Balamuthia mandrillaris: its Pathogenic Potential
INTRODUCTION AND HISTORICAL ASPECTS:
In 1958, Clyde G. Culbertson predicted the occurrence in humans of infection by free-living amebas (6). A few years later, Rodney F. Carter and Malcolm Fowler, in Adelaide, Australia, reported the first human cases of primary amebic meningoencephalitis (PAM) caused by Naegleria fowleri [2,9]. Since then, hundreds of cases of infection caused by Acanthamoeba spp. and Naegleria fowleri have been published all over the world [20]. During the last 10 years, another opportunistic ameba, B. mandrillaris has been added to the list of pathogenic, opportunistic free-living amebas [1,3–5,7,8,10–12,15–27,31–33].
In the spring of 1986, one of us (GSV), isolated an ameba from fragments of brain tissue of a 3-year-10 month old pregnant mandrill baboon (Papio sphinx) that died of a neurological disease at the San Diego Zoo Wild Animal Park [32,33]. Since this ameba resembled a free-living ameba belonging to the order Leptomyxida, it was named leptomyxid ameba. This ameba produced in that mandrill's brain a necrotizing hemorrhagic encephalitis similar to granulomatous amebic encephalitis (GAE) caused by Acanthamoeba. Based on light and electron microscope studies, animal pathogenicity tests, and antigenic analysis, a new genus, Balamuthia, was created for this ameba [34]. The name Balamuthia mandrillaris was given to this free-living ameba to reflect the origin of the type species. B. mandrillaris is a pathogenic ameba that can produce lesions in the CNS of humans and other animals. Based on light microscopic studies and immunofluorescence testing, more than 85 cases of B. mandrillaris encephalitis in humans have been identified worldwide, at least 40 in the USA, including 10 in HIV/AIDS patients [22,36,37]. There is good reason to believe that the number of cases of GAE caused by B. mandrillaris is significantly underestimated [22]. Additionally, there have been several reports of B. mandrillaris GAE in mammalian animals [17,24].
B. mandrillaris: PROTOZOOLOGICAL ASPECTS
B. mandrillaris, like Acanthamoeba, has two stages, a vegetative trophic stage and a dormant cyst stage, in its life cycle. The trophozites measure 15 jam to 60 (j.m in diameter and are characterized by a round nucleus with a large spherical, densely staining nucleolus. Occasionally, binucleated forms are seen. In some cases the nucleus may have more than one nucleolus and, when present, this feature distinguishes B. mandrillaris amebas from Acanthamoeba, especially in tissue sections [20,22,33,35]. During the early stages of mitosis both the nucleolus and the nuclear membrane remain intact but disappear as mitosis progresses. The nucleus is surrounded by abundant cytoplasm containing empty vacuoles, numerous mitochondria, ribosomes, and endoplasmic reticulum (Fig. 1). The trophozoites form broad pseudopodia and exhibit spider-like walking movements across the tissue culture monolayer on which they are feeding [35]. The cysts of B. mandrillaris are usually spherical and measure from 6 μm to 30 μm, with a mean of 15 μm in diameter. They are usually uninucleate and posseses a layer of refractile granules underneath the inner cyst wall. Although they appear to possess an outer wrinkled wall, the ectocyst, and an inner thin wall, the endocyst, and look like Acanthamoeba cysts under the optical microscope, ultrastructurally, however, they are tripartite and possess a thick amorphous, fibrillar middle layer, the mesocyst, in addition to the ecto- and endocyst [33,35] (Fig. 2). Unlike Acanthamoeba spp. and N. fowleri, that usually grow on non-nutrient agar plates coated with Gram-negative bacteria, B. mandrillaris does not grow on bacteria-coated non-nutrient agar plates [33,35]. It is probably because of this reason B. mandrillaris has not been isolated from nature, since isolations of free-living amebas are usually done by inoculating environmental samples on to bacteria-covered agar plates. B. mandrillaris, however, grows well at 35–37°C, on mammalian cell cultures (monkey kidney cells, human lung fibroblasts, or rat glioma cells) as well as in a complex chemical medium containing fetal bovine serum [28,29], which is helpful in the screening of various drug formulation for their activities against these amebas.
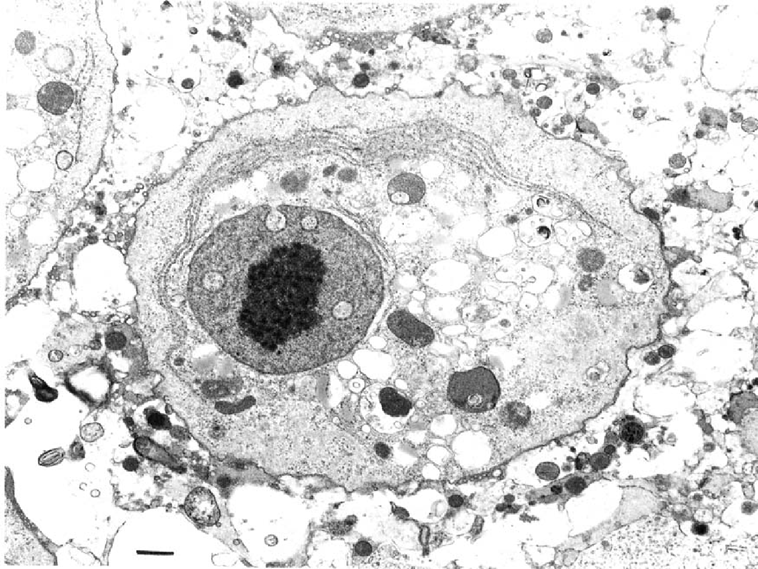
Trophozoite of B. mandrillaris with the characteristic dense nucleolus, granular-membrane-bound nucleus, and abundant cytoplasm containing empty vacuoles, lysosomes, inconspicuous mitochondria and large, parallel arrays of endoplasmic reticulum. EM; Bar = 2u,m.
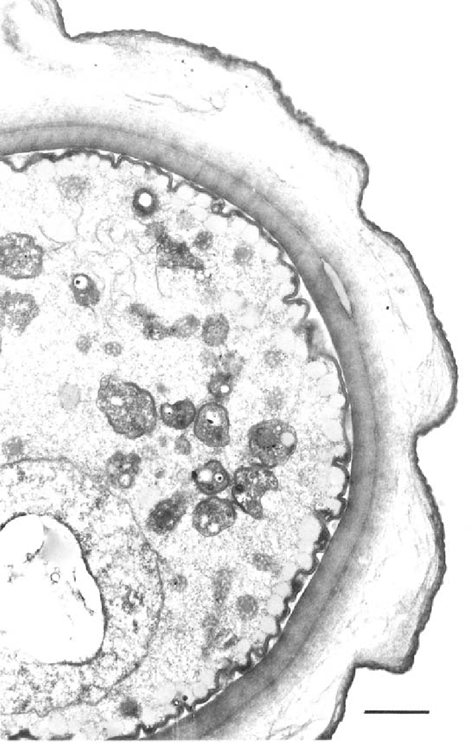
Typical cyst of B. mandrillaris showing the tripled-walled composition. There is a thick inner-wall, thin wavy outer layer and a clear-granular area between them. The cytoplasm contains numerous organelles, swollen mitochondria, lipid-like and lysosomal bodies. EM; Bar=2μm.
Experimental animals such as out-bred mice have been infected by intranasal instillation of culture-grown B. mandrillaris resulting in disease and death of the animals, similar to that in humans [33]. Further, an animal model using a congenitally immunodeficient mouse has been described confirming the “opportunistic” charactersisics of B mandrillaris [14].
B. mandrillaris: EPIDEMIOLOGICAL ASPECTS; DISTRIBUTION IN NATURE
It is well known that both N. fowleri and Acanthamoeba spp. are ubiquitous in nature and widely distributed in fresh water, soil, and dust throughout the world, thus providing a potential source of infection for humans and other animals. The environmental niche of B. mandrillaris is not known; however, it is believed to occupy the same environmental locations as N. fowleri and Acanthamoeba spp. [20,22,33–35].
Free-living amebas have emerged as important opportunistic protozoa, associated with diseases with defective immune system. Additionally, subclinical infections due to free-living amebas may occur in healthy individuals, with these protozoa living as a “normal flora” in the throat, oral mucosa, and nasal cavities. It is possible that in humans and animals, antibodies and cell-mediated immunity protect the host in ordinary circumstances against invasive infection. But in individuals whose immune system has been compromised and in debilitated, chronically ill individuals with depressed cell-mediated immunity, the free-living amebas, viruses, or fungi may proliferate, and thus allowing for a fulminant “opportunistic” infection to develop [20].
B. mandrillaris: THE DISEASE: GRANULOMATOUS AMEBIC ENCEPHALITIS (GAE)
The incubation period of GAE caused by B. mandrillaris in humans is unknown. However, in the experimental animals it takes from 1 week to 3 weeks to produce extensive tissue necrosis. B. mandrillaris is capable of infecting both healthy and immunosupressed hosts of both sexes, without a history of swimming or exposure to contaminated water. GAE caused by B. mandrillaris usually runs a protracted and insidious clinical course with an incubation period, probably lasting from 1–2 weeks to several months but definitely longer than 10 days [20].
The portal of entry into the CNS is believed to be the lower respiratory tract or an ulceration of the skin with subsequent hematogenous dissemination to the brain. However, the skin lesions may be either the presenting infection, or a terminal “metastatic” dissemination of the ameba [20].
CLINICAL MANIFESTATIONS
Clinically, patients present with sleepiness, mood swings, seizures, low-grade fever, stiff neck, nausea and vomiting, headaches and focal neurologic signs and symptoms like hemiparesis, cranial nerve palsies, aphasia and seizures. Personality and mental status abnormalities, associated with headache and stiff neck may be present, in combination with cranial nerve palsies, mainly the third and sixth cranial nerves. Cerebellar ataxia, diplopia, and low-grade fever have been reported in some cases [20].
Balamuthia mandrillaris causes relentless subacute or chronic, usually granulomatous encephalitis (GAE), in patients who have lost metabolic, physiologic or immunologic integrity. Among the commonly recognized causes of susceptibility are diseases such as diabetes mellitus, hematologic malignancies; cancer; acquired immunodeficiency syndrome (AIDS); pregnancy; treatment with cytotoxic agents and with broad spectrum antibiotics; immunomodulating drugs such as corticosteroids, cyclosporine, and FK-506 after organ transplantation.
Some patients with “spontaneous” GAE could possibly have had an underlying inherited immunodeficiency but did not have an immunologic evaluation performed. Impairment in cell mediated and humoral immunological defects could be present in some patients [20].
DIFFERENTIAL DIAGNOSIS
The diagnosis of GAE is made when the amebic trophozoites and cysts are identified in tissues (Fig. 3) or the agent is isolated in culture. Brain and skin biopsies are useful methods for culture and for histologic examination, using H&E or special stains such as Gomori's methenamine silver or periodic acid-Schiff. However, immunohistologic techniques, for example, indirect immunofluorescence assay or electron microscopy, is necessary to identify B. mandrillaris in tissue sections. Molecular biologic methods such as polymerase chain reaction or oligonucleotide probes are not currently available. Biopsy or autopsy tissue fixed in 10% buffered formalin or in Karnovsky's fixative can be embedded in plastic for electron microscopy to show the ultrastructural features of trophozoites and cysts, characteristic of B. mandrillaris. Recently, a serum antibody test that may be helpful in the diagnosis of B. mandrillaris infection has been developed [13,30].
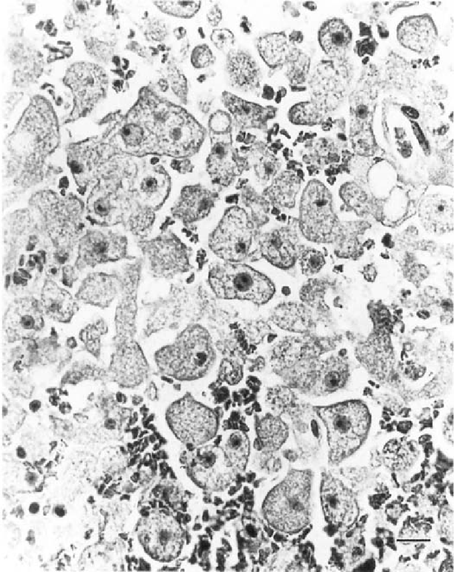
Infected CNS depicting numerous trophozoites of B. mandrillaris. H&E; Bar = 20μm.
The cerebrospinal fluid (CSF), removed by lumbar puncture, may show lymphocytic pleocytosis, normal or slightly low glucose level, and mild elevation of proteins, usually without amebic trophozoties or cysts. The CSF resembles that of aseptic meningitis in most cases.
Neuroimaging studies using computed tomography and magnetic resonance imaging of the head are helpful in the diagnosis. Ring-enhancing masses after injection of intravenous contrast material may be suggestive of a brain abscess or a brain tumor. Chest films may show focal areas of consolidation in the pulmonary tissue.
Clinically, GAE caused by B. mandrillaris resembles that produced by Acanthamoeba spp, namely the presence of a single or multiple lesions, suggesting space-occupying lesion(s), such as a brain tumor or intracerebral hematoma. On the other hand, these symptoms may also mimic, and are often mistaken for bacterial pyogenic or viral encephalitis, leptomeningitis, and tuberculous meningitis. Elevated intracranial pressure can lead to papilledema, coma, and herniation of cerebellar tonsils or death. The direct cause of death is usually acute bronchopneumonia, hepatic or renal failure, septicemia, and brain edema leading to uncal and cerebellar tonsillar herniation [20].
The isolation of B. mandrillaris initially from the brain tissue of a mandrill and subsequently from human and other animals brain tissue, as well as the production of specific anti- B. mandrillaris serum have made it possible to differentiate GAE caused by Balamuthia from that caused by Acanthamoeba spp. Immunofluorescence antibody technique is used currently to confirm the diagnosis of GAE due to B. mandrillaris.
PATHOLOGICAL FEATURES
GAE due to B. mandrillaris is characterized by multifocal areas of CNS necrosis and hemorrhages, mainly on the cerebral cortex, the basal ganglia, brain stem and cerebellum associated with focal edema. Numerous amebic trophozoites and few cysts may be seen within necrotic CNS tissues (Fig 3), and perivascular spaces. Occasional amebic trophozoites with two nucleoli may be seen.
Multinucleated giant cells and amebic trophozoites and cysts are usually seen in stained sections. The granulomatous component may be very subtle and composed of CD4 and CDS T-cells as well as B-lymphocytes, modest number of plasma cells, and some macrophages. Granulomas may be absent in severely immunocompromised hosts, indicating an impairment of the cell-mediated immunity or a defect in histiocytic response with failure to produce multinucleated giant cells. Lesions in the skin are characterized by the ulceration with the presence of trophozoites and cysts within chronic inflammatory reaction. The lungs demonstrate focal pneuminitis with polymorphonuclear leukocytes, lymphocytes, and macrophages [20].
TREATMENT, CONTROL AND PROGNOSIS
Up to the present, there is no known effective treatment for GAE caused by B. mandrillaris and the prognosis is grim. Recent experimental studies indicate that B. mandrillaris is sensitive to pentamidine isethionate, [28,29]. Recently, two patients with biopsy-confirmed B. mandrillaris GAE has so far survived this infection with the following therapy: Pentamidine IV for a short period followed by Clarithromycin, 500mg tid; Fluconazole, 400mg qd; Sulfadiazine 1.5g q 6h; 5-fluorocytosine 1.5g q 6h, although with severe neurological defects (Dr. Deetz, Santa Cruz Medical Center, Santa Cruz, CA. and Dr. Mark Sawyer, UCSD, San Diego CA; personal communication to GSV).
FUTURE DIRECTIONS
Clinical diagnosis of GAE is difficult. Most of the cases have been diagnosed postmortem. Obviously the practice of autopsies in suspected cases should be encouraged, particularly in patients with HIV/AIDS. Efforts should be made to develop methods for the isolation of Balamuthia from the environment, so that strategies for control could be developed. Additionally, efforts should be made to identify suitable therapeutic agents that cross the blood brain barrier and neutralize Balamuthia.




