Delta-9-Tetrahydrocannabinol (THC), the Major Psychoactive Component of Marijuana, Exacerbates Brain Infection by Acanthamoeba
The genus Acanthamoeba consists of free-living amebae associated with human infections. Acanthamoeba spp. are the causative agents of Granulomatous Amebic Encephalitis (GAE), a progressive disease of the central nervous system (CNS) which often is diagnosed post-mortem. An increase in the number of GAE cases has been reported world-wide [9,10]. GAE occurs primarily in hosts whose immune systems have been suppressed from cancer chemotherapy, steroid therapy, HIV infection, or other debilitating diseases [6,9]. Antibodies to Acanthamoeba are found frequently in human serum suggesting that exposure is common [8].
Marijuana has been advocated for therapeutic application to individuals suffering from debilitating disease characterized by cachexia, chronic pain, or nausea. However, marijuana also has been reported to exert deleterious effects on the immune system [2]. The majority of these effects have been attributed to delta-9-tetrahydrocannabinol (THC), its major psychoactive component. THC has been shown to increase the susceptibility of experimental animals to infection with viruses and bacteria [1–4,7,13]. Thus, individuals who utilize marijuana or its psychoactive component, THC, and have compromised immune systems, such as AIDS patients, could be at greater risk of infection with Acanthamoeba. Thus, the goal of this study was to examine the effect of THC on susceptibility to infection with Acanthamoeba, an opportunistic pathogen of the central nervous system (CNS). A murine in vivo model of CNS infection with Acanthamoeba was employed to assess effects of THC on the host response.
MATERIALS AND METHODS
Acanthamoeba castellanii (ATCC 30010) and Acanlhamoeba culberisoni (ATCC 30171) were maintained at 37 °C in Oxoid medium supplemented with calf serum and hemin. Amebae were harvested by washing with Hanks' Balanced Salt Solution (HBSS), counted using a hemacytometer, and adjusted to 3 × 106/10 1 for A. castellanii and 1 × 103/10 1 for A. culbertsoni. A second strain of A. castellanii, (ATCC 50494), was used to examine the antibody response to Acanthamoeba in THC-treated and untreated mice because this strain lost its virulence after continuous axenic culture. Use of this latter strain allowed for survival of mice and assessment of immune responsiveness uncompromised by animal mortalities.
Three week-old female B6C3F1 mice were used to determine the effect of THC on infections with A. castellanii or A. culbertsoni. Three week-old mice were inoculated once a day with 40 mg/kg of THC by the intraperitoneal (ip) route for three rounds of four consecutive days of treatment with three days of rest between each treatment. This protocol of four consecutive single daily injections interspersed with a three day rest period minimizes the development of tolerance to THC as relates to the immune system [2]. Mice were infected via the intranasal route with 20 1 HBSS-containing amebae after the second round of drug treatment. Control mice were subjected to a similar regimen of ip injections with vehicle (ethanol: emulphor: saline, 1: 1: 18). Cyclophosphamide (CPA), a powerful immunosuppressive drug that is more toxic for B lymphocytes than for T lymphocytes or macrophages, was injected ip one day prior to inoculation with Acanthamoeba as an alternative immunosuppressive agent [12]. Folloowing the last THC injection, mice were observed for 30 days for morbidity and mortality. Brain and lungs were removed from moribund animals and maintained in ameba growth medium to allow for isolation of Acanthamoeba (Table 1).
| Treatmenta | # of Animals | # Dead (%)b | Amebaec |
|---|---|---|---|
| A. casteUanii | |||
| Vehicle | 8 | 1(12) | Brain |
| CPA 200 mg/kgd | 8 | 0(0) | None |
| THC40mg/kg | 8 | 4(50) | Brain/lungs |
| A. culberlsoni | |||
| Vehicle | 8 | 4(50) | Brain |
| CPA 200 mg/kg | 8 | 4(50) | Brain |
| THC 40 mg/kg | 8 | 7(85) | Brain/lungs |
- aFemale mice (3 weeks old) were injected ip with vehicle (ethanol:emulphor:saline), cyclophosphamide (CPA) or THC prior to inoculation via the intranasal route with A. castellanii (3 × 106) or with A. culbertsoni (1 × 103).
- bThe number in brackets represents the percentage of mice that died over a 30-day period.
- cAt the time of death, brain and lungs were moved and cultured for isolation of amebae.
- d200 mg/kg of CPA was injected ip one day prior to challenge with Acanthamoeba.
Serum was obtained from THC-treated mice, drug-free control mice, and vehicle-treated mice infected with A. castellanii at Day 7 and Day 14 post infection to evaluate the effect of THC on the antibody response to Acanthamoeba. Extracts prepared by glass bead disruption of ameba trophozoites were used as the antigen source in Western blot analysis. Antibodies to Acanthamoeba were detected using goat anti-mouse IgM (ICN, Costa Mesa, CA) and rabbit anti-mouse IgG (Sigma-Aldrich, St. Louis, MO) (Fig. 1 and Fig. 2).
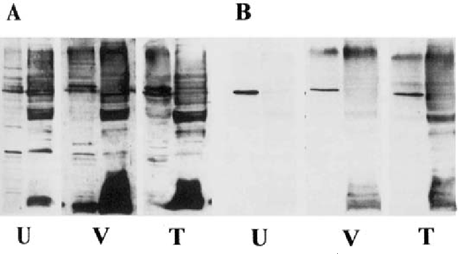
Western blots with serum antibodies against proteins from A. castellanii. serum was obtained 7 days after infection. A. IgM response of untreated infected mouse (U), vehicle-treated infected mouse (V), and THC-treated infected mouse (T). IgG response using the same serum samples. For each group, the left panel contains soluble cytoplasmic proteins while the right panel contains membrane proteins.
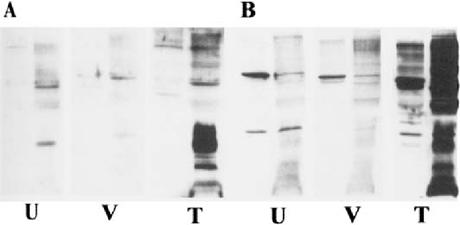
Western blots with serum antibodies against proteins from A. castellanii. serum was obtained 14 days after infection. A. IgM response of untreated infected use (U), vehicle-treated infected mouse (V), and THC-treated infected mouse (T). IgG response using the same serum samples. For each group, the left panel contains uble cytoplasmic proteins while the right panel contains membrane proteins.
RESULTS AND CONCLUSION
Using the B6C3F1 mouse model, we have examined differences between immune competent and immune suppressed mice infected with two species of Acanthamoeba. A. culbertsoni is more pathogenic in B6C3F1 mice (LD50 1 × 103) than A. castellanii (LD50 3 × 106). We have demonstrated that THC exacerbates Acanthamoeba-induced pathogenesis induced by both species. Mice treated with THC exhibited higher mortalities from infection with Acanthamoeba than similarly infected vehicle controls (Table 1).
At the time of death, lungs and brains were removed and cultured to determine whether amebae were present in these organs. Amebae were isolated from brain tissue of all vehicle-treated animals that died. In contrast, amebae were cultured from both brain and lung tissue of THC-treated animals that died, indicating colonization of multiple sites. Infection of CPA-treated mice with Acanthamoeba did not increase mortality when compared to untreated infected mice suggesting that B lymphocytes are not a major immune effector cell in Acanthamoeba infection. We also examined the IgM and IgG antibody response to Acanthamoeba in THC-treated and vehicle treated mice at 7 and 14 days post infection by Western blot. IgM and IgG antibodies to Acanthamoeba were detected in serum from survivors and animals that died. The IgM and IgG response to Acanthamoeba antigens were slightly higher in THC-treated mice indicating that THC did not inhibit the antibody response to Acanthamoeba in these animals (Fig. 1 & Fig 2). This observation is consistent with a previous report indicating that, although THC is immune suppressive, cannabinoids enhance B-cell growth at low nanomolar concentrations [5], Thus, stimulation of B cells by THC could account for the increased production of antibodies in anthamoeba infected animals. Alternatively, the increase in antibody gels for THC-treated infected animals could be reflective of a more seminated disease.
THC has been reported to have profound effects on the functional status macrophages including that of microglia, which constitute a resident pulation of macrophages in the brain. It suppresses macrophage mediated cytotoxicity against tumor cells, virus infected cells and amebae [2–4]. We have shown that microglia are capable of destroying Acanthamoeba in vitro and produce cytokine mRNA in response to Acanthamoeba [8]. THC has been shown to inhibit cytokine mRNA production and inhibit nitric oxide production by rat microglia [14]. Since macrophage-like cells constitute a major cell type in the formation of granulomas, which form around amebae in tissue, THC may cause higher Acanthamoeba-induced mortality of mice by inhibiting macrophage amebicidal activity. Studies are in progress to determine the effect of THC on microglial soluble and cell contact-dependent amebicidal activity in vivo and in vitro.
ACKNOWLEDGMENTS
This work was supported by NIH awards DA05274 and DA05832. T. Ferguson was supported by Training grant T32 AI 07407.




