomonas alcaligenes; #26: Bacillus cereus', #27: Bacillus cereus. They were originally isolated from dairy cattle or swine manure and their BCFs displayed considerable anti-viral activities [6]. Bacteria were grown in brain heart infusion (BHI) broth and bacterial cell-free filtrates (BCFs) were prepared by centrifuging an overnight culture and filtering through 0.2-μm membrane filter.
Screening of bacterial strains with anti-cryptosporidial activities
C. parvum oocysts were purified from naturally infected bovine calves by Sheather's sucrose flotation and CsCl gradient centrifugation [4]. They were added into 200 ml of BCFs at 3×104/ml, with antibiotics and fungizone also added to suppress the growth of incidental contaminating bacteria and yeasts. Alternatively, 0.5 ml of fresh culture of each bacterial strain was inoculated into 200 ml of BHI containing 3×104/ml oocysts. Antibiotics and fungizone were not added since the rapid growth of the specific bacterial strain inhibited the growth of accidental contaminant bacteria and yeasts. For the negative control, oocysts and antibiotics were added into sterilized BHI. Inoculated BCFs, bacterial cultures, and BHI were then incubated at 37°C or at room temperature for 3 weeks and portions of 40-ml samples were taken weekly. Oocysts were recovered from BCFs or BHI by a simple centrifugation and washing procedure whereas a modified membrane filtration procedure, developed for recovering oocysts from water samples [3], was used to recover oocysts from bacterial cultures. Oocyst viability was determined by propidium iodide (PI) staining [7] and oocyst inactivation (%) was calculated as (V0Vt)/V0× 100, in which V0 was the viability of oocysts of the zero time sample. Bacterial strains showing more obvious anticryptosporidial activities were chosen for further analyses.
Inactivation of C. parvum oocysts by BCFs
C. parvum oocysts were inoculated into BCFs of bacterial strains chosen from the above screening at 3 × 106/ml and incubated at 37°C for 2 weeks. Samples (1-ml) were taken weekly, and oocyst inactivation was measured by using an in vitro infectivity assay and exposure to bleach treatment.
a) In vitro infectivity assay
Oocysts from the 1-ml sample were pelleted, treated with trypsin-taurocholic acid, diluted, and inoculated onto monolayer Madin-Darby bovine kidney (MDBK, American Type Culture Collection) cells grown in wells of glass chamber slides (Nalge Nunc International), as previously described [5]. Five oocyst inoculum levels of 3 × 104, 3 × 103, 300, 30, and 3 oocysts per well respectively, were used and there were triplicate wells for each inoculum. The remaining well on each 16-well chamber slide received no oocyst, serving as a negative control. Infected cells were examined at 48 h post-infection by staining with rabbit anti-C. parvum antiserum and then goat anti-rabbit IgG conjugated with fluorescein isothiocyanate [4]. Each well was recorded as positive or negative, based on the presence or absence of fluorescently stained C, parvum life stages. The same numbers of oocysts stored at 4°C were also used to infect MDBK cells, serving as positive controls.
b) Bleach treatment of oocysts
This was to model the oocyst susceptibility to common disinfectants. Briefly, pelleted oocysts from 1-ml samples were incubated with I ml of freshly-prepared 5% Clorox bleach at room temperature for 30 min, washed twice with distilled water, and assessed by PI staining.
Scanning electron microscopy (SEM)
After 2-week incubation with BCFs or BHI, oocysts in 45 ml of sample were pelleted, stained with PI, and stained (nonviable) oocysts were separated from unstained ones on a Becton Dickinson FACScan flow cytometer. Sorted oocysts were washed with 0.1 M PBS (pH 7.4) and fixed in 2% of glutaraldehyde, and specimens were prepared by standard SEM procedures. The specimens were coated with 2.5 nm of gold and examined under a Hitachi scanning electron microscope. Oocysts stored at 4°C were processed and examined similarly.
To reveal more ultrastructural details of oocysts inactivated by bacterial activities, nonviable oocysts that resulted from incubation with BCFs were examined at higher resolution. The samples were prepared as above for normal SEM with the only exception that the mounted sample was not coated with gold, and SEM mounts were examined under a Hitachi S-4500 scanning electron microscope (Lawrence Livermore National Laboratory, Livermore, CA, USA). Oocysts freshly prepared from a naturally infected bovine calf were also examined by high resolution SEM, without undergoing PI staining and flow cytometry.
RESULTS AND DISCUSSIONS
Screening of bacterial strains with anti-cryptosporidial activity
There were generally no considerable differences between oocyst inactivation with a bacterial culture or with its BCF. In the BHI control, oocyst viability decreased from 94% to 43% and further to 18% after 1- and 2-week incubation at 37°C, respectively. In comparison, viabilities of oocysts incubated with bacterial cultures and BCFs were between 25% and 44% after 1-week and between 5% to 21% after 2-week incubation. Oocyst inactivation in cultures or BCFs of strains #14, #18, #26, and #27 was very similar to oocyst inactivation in the BHI control whereas the other 6 strains were used for further analyses. But oocyst inactivation by incubation at room temperature was less extensive than in its counterpart experiment conducted at 37°C as these 6 strains did not show much more inactivation than the BHI control, indicating that putative bacterial activities did not play as important roles in oocyst inactivation at room temperature as they did at 37°C.
Oocyst inactivation assessed by in vitro infectivity assay
As it is presented in Table 1, when refrigerated oocysts were used for cell infection, all wells receiving 30 or more oocysts were positive, whereas wells receiving 3 oocysts and the negative control well were negative, demonstrating the sensitivity and specificity of this assay. When oocysts incubated with BCFs of bacterial strains #7, #10, and #25 at 37°C for 2 weeks were used for cell infection, all wells receiving as many as 30,000 oocysts were negative, suggesting at least 3 log (99.9%) oocyst inactivation. For oocysts incubated with BCFs of #12, #13, and #22, developmental C. parvum life stages were observed only in 1 (#12 & #13) or 2 (#22) of the wells receiving 30,000 oocysts. In comparison, in the BHI control, wells receiving 2 300 oocysts were positive, indicating that oocyst inactivation by those bacterial strains was much more substantial than in the BHI control. It also indicated that the in vitro infectivity assay could be used for more accurate assessment of oocyst survival than PI staining.
| Number of oocysts inoculated per well | |||||
|---|---|---|---|---|---|
| 30,000 | 3,000 | 300 | 30 | 3 | |
| Controla | 3b | 3 | 3 | 3 | 0 |
| #7 | 0 | 0 | 0 | 0 | 0 |
| #10 | 0 | 0 | 0 | 0 | 0 |
| #12 | 1 | 0 | 0 | 0 | 0 |
| #13 | 1 | 0 | 0 | 0 | 0 |
| #22 | 2 | 0 | 0 | 0 | 0 |
| #25 | 0 | 0 | 0 | 0 | 0 |
| BHIc | 3 | 3 | 1 | 0 | 0 |
Oocyst susceptibility to bleach treatment after 37°C incubation with BCFs
Following incubation with 5% bleach solution at room temperature for 30 min, viability of refrigeration-stored oocysts decreased from 94% to 78%, representing a 17% oocyst inactivation. Viability of oocysts incubated at 37°C in BHI decreased from 43% to 30% with bleach treatment after 1 week and from 18% to 8% after 2 weeks, representing 30% and 56% inactivation and indicating that oocysts became more susceptible to bleach treatment during incubation at 37°C (table 2).
Oocysts incubated with BCFs at 37°C were more susceptible to bleach treatment than those in BHI. Following 1 week incubation, bleach treatment decreased oocyst viability from 26–38% to 11–17%, representing oocyst inactivation rates of 47% (for strain #13) to 66% (for strain #7). After 2 week incubation, no oocysts survived bleach treatment, indicating 100% inactivation. These data showed that oocyst membrane permeability increased more by incubation with BCFs than by incubation with BHL
Scanning electron microscopy of oocysts
Scanning electron microscopy of oocysts. Typical Oocyst stored at 40°C appeared spherical or slightly oval shaped (data not shown). Nonviable oocysts from 37°C incubation with BHI were distorted, wrinkled, and slightly shrunken (Fig. 1A). Similar to oocysts stored at 4°C, they still appeared to contain sporozoites. However, oocysts that had been incubated with BCFs mostly showed concavities, indicating that they were hollow and that sporomites were no longer inside these oocysts (Fig. 1B). As sporomites can leave oocysts only during excystation, it was clear that these oocysts had already excysted. However, no free sporomites were observed, due to the fragility of sporozoites in the external environment. When nonviable oocysts from incubation with BCFs were viewed under high resolution SEM, more morphological details could be observed, especially the oocyst suture (Fig. 2A). In addition, some oocysts had readily visible wrinkles on their surfaces, a feature similar to that of nonviable oocysts from incubation with BHI (Fig. 2B).
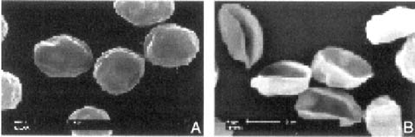
Scanning electron microscopy of nonviable oocysts from incubation in brain heart infusion broth (A) or in bacterial cell-free filtrates (B) at 37°C for 2 weeks. Bars = 2 m. Note distorted and wrinkled oocyst surface in panel A and empty oocysts in panel B.
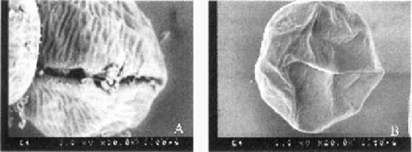
High resolution scanning electron microscopy of nonviable oocysts from incubation in bacterial cell-free filtrates at 37°C for 2 weeks. Bars = 1.5 μm. study that significant anti-hepatitis A virus activities of some of these bacterial strains were due to proteolytic activities [6], whether this was true for oocyst inactivation is yet to be determined in future studies.
The results of this study demonstrated that certain bacterial strains isolated from animal manure were active against C. parvum oocysts and that they caused oocyst inactivation by inducing oocyst excystation. At present it is unclear how oocysts were induced to excyst during incubation with BCFs, one possibility was that oocyst enzymes or surface proteins on the oocyst or its internal sporozoites possibility was that oocyst enzymes or surface proteins on the oocyst or its internal sporozoites were somehow activated by substances in BCFs. Although it was demonstrated in the previous study that significant anti-hepatitis A virus activities of some of these bacterial strains were due to proteolytic activities [6], whether this was true for oocyst inactivation is yet to be determined in future studies.
ACKNOWLEDGMENTS
We thank Ms. Carol Oxford of the Flow Cytometry Laboratory, Department of Pathology, School of Medicine, University of California-Davis for performing flow cytometry cell sorting; Mr. Bob Munn and Mr. Paul Lam of the Electron Microscopy Laboratory, Department of Pathology, School of Medicine, University of California-Davis for preparing SEM samples; and Mr. James Ferreira of Lawrence Livermore National Laboratory, Livermore, CA for conducting high resolution SEM.




