Desensitization of Thermal Hyperemia in the Skin is Reproducible
Abstract
Please cite this paper as: Frantz, Engelberger, Liaudet, Mazzolai, Waeber and Feihl (2012). Desensitization of Thermal Hyperemia in the Skin is Reproducible. Microcirculation 19(1), 78–85.
Objective: Local heating increases skin blood flow SkBF (thermal hyperemia). In a previous study, we reported that a first local thermal stimulus could attenuate the hyperemic response to a second one applied later on the same skin spot, a phenomenon that we termed desensitization. However, other studies found no evidence for desensitization in similar conditions. The aim of the present work was to test whether it was related to differences in instrumentation.
Methods: Twenty-eight healthy young males were studied. Two pairs of heating chambers, one custom-made (our study) and one commercial (other groups), were affixed to forearm skin. SkBF was measured with single-point laser-Doppler flowmetry (LDF) (780 nm) in one pair, and laser-Doppler imaging (LDI) (633 nm) in the other. A temperature step from 34 to 41°C, was applied for 30 minutes and repeated after two hours.
Results: During the second thermal challenge, the plateau SkBF was lower than during the first thermal and was observed with each of the four combinations of SkBF measurement techniques and heating equipment (p < 0.05 for all conditions, range −9% to −16% of the initial value).
Conclusion: Desensitization of thermal hyperemia is not specific to peculiar operating conditions.
Abbreviations used:
-
- SkBF
-
- skin blood flow
-
- LDI
-
- laser-Doppler imaging
-
- LDF
-
- single-point laser-Doppler flowmetry
-
- PU
-
- perfusion unit
-
- T0
-
- time of protocol start
-
- T2
-
- two hours after T0
Introduction
In nonglabrous human skin, a local rise in temperature is a powerful stimulus for local vasodilation, mediated by neurogenic reflexes and locally released substances [12,13,15,16]. The mechanisms implicated in this so-called thermal hyperemia remain incompletely defined. In contrast with thermoregulatory skin vasodilation, it is not mediated by central reflexes because it is unaffected by regional nerve block [17] and is preserved in grafted skin [5]. The response of skin blood flow (SkBF) to a step increase in local temperature is biphasic, with an early peak occurring within minutes, followed by a nadir, and then a late phase with a progressive rise to a plateau in 20–30 minutes response [3,10–12,16]. The plateau seems to depend on the local, non-neurally mediated release of nitric oxide (NO), because it is suppressed by inhibitors of NO synthase [11,12,16] and insensitive to local anesthesia [16]. In contrast, the early peak shows little dependence on NO, and is largely mediated by the stimulation of nociceptive C-fibers that trigger vasodilation through an axon reflex [13]. Accordingly, it is diminished by local anesthesia [7,16,21]. In short, the prevailing view [15] is that the early part of thermal hyperemia is due to the transient activation of an axon reflex, which progressively gives way, as heating is pursued, to a non-neural, NO-dependent mechanism.
Thermal hyperemia can easily be recorded in the skin in a non-invasive fashion, using laser-Doppler flowmetry to evaluate SkBF. Indeed, thermal hyperemia has been proposed as a test of microvascular function. This test has been used to document microvascular dysfunction in diabetes [1,22,23] and other conditions [14,19].
In a previous study, we found that the repeat application of a local thermal stimulus on the same skin patch was associated with a reduction in the elicited vasodilatory response, a phenomenon hereafter termed desensitization [3]. This result is of some practical importance, for example, if thermal hyperemia is to be used as an end point in acute interventional trials. However, other groups [4,20] found no evidence for desensitization, when recording two thermal hyperemia either one or two hours apart on the same skin site, as we had done. The aim of this study was to understand the reasons for this apparent discrepancy and, more specifically, to test whether it was related to differences in instrumentation. We had measured SkBF with laser-Doppler imaging (LDI) at a wavelength of 633 nm [3], whereas the cited studies used single-point laser-Doppler flowmetry (LDF) at 780 nm [4,20].
In comparison with 633 nm, the latter wavelength has greater skin penetration, and thus the potential to explore different vessels. In addition, the heating chambers used in our study were custom-made, as opposed to the commercial equipment employed by these other authors.
We therefore set out to establish whether desensitization to thermal hyperemia occurred under four sets of conditions, i.e., measuring SkBF with LDI or LDF, and heating the skin with our custom-made or with commercially available chambers.
Methods
Subjects
Twenty-eight healthy male subjects, aged from 18 to 32 years, were included. They were all non-smokers, had no personal history of hypertension, diabetes, or hypercholesterolemia, and no dermographism. None took any drugs or reported being sick in the last 15 days before the start of the study. The volunteers were fully informed about the protocol, and gave their written informed consent. The study conformed to the principles outlined in the Declaration of Helsinki, and the protocol was approved by the local Ethical Committee.
Assessment of Skin Microvascular Reactivity
Measuring devices. To investigate forearm SkBF, we used two different laser-Doppler measuring devices. The first one was a laser-Doppler imaging system (LDI; Moor Instruments, Axminster, UK) and the second one, a single-point dual-channel laser-Doppler flowmeter (PF4001; Perimed, Järfalla, Sweden).
Laser-Doppler imaging system (LDI). The LDI system used a beam of coherent red light generated by a 633-nm-helium–neon laser. In this system, the beam is directed by a moving mirror whose rotations around two perpendicular axes are controlled by a computer, allowing the scanning of a delimited area. The analysis of the backscattered Doppler-shifted light results in a computer-generated, color-coded image of the spatial distribution of microvascular blood flow over the scanned area. No direct contact with the skin is required. The scanned area can be chosen in a range from a few mm2 to a complete body part such as the hand or thorax, depending on angular amplitudes of mirror movements and distance of the latter to the skin.
In the present study, the scanned area was about 3 × 7 cm, and the distance travelled by the incident laser beam from the device shutter to the skin was set at 41 cm. SkBF was expressed in perfusion units (PU).
Single-point fiber-optic laser Doppler (LDF). The LDF system used infrared light produced by a 780-nm-helium–neon laser. In this system, two optical fibers are embedded in a probe placed in contact with the skin surface. One fiber is used to transmit a laser beam and the other to detect the back-scattered light. The measurement depth varies according to the distance between the fibers. The probes used in this study (PF408; Perimed) had diameter and a fiber separation of, respectively, 6 and 0.25 mm. SkBF was expressed in volts.
Assessment of thermal hyperemia response. We used two different systems for the local heating of the skin. The first one, custom-made, had been used in our previous study [3]. It comprised a stainless steel, temperature-controlled, ring-shaped chamber with inner diameter, outer diameter, and thickness of 8, 25, and 8 mm, respectively, affixed to the skin with double-sided tape [3,7].
The second system was commercially available (Perimed). It comprised a thermostatic probe holder (PF450; Perimed), which is a ring-shaped chamber, whose visible part is in plastic with inner diameter, outer diameter, and thickness of 6, 32, and 12 mm, respectively, and is also affixed to the skin with a double-sided tape. The chamber was connected to an analog dual-channel temperature controller with adjustable set point (Peritemp 4005 Heater; Perimed).
The present study aimed at comparing results obtained with each of the four combinations of measuring systems (LDI or LDF) and heating devices (commercial or custom-made). The required adaptations are described below (also see Figure 1).
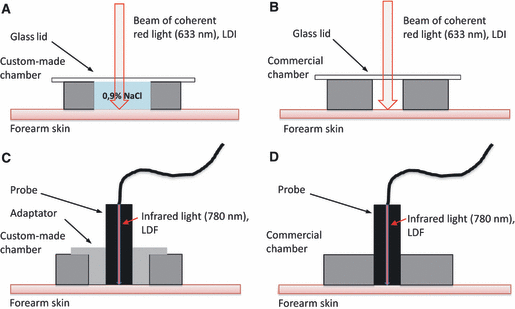
The four different setups used for recording local thermal hyperemia in forearm skin. Each thermo-controlled chamber was annular, with the lower face affixed to the skin with double-sided tape. (A) Custom-made chamber filled with 0,9% NaCl and overlaid with a transparent glass lid. (B) Commercial chamber, with a narrower well, not filled with liquid, because it was not watertight, and overlaid with a transparent glass lid. (C) Custom-made chamber with an adaptator to hold a single-point probe for laser-Doppler flowmetry (LDF). (D) Commercial chamber, able to hold an LDF probe without an adaptor. On sites (A and B), skin blood flow was measured using laser-Doppler imaging (LDI). The upper face of the chambers were covered with black neoprene to avoid reflection artifacts, which otherwise might contaminate the LDI measurements, and overlaid with a glass lid. On sites (C and D), skin blood flow was measured with LDF.
The LDI device was programmed to repetitively scan a 3 × 7 cm area sufficient to encompass two chambers, one custom-made and one commercial (see below). Repetition time was every 60 seconds, with each scan being accomplished in about 40 seconds. The central well of the custom-made chamber was filled to the rim with isotonic saline and overlaid with a transparent glass cover slip. The skin underneath the cover slip and water was thus accessible to the laser light (as in our previous study [3]).
The commercial chamber was just overlaid with a transparent glass cover slip and not filled with liquid, because it was not water-resistant.
For the measurements with the LDF device, probes were fitted into either a custom-made or a commercial chamber. An adaptator was required to hold the PF408 probe in the custom-made chamber (Figure 1C).
In preliminary experiments, a small-size thermistor (length and diameter of 0.3 cm and 0.01 cm, calibrated with a mercury thermometer) was used to check the skin temperature underneath each chamber at settings of 34°C and 41°C. This thermistor (custom prepared from a recycled 2F Swan-Ganz catheter, Edwards, Irvine, United States) was placed between the skin and the double-sided tape within a tad of heat-conducting paste.
The sequence for inducing thermal hyperemia was as follows. The temperature was set at 34°C during about three minutes to ensure thermal stability. Then, SkBF was recorded for five minutes at 34°C, after which the temperature was raised to 41°C and maintained at this level for the next 30 minutes [3]. The time required to reach the final temperature was slightly shorter with the commercial (30 seconds) than with the custom-made system (60 seconds). This difference between devices was inherent to their design and thus could not be avoided.
Protocol. The experiment was completed in a single visit. The subjects were examined in a quiet room with an ambient temperature ranging from 21 to 25°C (systematically controlled and kept in that range with air conditioning). The ambient light level was daylight with the blinds half pulled down and artificial light turned off to avoid any confounding of laser-Doppler measurements by changes in background lighting levels [6].
The volunteers reported to the laboratory at 1:30 pm. They had abstained from caffeine-containing beverages since the night before the experiment, had taken a lunch two hours before the study, had been instructed to avoid exposing themselves to important physical exercise, mental stress, or changes in ambient temperature, just before the beginning of the study. Their weight and height were measured on arrival.
Body temperature was taken with an ear thermometer (ThermoScan Braun, Switzerland). Forearm skin temperature was obtained with the thermistor described above near the sites of SkBF measurement. The arm circumference was taken too, to choose a cuff of the right size for the oscillometric measurement of blood pressure and heart rate (StabiloGraph, IEM, Deutschland).
The subjects were examined in the supine position with their forearms supported by vacuum cushions. They remained quiet in that position for about 15 minutes before the taking of any recording.
On the ventral surface of one forearm (dominant or not), two sites (A, B) were selected, distant from each other by 2–3 cm and excluding visible veins. The site A received the custom-made chamber, which was filled with saline and overlaid with a transparent glass cover slip (Figure 1A). Site B, was placed an empty commercial chamber, overlaid with a transparent glass cover slip, too (Figure 1B). It was not feasible to fill this chamber with water, as it was not watertight. SkBF was measured by LDI, simultaneously in both chambers.
Two other sites (C, D) were chosen, in a similar position, on the ventral surface of the other forearm, to receive either a custom-made chamber with the adaptator (Figure 1C), to hold the LDF probe, or a commercial chamber (Figure 1D). Neither chamber contained any liquid. SkBF was measured by LDF, simultaneously on sites C and D, using the two channels of the Periflux 4001. Care was taken that the probes did not exert any pressure on the skin. With this experimental design, the conditions of our previous study [3] were exactly reproduced on site A, and those of site D were analogous to those used by Cracowski et al. or Shastry et al. [4,20].
At T0 (time zero), the temperature of the four chambers was raised from 34°C to 41°C and maintained at this level for the next 30 minutes. At T0 +30 minutes (time zero plus 30 minutes), the heating was turned off. The chambers on sites A and B were uncovered, and saline was emptied from the chamber located on site A. Blood pressure and heart rate were measured on the arm on which SkBF was assessed with the LDI. The other arm was not used due to the danger of cuff inflation causing small movements that might have perturbed the position of the LDF probes.
Two hours after T0 (T2), all these maneuvers were repeated. At the end of the experiment and while the controllers were still set at 41°C, the temperature in the custom-made chamber filled with saline was checked.
The total duration of the protocol was three hours. The volunteer had to remain strictly immobile at least during both periods of thermal hyperemia, with particular attention paid to the arm bearing the LDF probes, which was left untouched during the whole protocol. From T0 +30 to T2 −15 minutes, the subject was allowed to watch a movie on a DVD player.
Data Processing
The raw flow images generated by the LDI device were processed with the image analysis software provided by the manufacturer (Moor LDI Image Review, V5.0). Each image contained two areas of non-zero flow, corresponding to the custom-made and the commercial chamber, simultaneously scanned as described above. Separate regions of interest were defined around each of these areas, to calculate in each, the spatial average of non-zero pixels. The software has a facility to exclude zero-valued pixels from the computation, so that the result is insensitive to small variations in the shape of the region of interest. The thermal hyperemia elicited by each chamber is thus reduced to a series of average flow values, separated by time intervals of one minute (as scans are repeated at a rate of 1/minute).
The PF4001 laser-Doppler flowmeter generates analog DC output voltages proportional to the detected flow, which were digitized at a sampling frequency of 40 Hz and stored on computer disk, using the Powerlab 8/35 hardware and the Labchart V5.0 software by ADInstruments (Spechbach, Germany). These signals were then time-averaged over successive, contiguous periods of one minute.
In this fashion, whether evaluated with LDI or LDF, all thermal hyperemias were expressed in time series of identical format. The last step in data reduction was then the calculation of the following variables: baseline flow (average of five values corresponding to the five minutes preceding the rise in local temperature), early peak response (maximal flow during the 10 minutes following the rise in temperature, minus baseline flow), nadir response (minimal flow from the time of early peak to the 15th minute of recording, minus baseline flow), and plateau response (mean of the last five flow values, recorded from 25th to 30th minute following the rise in temperature minus baseline flow).
Data Analysis
As measurements obtained with the two laser-Doppler techniques are not in the same units (i.e., volts vs PU), statistical analysis was carried out separately for LDI and for LDF data. Baseline flow, early peak response, nadir response, and plateau response were tested with analysis of variance for repeated measures. The model included time (T0 or T2), chamber type (custom, commercial), and their interaction as repeated factors. The alpha level of all tests was set at 0.05. Data are presented as the mean and SD, unless specified otherwise.
Results
The 28 subjects were healthy men, aged 19–32 years. Fifteen of them were lean (BMI <25 kg/m2) and the others were overweight, but not obese (BMI 25–29 kg/m2). The mean skin temperature measured in the immediate vicinity of sites A, B, C, and D was 32.8 ± 0.8°C.
Between T0 +30 and T2 +30 minutes, HR did not change (65 ± 8 vs 64 ± 9 beats/minute), but the mean BP slightly increased (from 80 ± 7 to 87 ± 6 mmHg, p < 0.001), a difference that may be explained by the discomfort induced by lasting bilateral arm immobilization, as expressed by several subjects.
Figure 2 shows the mean time courses of SkBF responses to local heating, observed in the four experimental conditions. As expected, the general shape was biphasic with an early peak of SkBF occurring between 0 and 5 minutes after the onset of local heating, followed by a nadir during about five minutes and later a secondary progressive increase, which stabilized between 25 and 30 minutes (plateau). The most obvious feature is a decrease in the plateau SkBF contrasting with a slight increase in the early peak, from T0 to T2. These changes were consistently observed in the four conditions.
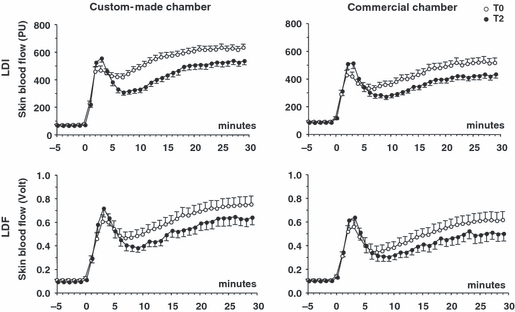
Time course of thermal hyperemia responses recorded on the same skin site carried out on two occasions separated by two hours (T0, T2), using two different heating chambers (custom-made, commercial) and two methods for the measurement of skin blood flow (LDI, laser-Doppler imaging; LDF, laser-Doppler flowmetry). PU, perfusion units. Data points are means of 28 subjects. Error bars are SE rather than SD for graphical convenience.
Figure 3 shows the mean values of baseline, early peak, nadir, and plateau SkBF. The visual impression conveyed by Figure 2, was confirmed by the statistical analysis of these parameters. From T0 to T2, the mean decrease in the plateau SkBF and increase in early peak were, respectively, −9% to −16% and +10% to +18% of the value at T0 (p values below 0.05 for all conditions). As occurred with the plateau, the nadir tended to be lower at T2 than at T0 in the four conditions tested, but the difference reached statistical significance only in the case of the custom-made chamber probed with LDI. Finally (and not obvious in Figure 2), the baseline SkBF, in all conditions, was slightly and significantly lower at T2, in comparison with T0.
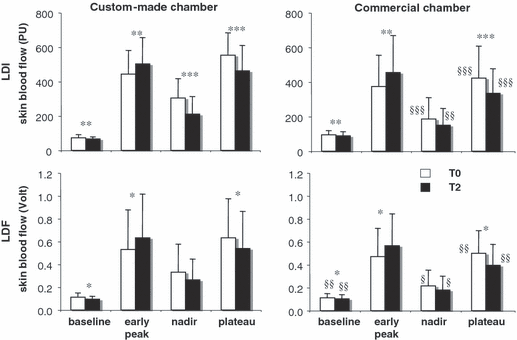
Summary variables derived from thermal hyperemia responses recorded on the same skin site carried out on two occasions separated by two hours (T0, T2), using two different heating chambers (custom-made, commercial) and two methods for the measurement of skin blood flow (LDI, laser-Doppler imaging; LDF, laser-Doppler flowmetry). PU, perfusion units. Data are reported as mean of 28 subjects. Error bars are SD. *p < 0.05, **p < 0.01, ***p < 0.001 T2 vs T0. §p < 0.05, §§p < 0.01, §§§p < 0.001 commercial vs custom-made chamber.
At T2, the higher peak response was associated with a higher mean BP and therefore could reflect a change in perfusion pressure rather than in vascular tone. Against this interpretation, cutaneous vascular conductance (i.e., SkBF divided by mean BP) consistently increased from T0 to T2 (LDI custom-made chamber: from 4.7 ± 1.5 to 5.8 ± 1.9 PU/mmHg, p < 0.001; LDI commercial chamber: from 4.0 ± 2.0 to 5.3 ± 2.6 PU/mmHg, p < 0.001; LDF custom-made chamber: from 6.8 ± 4.0 to 8.3 ± 4.7 mV/mmHg, p = 0.001; LDF commercial chamber: from 6.2 ± 2.7 to 7.7 ± 3.4 mV/mmHg, p = 0.001).
Finally, the plateau response was somewhat lower with the custom commercial, when compared with the custom-made chamber. Although statistically significant, this effect was minor and could have been related to small differences in heating rate and temperature reached.
Discussion
The present study confirms our previous observation that the repeated application of a local thermal stimulus on the same skin patch, at least when carried out within two hours, leads to a reduction in the elicited vasodilatory response. However, two studies [4,20] have not noticed this phenomenon. Therefore, the questions that must be asked are the reasons for this apparent discrepancy and whether differences in methods could be involved. The major difference relates to the equipment, both for measuring SkBF (LDF vs LDI) and for local heating (commercially available vs custom-made chambers, which may not have the same surface area and heating rate). Any of these factors could have contributed to the discrepancy between our previous observations [3] and those made by these other groups [4,20]. However, in the present study, desensitization clearly occurred in all tested conditions, supporting its independency from the measuring equipment and heating system used in the experiment.
In the work by Shastry et al., 10 subjects participated, five men and five women. The laser-Doppler flowmeter (PF 5010; Perimed) was single point at 780 nm, based on exactly the same technology as the less recent Perimed 4001 used in the present study. The local heaters (also by Perimed) were set at 42°C until SkBF had reached a plateau and then turned off. SkBF values were allowed to return to baseline (in about one hour) and the test was repeated [20] with a plateau response somewhat lower than the first one (94%), a difference that was not statistically significant.
In the protocol by Cracowski et al. [4], six subjects were enrolled, three men and three women. The laser-Doppler flowmeter (MoorLAB; Moor Instruments, Devon, UK) was also single point at 780 nm, and associated with integrated local heaters (SH02; Moor Instruments). Heating was carried out to 42°C until SkBF reached a plateau (30 minutes), on two occasions separated by two hours [4].
Thus, the set of conditions in the present study essentially included those used by both authors, in terms of equipment and timing. And nevertheless, desensitization of the plateau response was systematically observed.
The major remaining difference is the much larger size of our study, compared with these others. It must be underscored that the primary aim of these two studies was not to test the reproducibility of thermal hyperemia. Rather, they were powered to detect effects of locally administered pharmacological agents, with sites that were either untreated [4] or treated with placebo [20] used as controls. The data just cited from these two studies exclusively concern the control sites.
With relatively few subjects, the desensitization effect could have been missed, considering the variability of SkBF measured with LDF, which is much higher than with the LDI, as clearly demonstrated by Roustit et al. [18]. Indeed, we carried out a preliminary analysis of our data after the inclusion of the first 12 subjects (not shown), with results qualitatively similar to those shown in 2, 3, and statistical significance for desensitization attained on sites evaluated with LDI (p = 0.001), but not with LDF (p = 0.13). Power calculations then induced us to include 16 more subjects to settle the matter and safely conclude that desensitization is not specific to the particular conditions of our previous study. That it took fewer subjects to detect the same effect with LDI than with LDF instrumentation suggests an advantage in terms of study size of using the former, if available, in future studies, which would employ thermal hyperemia as a tool for probing the skin microcirculation in humans.
The mechanisms implied in desensitization remain incompletely defined. In our previous study [3], we found that local heating desensitized forearm skin to the vasodilatory effects of NO, as administered exogenously by iontophoresis of sodium nitroprusside, a donor of NO. This effect of local heating was transient, being observed in 2, but not four hours after the thermal challenge. On the basis of this observation, we postulated that local heating could down-regulate NO signaling somewhere downstream from the endogenous production of this mediator. In further support of this hypothesis, we had detected a modification of only the NO-dependent (plateau), but not the NO-independent part (early peak) of the induced vasodilation on the second thermal challenge carried out two hours after the first one.
In the present experiments, baseline SkBF was lower and the early peak was higher at T2, in comparison with T0, in apparent contradiction with our previous study, where no change in any of these two variables could be detected [3]. The most likely explanation for this apparent discrepancy is the higher number of enrolled subjects (28 vs 12), leading to a greater power to detect relatively small effects. Desensitization to NO could account for the observed modification of baseline SkBF if, in these thermal conditions (i.e., 34°C), NO actually contributed to lower dermal microvascular tone, as suggested by some [12,16], although not all studies [10,11]. More difficult to understand in this context is the increase in the early peak response observed from T0 to T2.
As the early peak is not caused by NO, it should not be affected by removing or attenuating (by desensitization) the action of this mediator. One might argue that the basal level of NO-dependent vasodilation (i.e., in normothermia, prior to heating and during the first few minutes of heating, when it would remain unaffected) might still modulate the early peak. In that case, however, the expected result of desensitization to NO would be a decrease, not an increase of the initial vasodilatory response to the thermal stimulus.
Some insight into this matter may be provided by data indicating that local heating activates sympathetic nerve endings in the skin microcirculation, with potentially a dual effect on vascular tone, vasodilator on one hand through stimulation of endothelial alpha2 adrenergic receptors leading to enhanced activity of eNOS, vasoconstrictor on the other hand through a direct action on vascular smooth muscle [8,9]. Importantly, the local thermal challenge seems to dynamically alter the balance between these two effects, tipping it in favor of vasodilation during the first 30 minutes, and in the opposite direction later on, accounting for a progressive decline of SkBF even when local heating is maintained (the “dying out” phenomenon) [8]. We speculate that, in the present study, the first thermal challenge at T0 had a persistent influence on local adrenergic mechanisms, such that, on the second thermal challenge at T2, the balance was more intensely tipped toward vasodilation at the time of the early peak. Following this line of thought, one might also wonder whether the later tipping of sympathetic influences toward vasoconstriction might not have contributed to lower the plateau response at T2. Clearly, further studies are warranted to test these hypotheses.
A final note is required regarding the fact that both the nadir and the plateau responses were somewhat lower when thermal hyperemia was elicited by the commercial, in comparison with the custom-made chamber (Figure 3). The amplitude of SkBF responses to local heating is very sensitive to actual skin temperature reached in the steady state, at least in the range of 37–41°C [2]. It could be that, in spite of identical set points, the two systems for local heating slightly differed in that respect. In our preliminary checks, the temperatures achieved by each system were verified by placing a thermistor probe underneath the adhesive tape affixing the chamber to the skin, i.e., not on the exact sites where SkBF was measured (see Methods). At these sites, a small systematic temperature difference between heating systems therefore cannot be formally excluded.
In summary, we confirmed that the hyperemic response of skin microcirculation to local heating is subject to desensitization, at least in young men and with protocols in which temperature is increased rapidly. Desensitization was observed with two different methods of measuring skin blood flow and two different equipments for carrying out local heating, making it likely that our observations reflect a general physiological phenomenon.
Perspective
Although its mechanisms remain to be defined, desensitization should be taken into account by studies using thermal hyperemia to probe the physiology or pharmacology of microcirculation in human skin.
Acknowledgment
The authors wish to thank Guy Berset, Emmanuel Fluck and Danilo Gubian for their excellent assistance.




