Risk Communication Planning for the Aftermath of a Plague Bioattack
Department of Social and Decision Sciences, Carnegie Mellon University.
Abstract
We create an influence diagram of how a plague bioattack could unfold and then use it to identify factors shaping infection risks in many possible scenarios. The influence diagram and associated explanations provide a compact reference that allows risk communicators to identify key messages for pre-event preparation and testing. It can also be used to answer specific questions in whatever unique situations arise, considering both the conditions of the attack and the properties of the attacked populations. The influence diagram allows a quick, visual check of the factors that must be covered when evaluating audience information needs. The documentation provides content for explaining the resultant advice. We show how these tools can help in preparing for crises and responding to them.
1. INTRODUCTION
In a public health emergency, there is little time to develop health communications. Unless that work has been done in advance, public health officials must improvise—at the risk of saying wrong things (because the situation has not been analyzed properly) or of saying right things wrongly (because messages have not been tested for effectiveness). If officials fail the public, then they can cede the stage to less qualified voices, offering confident, incompetent, and contradictory messages.
There are guidelines for systematically developing and evaluating communications for well-specified risks.(1,2) But what happens when a threat's details cannot be predicted in advance? We propose a method for developing communications for such situations. It uses an influence diagram to organize the facts relevant to the decisions that individuals might face. In advance of an emergency, prototype messages would be developed and evaluated for scenarios spanning the range of possible emergencies. When an actual emergency arose, the prototype messages would be adapted to the specific circumstances, drawing on the information organized with the influence diagram. We demonstrate the approach with a plague bioattack, one threat with multiple possible scenarios.
1.1. Current Risk Communication Planning for Plague Attack
In focus groups convened by the Centers for Disease Control and Prevention (CDC), participants reported wanting information that would help them to prevent and detect exposures, identify symptoms, and treat infections, along with background information providing them with basic understanding of the hazard.(3) Such information is available on the CDC bioterrorism website.(4)
As seen below, plague risk reflects the interaction of multiple, complex processes. Without decision-focused analysis, communications can miss critical facts or bury them in irrelevant details. Members of the public cannot be expected to set information priorities about topics where they lack expertise, even with more systematic data-collection methods than focus groups, whose only proper use is in the most preliminary, formative stages of research.(5)
1.2. Influence-Diagram-Based Rapid Communication Method
There are large peer-reviewed and gray literatures about plague, its natural ecology, and control with contributions from many disciplines. Influence diagrams can organize such disparate facts,(6,7) representing critical factors as nodes and their dependencies as connecting arrows.
Section 2 gives an overview of naturally occurring and postattack plague risk. Section 3 presents a basic influence diagram model of a plague bioattack, focused on factors relevant to decision making. Section 4 focuses on measures that disrupt model links. Section 5 describes how the model can be used to identify and organize facts needed for effective communication. We focus on the United States, although many conclusions apply elsewhere.
2. PLAGUE ATTACK SCENARIO AND BACKGROUND INFORMATION
Plague is a rapidly progressing, often fatal disease caused by the bacterium Yersinia pestis. It is a CDC Category A select agent, i.e., an organism suitable for bioterrorism. Plague can infect many warm-blooded animals, often lethally.(8,9)
In order to infect humans, plague bacteria must be inhaled, swallowed, or enter broken skin. Naturally occurring cases are mostly bubonic, transmitted by flea bite and characterized by painful swollen lymph nodes (buboes). Some flea-borne infections become septicemic, infecting the bloodstream. Pneumonic plague is an infection of the airways, and is usually contracted by inhaling infectious fluid droplets.
If begun within 18 hours of the first symptoms, antibiotics can treat most naturally circulating plague strains,(4) though some drug-resistant strains have been observed in Africa.(10) The Soviet Union is thought to have developed multidrug-resistant strains. Because creating antibiotic-resistant bacteria is straightforward, preventing transmission is critical in bioterror attacks. That requires behavioral measures—and communications supporting them.
2.1. Naturally Occurring Plague in the United States
Plague arrived by ship from China more than a century ago, causing rat-borne human epidemics in port cities on the Pacific and Gulf coasts. Aggressive rat control and plague surveillance stopped its spread.(11) Similar measures prevented further urban outbreaks, but not before plague had moved into native rural rodent populations in grassland, forest, and shrubland habitats, where it is now endemic in the western United States.(9,12)
CDC receives about a dozen reports of human plague cases annually, with 78% traced to flea bites, 20% to direct contact with infected animals, and 2% to inhalation (the latter almost always involving domestic cats).(13–15) Although epizootics (epidemics in animals) can be geographically widespread, few human cases have resulted.
2.2. Zoonotic Potential of a Plague Bioattack
Most analyses of plague bioattacks have ignored the zoonotic dimension. For example, a major World Health Organization assessment assumed no animal uptake in a scenario involving 50 kg of plague bacteria dropped from a plane.(16) One of the Department of Homeland Security's (DHS) 15 disaster planning scenarios has aerosol releases causing thousands of human cases of pneumonic plague, but no zoonotic involvement;(17) DHS's first three TOPOFF planning exercises also had no zoonotic dimension.
In nature, plague is a zoonotic disease of rodents (rats, mice, chipmunks, squirrels moles, voles) and lagomorphs (hares, rabbits, pikas), presumably susceptible to aerosol infection.(18) Flea bites and contact with dead animals can infect humans and companion animals like cats and dogs. The risk to humans lasts until an epizootic burns through susceptible animal populations.(13) That could be prolonged, if illness (or fear) undermined the municipal services that control plague risk: pest extermination, garbage collection, lawn mowing, sewer maintenance, animal shelters, etc.(19–21)
3. INFLUENCE DIAGRAM DOCUMENTATION FOR A PLAGUE BIOATTACK
Fig. 1 shows the basic relationships between human and animal plague infections. An aerosol release (top) causes pneumonic plague in humans and animals, initiating a zoonotic cycle that can then cause bubonic plague in humans with the health outcomes that follow. Fig. 2 elaborates these relationships, including bacteria transfer between hosts; factors that affect exposure, detection, countermeasures; and information flows.
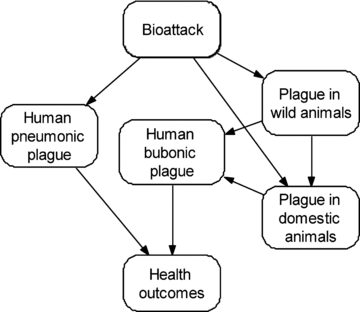
Basic structure of plague influence diagram.
Influence diagram of a plague bioattack, including consequences and public health responses. Numbers refer to text sections.
We now summarize the science underlying zoonotic aspects of a bioattack. Others have summarized the pneumonic side.(4,22–26)
3.1. Plague Release: The Fate of the Plague Aerosol
In air, plague bacteria die in less than an hour, although additives can extend their survival time.(27) Survival also depends on the surfaces that bacteria settle on and ambient conditions, including light, heat, and drying. For example, bacteria on nutrient agar die within 5 hours in direct sunlight, but can survive 4 days in diffuse sunlight. Survival in crushed fleas or their feces ranges from 1 day (at 30°C and open to drying) to 120 days (at 4–5°C in a moist chamber). Desiccation kills quickly.(27) In a study of common work and kitchen surfaces, plague bacteria survival ranged from 2 to 4 hours on stainless steel, polyethylene, and glass to more than 2 days on paper (at 55–60% relative humidity). Suspending cells in broth (rather than nonnutrient buffers) increased viability to 3+ days on stainless steel, polyethylene, and glass and 5 days on paper.(28) These results contradict common assurances that surfaces need not be decontaminated immediately after an aerosol attack.(22–24)
3.2. Plague in Animal Populations
3.2.1. Enzoonotic (Maintenance) Cycle
In nature, plague can persist at low levels of infection in reservoir hosts and their fleas, in the enzootic cycle. In the western United States, reservoir hosts include wood rats (Neotoma species), deer mice (Peromyscus species), voles (Microtus species), kangaroo rats (Dipodomys species), and grasshopper mice (Onychomys leucogaster).(29,30)
Plague can persist in the environment without living rodents in burrows,(31) carcasses,(32) soils,(33) grains, dry sputum, flea feces, and buried human bodies.(27,34) At near-freezing temperatures, it can live for years. Animals digging through contaminated soil can become infected, initiating new enzootic cycles.(35–37) Reported survival times in animal and human remains indicate that cold temperatures increase bacterial persistence. Plague was recovered from exhumed human bodies in SE Russia and Manchuria after 180 days in winter and 30 days in summer. More relevant to bioattacks, plague persisted in guinea pig carcasses after 109 days at –3 to –5°C and mouse carcasses after 22 days at 1–10°C, after 9 days at 10–22°C, and after 7 days at 22–30°C.(27)
Wild animals can transmit plague to urban rodents when their habitats overlap, as has occurred around San Francisco Bay.(38,39)
3.2.2. Epizootic (Amplification) Cycle
When plague crosses into less-resistant amplification hosts, massive outbreaks with high mortality occur, in the epizootic cycle. Ground squirrels (Spermophilus species), chipmunks (Eutamias species), and prairie dogs (Cynomys species) are examples of amplification hosts. They are highly susceptible to plague and experience devastating outbreaks.(40) In addition to decimating the host population, such outbreaks can expand plague into new territories, increasing the chance of incidental human infection.
3.2.3. Incidental Hosts
Only rodents and lagomorphs participate in plague enzootic and epizootic cycles. Other animals, including humans, can be infected, but do not efficiently infect the fleas that feed on them, thereby breaking the cycle of infection.
Carnivores, such as domestic dogs, domestic ferrets (but not the indigenous black-footed ferret), black bears, badgers, coyotes, and skunks, can be infected by flea bite, inhalation, wound, or ingestion. They have fairly strong resistance to plague infection, typically exhibiting mild or no symptoms, after ingesting plague-infected rodents. Felines and black-footed ferrets are exceptions; they become mortally ill when infected. Even if uninfected, carnivores can physically transfer plague bacteria and plague-infected fleas from other animals.
In a study performed in states where plague is endemic, 16% of tested carnivores had antibodies to Y. pestis, evidence of plague infection. The sample included coyotes, badgers, raccoons, foxes, weasels, martens, skunks, bobcats, lynxes, mountain lions, and wild boars. Rates for some individual species were: badger 55%, weasels 43%, coyotes 13–14%, raccoons 6–14%, and bears 3%.(41)
Hoofed animals are rarely infected, hence pose little threat to humans. Birds are resistant to plague, but may transport infected fleas between susceptible hosts. Reptiles and fish are resistant.(9)
If infected, these incidental hosts can pose some risk to humans by transporting infected fleas, infecting wounds and scratches, or emitting respirable infectious droplets.(42)
3.2.4. Zoonotic Flea-Borne Transmission
Flea bite is the main transmission mode for both enzootics and epizootics with their course depending on the flea species. Although fleas have distinct preferences among animal hosts, most infested animals carry several species, including species that prefer other hosts.
About 80 flea species are susceptible to Y. pestis infection.(43) In a few species, after an infected blood meal, Y. pestis clogs the entrance to the midgut with a biofilm-and-bacteria plug.(44) This blockage not only increases the number of bacteria transmitted per bite, but starves the flea, encouraging it to feed more aggressively. When the flea bites, bacteria flow from the plug into the bite wound. Oriental rat fleas (Xenopsylla cheopis), associated with historical plague pandemics, are particularly prone to blockage. Although blocking greatly enhances transmission, most U.S. human cases come from unblocked ground squirrel fleas (Oropsylla montanus), which become infectious immediately after feeding, and then transmit plague efficiently.(45)
3.2.5. Fleas and Climate
Seasonal variation is common in both the presence of different flea species and their infestation levels on different hosts.(46–49) High temperature and low humidity reduce adult flea survival, especially when they leave their hosts.(50) Flea infestations are generally higher around nests and burrows than on roaming adult rodents. In hot weather, burrows and nests have moderate temperature and humidity, thereby improving juvenile flea survival.(51) As a result, environmental conditions cause seasonality in flea-borne human plague coincident with local “flea seasons.” Interannual variation in human plague cases has been linked to precipitation effects on vegetation, rodent abundance, and flea survival.(52)
3.3. Flea-to-Human Transmission
Human plague cases are often associated with epizootic die-offs of rodent hosts after fleas leave dead animals to seek new hosts, including humans within jumping range.(53) Cat fleas can jump 50 cm horizontally and 25 cm vertically in a single jump.(54)
Any flea that can bite through skin can transmit plague, with fewer than 100 bacteria needed to infect a human. Scratching bites can introduce bacteria left by fleas into abrasions. The threat posed by flea vectors depends on their access to humans and their plague transmission efficiency. That said, the inefficient cat flea (C. felis) and human flea (P. irritans) have both been involved in outbreaks, including Japan's use of weaponized plague-infected P. irritans during World War II.(55)
Fleas' biting ability and host preferences also affect their risk to humans.(47,51,56–58) For example, although prairie dogs have been involved in the most widespread U.S. plague epizootics, they account for only 3–6% of human cases, as their fleas prefer other hosts.(9) In contrast, 40% involve the ground squirrel flea, which readily bites humans. Although cat and dog fleas feed on humans, they cause less than 5% because they are poor vectors, with limited access to plague-infected rodents.(51)
Most human-flea interactions in the United States involve companion animals. In the humid east, cat fleas infest cats, dogs, rabbits, and other species, typically feeding on humans only when infestations are high.
In the arid west, the cat flea cannot survive. There, the most problematic fleas infest wild animals, biting humans when those animals die or abandon nests near homes. These species include the human flea, which lives on skunks, opossums, and foxes, and Orchopeas howardi, a fox squirrel flea, which dogs can carry into homes.(59)
Several factors inhibit flea-to-human transmission. One is that only a fraction of the fleas from infected hosts and burrows are typically infected with plague.(60,61) Second, although most mammals can contract plague, only certain species can infect fleas.(62) Third, most flea species transmit plague poorly, either because their mouthparts cannot penetrate human skin or because they prefer other hosts. Fourth, plague bacteria kill many infected fleas before they can transmit disease.(55)
City dwellers typically have little contact with fleas, except for the homeless, shelter workers, and people living in substandard housing. Hikers, campers, and rural residents are sometimes bitten. Small animal veterinarians and assistants, animal shelter workers, and exterminators also face exposure. The largest group of people with regular flea exposure is owners of outdoor dogs and cats.(14,63,64)
For humans in plague-endemic areas, the greatest risks come from contact with infected animal tissues or fluids, rodent harborage, and food sources around the home (e.g., pet food), and fleas on roaming pets.(14,64) Minor risk factors are skinning or cooking rabbits, coyotes, and foxes, and camping.(15,65–67)
3.4. Urban Rodents
3.4.1. Risks to Humans from Rats
Domestic rats are not currently involved in plague transmission in the United States. The two common urban rat species are, however, competent vectors. The black rat, Rattus rattus, inhabits southeastern coastal states from southeast Virginia to Texas and all western coastal states and Hawaii. The larger Norway rat, Rattus norvegicus, is found in all states.(68) Norway rats nest mostly in burrows, usually close to human habitation. They are well adapted to living with humans, frequenting trash heaps, alleys, and sewer systems, especially older combined storm and sanitary sewers. Black rats are climbers, nesting mainly in roofs, attics, and trees.
Rat population size is limited principally by food. A female Norway rat can, theoretically, produce 180 offspring a year, with a gestation period of 23 days and estrus 18 hours after giving birth. The actual annual number of successful weanlings is 10–20.(69,70) Female pups reach sexual maturity in four months, males in three.
The United States has not had a case of human plague from urban rats since 1924. Rats are occasionally involved in epizootic-plague transmission.(11,13) For half a century, densities of rats and transmitting flea species have remained steady, with episodic “hot” spots or seasons.(49) A city's urban rat population is said to be roughly equal to its human population, with great local variation in density.(71)
A 1990 survey in Baltimore found that rats and mice were often seen outdoors, but seldom inside residences; only 1.2% of respondents reported ever being bitten by a rodent.(72) Estimating rat-human interaction from rat-bite frequencies is difficult, as few cases are reported to health authorities.(70) In New York City between 1974 and 1978, the annual average incidence of reported rat bites was 2/100,000, ranging from 8.5/100,000 (Lower East Side) to 0.3/100,000 (Forest Hills, Queens). About half of the incidences occurred while people were asleep.(73–75)
No U.S. city appears conducive to sustained rat-mediated flea-borne transmission to humans, given current rat densities, flea species densities, and contact frequency with other potential hosts,(9) although some neighborhoods may be exceptions.(49) As a result, if the plague infected the urban rats, it would, in most instances, eventually disappear or retreat to rural hosts.
3.4.2. Risk to Humans from House Mice
Although house mice, Mus musculus, can be infected with Y. pestis, they have not been implicated in human plague.(76,77) Feral house mice sometimes migrate seasonally to human dwellings, where they can exchange fleas with domestic mice. However, this transmission pathway is probably very minor.(78) The largest risk to humans comes from cats infected from eating plague-killed mice.(79)
3.4.3. Risks to Humans from Other Urban and Suburban Fauna
Other urban and suburban animals involved in plague transmission include squirrels, chipmunks, voles, and rabbits. The fox squirrel (Sciurus niger) has participated in urban plague circulation in Colorado for at least the last 40 years, with few jumps to humans.(9,13,80,81)
3.5. Companion Animals
More than half of American households have dogs or cats, with more than 30% having at least one cat.(82) Pets living entirely indoors (e.g., rabbits, gerbils, hamsters) have little chance of exposure unless they interact with infectious outdoor pets.
3.5.1. Plague in Cats
Unlike other carnivores, cats are highly susceptible to plague. Like humans, they can develop bubonic plague (sometimes progressing to secondary pneumonic plague), septicemic plague, and primary pneumonic plague. Recognizing plague in cats can be difficult, as typical symptoms resemble other feline diseases, fever (103–106°F; normal temperature is 101°F), anorexia, lethargy, and enlarged, sometimes abscessed lymph nodes (buboes), especially under the jaw (Table I). These abscesses rupture easily, producing exudates loaded with Y. pestis. The mortality rate is about 10% in cats treated with antibiotics, 14% for untreated bubonic, 70% for untreated septicemic, and 83% for untreated pneumonic plague.(42,83)
| Lethargy | 82% |
| Anorexia | 77% |
| Fever (greater than 39.2°C, 102.6°F) | 71% |
| Abscesses (open sores) | 34% |
| Difficulty breathing | 14% |
| Discharge from mouth or nose | 14% |
| Coughing or sneezing | 9% |
| Lethargy, anorexia, fever, buboes | 34% |
| Lethargy, anorexia, fever, abscesses* | 24% |
- *Sixty-one percent of abscesses were of buboes, that is, on lymph nodes. Other locations: on or beneath the tongue, mouth, face, lips, jaw, buttocks, hind limb, forelimb, chest, and abdomen.
Infected cats were the source of 8% of the 297 U.S. cases of human plague from 1977 to 1998, and accounted for all but one case of pneumonic plague.(42,67,84–87) Nearly all these cases involved close physical contact with sick cats; about a quarter occurred in veterinarians or assistants (Table II). Unlike most human plague, cat-related cases do not peak in the summer along with flea abundance.(42) Feces and urine from infected cats seldom contain Y. pestis.(79)
| Activity | Frequency |
|---|---|
| Cared for sick cat, handled cat, buried dead cat | 35% |
| Face-to-face contact, slept with cat, inhalation | 30% |
| Bite | 22% |
| Scratch | 13% |
3.5.2. Plague in Dogs
Dogs can become infected through flea bites, ingestion, or contacting infected animals. Most dogs recover without antibiotics.(67) Although rare, plague fatalities have occurred in both dogs and their owners, with owners being infected by fleas carried by dogs.(88) Plague-infected dogs have no symptoms or nonspecific ones, like moderate fever (105°F vs. normal = 100.5–102.5°F), lethargy, unresponsiveness, oral cavity lesions, anorexia, coughing, and drooling. A study found that dogs deliberately infected subcutaneously (to simulate flea-bite) developed swelling and inflammation within three days. All still had lesions with plague bacteria on Day 10. Plague bacteria were found after 10 days in throat swabs of half the dogs who were fed plague-containing rat viscera.(89) In another study, all dogs infected via inhalation died.(90)
3.6. Human-to-Human Transmission
Human-to-human plague transmission (pneumonic plague) is typically through infectious aerosol droplets.
4. RISK-REDUCTION MEASURES
Risks of human exposure are reduced by interrupting pathways of plague transmission in nature and in the urban environment including home.
4.1. Measures Focused on Wildlife
4.1.1. Environmental Monitoring
Plague epizootics typically go unnoticed, as the hosts are small or reclusive and die unobserved. In a bioattack, concerted environmental monitoring would be needed in order to formulate control strategies and determine when the danger has past.
4.1.2. Plague Suppression
Rodent eradication is not practical, or even possible, for wild species. However, traps and rodenticides can reduce rodent densities, while pesticides can reduce flea burdens (e.g., dusting burrow entrances and runways). Experimental live-virus, bait-delivered vaccines exist for prairie dogs.(91)
4.2. Measures Focused on Urban Rodents
After a plague bioattack, rats and mice, both live and dead, should be considered potentially infectious.
4.2.1. Rat Control
Effective methods include deploying poison bait and traps, destroying rat harborage, and repairing sewer pipes. These activities are usually municipal functions, and cash-strapped cities have experienced widespread problems after eliminating their rodent control programs.3 Poisoning rats without first controlling fleas can raise human risk by increasing the number of questing fleas.
4.2.2. Property Maintenance
Residents can discourage rodents by clearing debris, using metal garbage cans, removing food (e.g., pet food, bird seed, animal feces), and blocking entrances (e.g., holes in foundations, doors, windows).(70) Commercially available traps and poisons can reduce infestations. Burning rat harborage and debris, however, poses fire risks. In 1900, a large section of Honolulu burned down as a result of clearing debris while combating plague.(93)
4.2.3. Treating the Outside Environment
If plague is detected in wild rodents, yards with flea infestations will require pesticide treatment before reoccupation. Protective clothing (long pants with cuffs tucked into socks, long sleeves, gloves) and insect repellent with DEET should be worn.
Dead animals should not be buried, as roaming animals might exhume the infectious remains. Fleas can jump nearly 2 feet, so carcasses should be moved using a long handled shovel. Disposal of remains in sturdy plastic bags in outside garbage cans is recommended.(94)
4.3. Measures Focused on Companion Animals
4.3.1 Plague Prevention
If a bioattack infects urban rodents, roaming companion animals could be affected. Keeping dogs and cats from hunting and eating rodents and rabbits is critical to preventing Y. pestis infection. Free-roaming pets should be treated with quick-acting insecticide, preferably flea powder, and then kept inside. If symptoms appear, prompt treatment is advised. Until neighborhoods are declared plague-free, dogs should be walked with short leashes and kept from contact with rodents and dead animals. Normal waste disposal practices should suffice.
4.3.2. Preferred Methods for Flea Control
Control measures must work quickly. The quickest “knock down” of adult fleas is with insecticidal powders, shampoos, dips, and sprays. “Spot-on” systemic treatments, fast-drying liquids applied between pets' shoulder blades or along the backbone, take a day to kill all adult fleas (killing 98% within 12 hours), during which time some fleas may bite or move elsewhere. Thus, spot–on treatments leave some small risk, so treated pets should be isolated from humans for a day while the fleas die. When defleaing an animal, people should wear protective clothing and insect repellent (Section 4.3.5). To prevent reinfestation, the environment may need to be treated (Section 4.3.6).
4.3.3. Flea Treatments That Don't Work
As mentioned, some topical flea treatments work too slowly to provide instantaneous protection. All systemic pesticides are imperfect, as fleas ingest them when they bite, which is too late to prevent plague transmission. Gas-emitting flea collars work only around the neck, leaving fleas elsewhere. Herbal remedies (e.g., garlic, onions, thiamine, fleabane, brewer's yeast, eucalyptus) provide no protection. Flea pills do not kill adult fleas, but prevent flea eggs from emerging into the larval stage.
Insecticides can kill or sicken kittens younger than five months old, so nonpesticidal methods may be necessary. These include flea combs and pesticide-free shampoo.(95) These methods put the groomer at increased risk of flea bite and release host-less, live fleas. Nonpesticidal methods should be employed only if the animal has not been exposed to plague.
4.3.4. Illegal Pesticides
EPA's website warns about counterfeit flea treatments resembling registered products, which might become more common after a plague bioattack.(96) Illegally imported unsafe pesticides are another threat, especially to immigrants from the importing countries.(97)
4.3.5. Handling a Sick Pet
Only veterinarians can confirm plague. If they do, family members need prophylactic antibiotics. If professional help is unavailable, as might happen during a plague attack, symptomatic pets should be treated as though infected. After flea treatment, (potentially) sick animals should be isolated and allowed to recover or die by themselves. Antibiotics prescribed to humans should not be shared with animals, as neither humans nor animals will get proper doses.
People who handle sick pets should wear protective clothing, work gloves, insect repellent containing DEET, and eye and breathing protection. When done, they should wash immediately with soap and water, launder clothing in hot water and detergent, and disinfect any surfaces that animals have touched with a 10% solution of household chlorine-based bleach. Facemasks should be discarded in a plastic bag, along with other contaminated items that cannot be cleaned.
As a precaution, even asymptomatic pets should get flea treatment and be kept off beds. Owners should avoid nuzzling, scratches, bites, and contact with sores and saliva.
4.3.6. Treating the House for Fleas
If a house is infested with fleas, daily vacuuming is recommended, especially of carpets, under furniture, flooring cracks, baseboards, windows, doorframes, and places where animals rest. A flea collar or mothballs inside the vacuum bag will kill fleas caught there. Pet bedding should be washed or steam cleaned, and areal insecticidal sprays or flea bombs used for persistent infestations. Treatments must be repeated until all flea pupae have hatched, which could take months.
4.4. Measures Focused on Infectious Humans
Because human-to-human plague transmission (pneumonic plague) typically involves infectious aerosol droplets, barrier methods, like masks, could protect patients' caregivers.(98) If the strain is sensitive to antibiotics, prophylactic antibiotics could reduce disease incidence. A formalin-inactivated vaccine exists for bubonic plague. However, it requires several doses spread over months and does not protect against pneumonic plague.(14) New vaccines are under development, based on F1 and V antigens of Y. pestis. Once shown safe and effective, their usefulness in a bioattack will depend on how available they are and how quickly they stimulate an immune response.(99)
5. COMMUNICATIONS IN BIOATTACK RESPONSE
Focusing communications on the risk factors that determine human exposures and the measures that might control them makes best use of citizens' limited time, energy, and resources—while protecting them against the false security of intuitively appealing, but ineffective measures.
Once identified, plague-related advice is relatively easy to explain. The communication challenge is identifying the few critical facts, in this complex domain. Fig. 2 structured that process by summarizing the possible human exposures, the factors leading to them, and the opportunities for their reduction.
Once the content of communications has been selected, it must be made comprehensible. Often, that requires affording recipients a mental model of why the actions are recommended and how they can be adapted to specific circumstances. Fortunately, many plague facts are special cases of familiar processes. For example, plague is caused by a bacterium that antibiotics can treat (unless it has been genetically altered); that dies quickly in sunlight, but persists in cool, humid environments; and that is transmitted by close contact (fleas, droplets, wound exudates). For individuals who understand these core concepts, specific messages (e.g., not nuzzling pets, keeping a safe distance from dead animals) should take little additional explanation.
5.1. Communications Based on Core Concepts
Our analysis points to the following core concepts:
- •
Plague is a deadly infectious disease caused by a bacterium.
- •
Early symptoms in humans resemble flu or digestive upset.
- •
Starting antibiotics as soon as symptoms appear increases the chance of survival.
- •
Plague can infect many mammals and fleas that can, in turn, infect humans.
- •
Plague can be spread by flea and animal bites, inhalation, and cuts.
- •
Plague bacteria can survive for days in humid, cool, dark places.
Before an emergency, research should establish empirically how well people understand these core concepts, and then find ways to make needed improvements. Research can then build on these core concepts to explain the measures(14) described in Section 4, with the key ones being:
- 1
reduce rodent harborage and food sources near the home;
- 2
use insect repellents when outdoors;
- 3
keep cats and dogs indoors;
- 4
use fast-acting insecticides to kill fleas on cats and dogs;
- 5
avoid sick or dead animals; and
- 6
avoid sick cats' fleas, open sores, or respiratory droplets.
When treatments have potential side effects, those should be acknowledged in quantitative terms showing the size of the risks and allowing comparisons to benefits.
5.2. Using the Diagram to Construct Communications
5.2.1. Exposure-Specific Messages
Fig. 2 highlights the exposure routes that these measures seek to control. Assembling the information regarding a particular exposure route involves tracing the arrows from the communication node to the relevant exposures (double oval nodes) and, then, to the factors contributing to that exposure. These factors are labeled with the numbers of the sections (in this article) providing explanatory material. Messages should convey this content, following the diagram's causal structure and invoking the core concepts. As ever, messages should be evaluated empirically. Communications currently used in plague-endemic areas might be adapted to bioattacks.(85,100)
As an example, Fig. 3 highlights the portion of the model for pet care advice. It shows three classes of exposures: “home,”“outdoor & recreational,” and “pet-related.” The arrows connect these exposures to the contributing risk factors (round-cornered oblong nodes). This information determines the relevance and effectiveness of possible protective measures (rectangles). Invoking the core concepts should give the recommendations credibility and help people to apply them appropriately.

Relevant nodes and text sections for pet care information.
5.2.2. Answering Context-Specific Questions
Any generic message will omit situations important to some people. The diagram can help to answer such questions. Consider, for example, hotline callers asking: “Is it OK for my children to play outdoors?” The hotline operator would locate the exposure node “outdoor & recreational exposure” in Fig. 2 and review the factors pointing to it (Fig. 4). The operator could then walk the caller through the potential risk factors (e.g., do outdoor cats or dogs frequent the play area?). Combining that information with other knowledge (e.g., whether a caller's area has an epizootic) allows assessing the value of measures like “using insect repellant” or “avoiding wild rodents.”
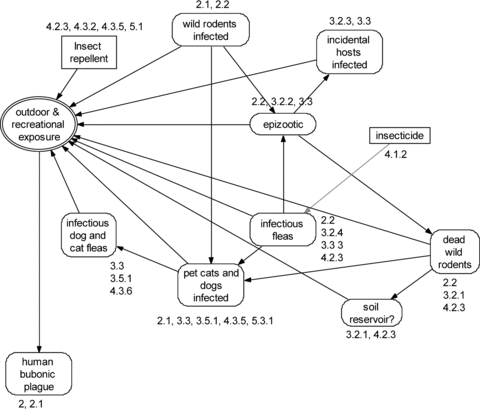
Information needed to answer the question “Is it OK for my children to play outdoors?”
5.3. Information for Heterogeneous Risk Groups
The risks faced by some special populations will be known in advance.(22) Messages should be developed for them, both to serve their needs and to avoid cluttering general messages with information that most people do not need. Trusted sources may be enlisted in designing and disseminating these messages.(101,102) If these audiences lack the material resources or physical abilities needed to protect themselves, communication is an incomplete solution.
5.3.1. Professions
Tailored messages are needed for people with jobs that create special exposures, such as laboratory technicians, health care workers, veterinarians and assistants, animal shelter workers, exterminators, morticians, coroners, medical examiners, dieners, and police. These individuals will often have the motivation and background needed to manage the extra information load.(103,104) Their professional communities should be able to help with message dissemination, comprehensibility, and realism (e.g., recognizing the constraints of their jobs).
5.3.2. Vulnerable Populations
Homeless and poorly housed individuals face elevated risk. Homeless shelters increase exposure to respiratory infections and diseases borne by rats, fleas, lice, and ticks.(105–107) Children might have limited understanding and unusual exposures (e.g., befriending animals that act strangely). Language barriers, social isolation, and distrust may increase the vulnerability of individuals in immigrant communities.
5.3.3. Transient Populations
An attack will catch some individuals away from their usual surroundings, without needed resources or knowledge (e.g., campers, hikers, hunters, travelers). These individuals will need special messages (e.g., about local medical resources) and help, perhaps conveyed through local professionals (e.g., rangers, police, hotel staff).
6. CONCLUSION
Urban plague risk has been eliminated in the United States for a century, thanks to citizens and professionals who have maintained clean surroundings and suppressed outbreaks. A bioattack could undermine these strategies (e.g., through absenteeism among sanitation workers), while introducing new vectors (e.g., pets). Although the full picture is complex (Fig. 2), each exposure route is much simpler (Figs. 3 and 4). Moreover, all routes share familiar processes, summarized in the core concepts, with specialized information available for dealing with specific exposures (e.g., how far fleas jump; how to handle a sick cat). As a result, this complex topic can be reduced to a small set of measures that should be relatively easy to understand and execute.
Whether that potential is realized depends on the quality of the research implementing it. Message testing is needed to reveal how robust existing beliefs are and where they require correction, as well as where messages make unrealistic demands (e.g., requiring material resources or physical abilities that people lack). Messages will undermine trust if they make no sense or ask people to do the impossible.(108) They will strengthen public morale if they afford a warranted feeling of self-efficacy, as well as confidence in authorities who have demonstrated their ability to meet the public's needs.
The method proposed here can facilitate anticipating information needs, composing messages, predicting noncompliance, and responding to emerging events. Combined with empirical message testing, it can help health communications officials get ahead and stay abreast of an attack.
Once derived, this advice may seem somewhat obvious. However, some of the advice in this article contradicts the assumptions in prominent plague bioterrorism scenarios. These scenarios assume that (1) aerosolized plague will die in an hour, ignoring conditions that increase survival times; (2) household surfaces need not be disinfected, ignoring substrate effects; (3) mice play no role, ignoring their ability to infect cats; (4) antibiotics will work, ignoring the possibility of engineered strains; (5) adequate personnel and supplies will be available, ignoring epidemics' disruptive effects; and (6) people will follow recommendations, ignoring barriers to understanding and execution. Most importantly, official scenarios ignore the roles of zoonotic processes. These questionable assumptions were revealed by analyzing these scenarios in terms of the advice that ordinary citizens need.
Footnotes
ACKNOWLEDGMENTS
This work was supported by EPA/DHS CAMRA Center Grant RD832362, NSF Grant SES-0433152, and the MacArthur Foundation. We thank David Wagner, Julie Downs, and Michael DeKay for their suggestions and insights.




