Survey of Sera from Encephalitis Patients for Balamuthia mandrillaris Antibody
More than 100 different infectious agents are known to cause encephalitis in humans, including viruses, bacteria, protozoa, and multi-cellular parasites. Balamuthia mandrillaris is a free-living ameba that causes granulomatous amebic encephalitis (GAE) in both immunocompetent and immunocompromised individuals. Identified and isolated about a dozen years ago from a mandrill baboon [12], >80 cases have since been reported globally with ∼ 40 cases in the United States. As part of the California Encephalitis Project (CEP) - a program to identify the etiologic agents involved in otherwise undiagnosed encephalitis cases - we undertook screening of serum samples of encephalitis patients to look for evidence of Balamuthia GAE or exposure to Balamuthia as indicated by elevated antibody titers demonstrated by indirect immunofluorescence (IIF).
MATERIALS AND METHODS
Serum samples, both acute and convalescent, were submitted by participating physicians throughout the state of California, to the Department of Health Services for diagnostic testing. The case definition for inclusion in the study was hospitalization with encephalopathy (depressed or altered level of consciousness ≥24 hrs, lethargy or change in personality) plus one of the following: fever (≥38°C), pleocytosis, EEG findings consistent with encephalitis, ≥6 months-old, and not severely immunocompromised. More than 300 samples were submitted, of which, criteria for IIF testing for Balamuthia antibodies were outdoor occupational (farming, construction work, exposure to dust) or recreational (camping, swimming) activities. In excess of 150 samples from 105 males (average age of 31.5 yrs, range 1–84 yrs) and 58 females (average age 28.5 yrs, range 1–78 yrs) were tested using IIF. In addition to serum samples, cerebrospinal fluid and brain tissue were also included when these were available for examination. Controls used included both negative serum samples (samples collected at Health Services to monitor health care professionals), and positive serum samples (from Balamuthia GAE cases submitted to and diagnosed at the Centers for Disease Control).
B. mandrillaris isolates for IIF were grown in a cell-free medium at 30°C [9]. Three isolates were routinely used for staining: the strain isolated from the mandrill baboon (V039), and two isolates from humans (V188, and V194). Other strains were used for testing, but less frequently. On occasion, axenically-cultured Naegleria fowleri and Acanthamoeba castellanii were used in IIF staining, performed as previously described [13].
RESULTS AND DISCUSSION
Of the -150 serum samples tested, 11 had reciprocal titers (RT) of ≥ 64 for Balamuthia antibody (Table 1). One of the submitted samples had a titer of 512, a strong indication of Balamuthia GAE. The tentative diagnosis was confirmed by direct immunofluorescence staining of sectioned brain stem, as well as isolation and cultivation of the ameba from brain tissue. Most of the cases included in Table 1 had elevated CSF protein and white blood cells levels, but normal CSF glucose levels.
| Case Number | Sex | Age | CSF Protein | CSF Glucose | CSF WBC Cells | Reciprocal Titer | Resolution |
|---|---|---|---|---|---|---|---|
| 10 | F | 33 | 45 | 63 | 63 | 64/32 | Chlamydia (?) |
| 11 | F | 54 | 55 | 63 | 12 | 32/64 | undiagnosed |
| 28 | F | 17 | 73 | 67 | 143 | 64/16 | undiagnosed |
| 32 | M | 11 | 280 | 34 | 5300 | 64/64 | undiagnosed |
| 57 | M | 31 | 51 | 60 | 65 | 64/32 | undiagnosed |
| 60 | M | 15 | 30 | 64 | 11 | 28/64/32 | undiagnosed |
| 78 | M | 22 | 232 | 68 | 1 | 64/32 | undiagnosed |
| 82 | M | 17 | 64 | 57 | 295 | 64 | undiagnosedb |
| 84 | F | 58 | NDa | NDa | 400 | 64/64 | undiagnosed |
| 104 | M | 17 | 70 | 60 | 19 | 64/32 | Baylisascaris |
| 128 | F | 4 | 1247 | 6 | 354 | 512/1024 | Balainitthia b |
| Normal | 15–45 | 40–80 | 0–5 |
- ano data
- bdeceased
Figure 1 shows the distribution of RT's for both acute and convalescent sera examined in the study. Sera from encephalitis patients peaked at an RT of 16 for both acute and convalescent samples. The range of RT's of negative controls was 0–32, while that of positive controls was 16–2048 (data not shown). Based on these results, we conclude that RT's of ≥ 64 suggest exposure to Balamuthia and subsequent antibody formation.
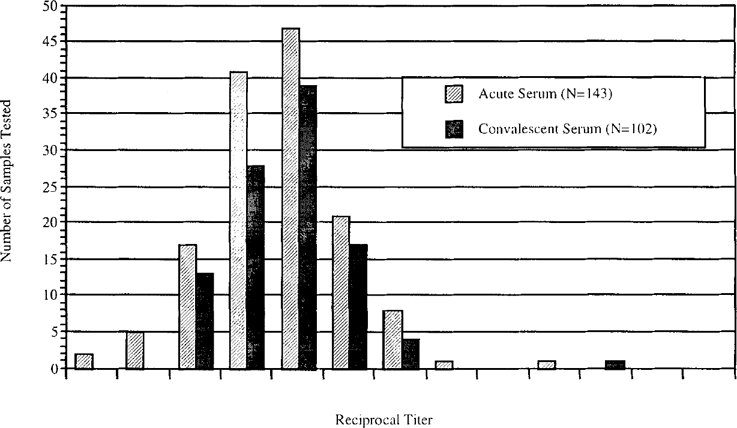
Encephalitic serum samples from the California Encephalitis Project tested for Balamuthia antibody. Reciprocal titers of both acute and convalescent sera are shown in the graph. Acute and convalescent reciprocal titers of the Balamuthia case are included in the graph, 512 and 1024, respectively; the “convalescent” sample, in reality, was taken shortly before death of the patient.
While certainly a factor to be considered in diagnosing encephalitis, Balamuthia GAE is not widespread in the California population, with 1 confirmed case in ∼150 cases studied. In a retrospective study of 1,100 brain lesions in a pediatric population, 4 were found to be positive for Balamuthia GAE by immunofluorescence [11].
As with many other maladies caused by infectious agents, there is a difference between infection and disease. Previous studies on the incidence of antibodies in humans to other free-living amebas, specifically Acanthamoeba and Naegleria, have been published. Marciano-Cabral, et al. [8] found evidence of exposure to Naegleria in otherwise healthy with areas of sampling (Virginia and Pennsylvania). Thus, local conditions (swimming in waters in which amebas are present, etc.) can characterize the exposure of the population to the agent. Studies by Cursons et al. in New Zealand [3], Cerva in Czechoslovakia [2], and Dubray et al. in Tennessee [4], all demonstrated elevated Naegleria titers, as well as Acanthcunoeba titers [2], in sampled populations. Other reports have shown elevated antibody titers to Naegleria in domesticated and wild mammals [1,7].
Of more relevance to the present study are the findings of Huang, et al [6] for incidence of Balamuthia antibodies in an Australian population sample. Using FACS technology, they found elevated antibody levels to Balamuthia amebas in their samples of 1: 64–1: 256. The difference between their incidence and that of the present study (< 0.01%) can be attributed to the different techniques used (FACS vs. IIF), or the degree of exposure to the amebas (reflecling distribution in soil, water, etc.).
We believe that the RT's of ≥64 are real and not due to cross-reactivity with other soil amebas. In several tests, sera showing titers ≥ 64 were adsorbed with Balamuthia amebas, thus eliminating the staining for antibody. In the case of the confirmed Balamuthia GAE in our sample (case number 128 in Table 1), testing for Acanthamoeba antibody gave a RT of 32 compared to the Balamuthia RT of 512. It has been reported that individuals with neurological disorders, tropical diseases, and tuberculosis can generate cross-reactions in tests for protozoal infections [5,10]. Five of the sera tested in our study were from individuals diagnosed wilh tubercular meningoencephalitis, and none had an RT for Balamuthia antibody higher than 16. Thus, cross-reactivity appears not to be a factor in the staining patterns in the current study.
In the course of the sludy, two addilional serum samples were submitted to the Department of Health Services, but not as part of the CEP. One was from a California residenl and the other from a Texas resident. Both samples gave high titers by IIF for Balamuthia antibody (1: 512), and were subjected to further testing. Both California and Texas cases were confirmed by direct immunofluorescence and subsequent examination of hematoxylin/eosin-stained brain seclions; the Texas case awails further tesling for confirmation.
These cases are of particular interesl because of the similarity to the CEP case, number 128 (Table 1). All 3 occurred in otherwise healthy children (ages 3 to 8), with no apparent source of infection. All 3 cases had high CSF protein levels (> 1,000 mg/dL), and resulted in death due to infection. In only 1 of the 3 cases was an autopsy performed, and that led to the isolation of the ameba. A significantly elevated CSF protein level suggests that this might be a useful indicator for considering Balamuthia encephalitis. Previous cases in our sample population had elevated protein levels but none as high as these 3. However, these other cases with high CSF protein had essentially “normal”Balamuthia titers (1:8–1: 16).
We conclude that the IIF technique can be useful in antemortem identification of cases of Balamuthia GAE, as well as in determining exposure of the population to the ameba. Wider use of IIF in encephalitis cases with prognostic indicators can help to identify Balamuthia GAE, with the possibilily of initiating antimicrobial therapy before the infections reach an irreversible conclusion
ACKNOWLEDGEMENTS
We thank Drs. David Ascher and Negar Ashouri for providing information on the Texas and California Balamuthia cases, respectively, referred to in the text, and Dr. John Rowland (Children's Hospital in Oakland) for providing tissue samples for isolation of the ameba, and Dr. Andrew Bollen (University of California Medical Center in San Francisco) for his evaluation of pathology sections




