The JAK2 V617F mutation in patients with cerebral venous thrombosis
Abstract
Summary. Background: It is currently unclear whether or not cerebral venous thrombosis, such as splanchnic venous thrombosis, can be the first manifestation of an underlying myeloproliferative neoplasm.
Objective: To determine the prevalence of the JAK2 V617F mutation in patients with a first episode of cerebral venous thrombosis.
Patients: In this retrospective cohort study, patients with cerebral venous thrombosis were tested for the JAK2 V617F mutation and were followed until the development of a myeloproliferative neoplasm or censored at the end of follow-up.
Results: Ten of 152 patients (6.6%) carried the JAK2 V617F mutation. Three of them had known acquired risk factors for thrombosis, and five had thrombophilia. Six patients met the diagnostic criteria for myeloproliferative neoplasm at the time of cerebral venous thrombosis, and three additional patients developed the disease during the follow-up (median duration 7.8 years, range 6 months to 21.3 years), giving an annual incidence of 0.26% patient-years (95% confidence interval 0.05–0.64). The last patient has no evidence of disease after 3 years of follow-up. Patients without the JAK2 V617F mutation at the time of cerebral venous thrombosis were retested at the end of the follow-up and remained negative, with normal blood counts (log-rank test χ2: 159 [P < 0.0001]).
Conclusions: Cerebral venous thrombosis can be the first symptom of a myeloproliferative neoplasm. Patients with cerebral venous thrombosis can carry the JAK2 V617F mutation, irrespective of blood count.
Introduction
Thrombosis is the leading cause of morbidity and mortality in patients with chromosome Philadelphia-negative myeloproliferative neoplasms, i.e. essential thrombocythemia, polycythemia vera, and primary myelofibrosis [1]. Venous thromboses at rare sites are characteristics of these diseases. Splanchnic vein thrombosis occurs in 5–10% of patients with overt myeloproliferative neoplasms [2,3], and is the first clinical manifestation of a still undiagnosed myeloproliferative neoplasm in 25–65% of cases [4]. In 2005, the somatic gain-of-function mutation in the Janus kinase gene (JAK2), resulting in the replacement of valine by phenylalanine at codon 617 (V617F), has been described as contributing to the clonal expansion of hematopoietic cells in myeloproliferative neoplasms [5]. The JAK2 V617F mutation is detected in 50–60% of patients with essential thrombocythemia and primary myelofibrosis, and in 95% of those with polycythemia vera [1,5]. Hence, the presence of the JAK2 V617F mutation is one of the diagnostic criteria for myeloproliferative neoplasms, and is commonly looked for in patients with splanchnic vein thrombosis [6,7].
Cerebral venous thrombosis is another rare thrombosis, and occurs in 1% of patients with overt myeloproliferative neoplasms [3]. Whether or not, as shown for splanchnic vein thrombosis, it is an early sign of an underlying myeloproliferative neoplasm is not established. The JAK2 V617F mutation has been investigated in a few studies with small sample sizes, and these have reported a prevalence in patients with cerebral venous thrombosis varying from 0% to 6.2% [8–13]. The largest study, of 87 patients, found only one patient (1.1%) with the JAK2 V617F mutation [10]. In addition, a meta-analysis [14] and a literature review [15] were inconclusive regarding the association between the JAK2 V617F mutation and cerebral vein thrombosis, suggesting the need for further research. With this as a background and in order to assess whether or not patients with a first episode of cerebral venous thrombosis should be investigated for myeloproliferative neoplasms, we carried out a retrospective cohort study to assess JAK2 V617F status in a large cohort of patients at the time of their cerebral venous thrombosis. In addition, we looked at the possibility of them developing a myeloproliferative neoplasm over time during their follow-up.
Patients and methods
Patients
Patients with a first episode of cerebral venous thrombosis consecutively referred between January 1991 and October 2010 for thrombophilia screening to the outpatient clinic of our Thrombosis Center formed the initial cohort of this study. Patients with brain tumors or non-hematologic cancers, and patients with inadequate objective documentation of cerebral venous thrombosis, were excluded. Patients referred after more than 12 months from the onset of cerebral venous thrombosis were also excluded, to ensure the temporal association between the potential risk factor (JAK2 V617F mutation) and the disease (cerebral venous thrombosis). Demographic data, location of thrombosis, types of symptom, and medical history, focusing on potential risk factors for thrombosis other than the thrombophilia that was the original reason for of referral (infections, autoimmune diseases, trauma, surgery within 1 month, oral contraceptive use, pregnancy, or puerperium), were recorded on the day of blood sampling. In the absence of these predisposing factors, cerebral venous thrombosis was considered to be unprovoked.
For the purpose of this study, we diagnosed myeloproliferative neoplasms associated with cerebral venous thrombosis by applying retrospectively the 2008 World Health Organization diagnostic criteria to the data at the onset of cerebral venous thrombosis that were collected at the time of the first visit [6]. Patients were followed on an annual basis by means of a visit to the center, telephone contact, or a mail questionnaire on healthy status, followed by telephone contact. At the follow-up visits, blood was taken for whole blood counts. Patients unable to reach the center were asked to obtain the whole blood count in other laboratories and send its report to us by post, fax, or email. Follow-up started at the time of cerebral venous thrombosis, and ended at the time of diagnosis of myeloproliferative neoplasm or death, or on 20 March 2011 (administrative censoring). All patients had at least 6 months of follow-up.
The Institutional Review Board approved the study, which was carried out and reported according to the STROBE guidelines for observational studies [16]. Patients gave their written informed consent to participate in the study.
Laboratory tests
Blood samples were taken at the time of the first visit for whole blood count and thrombophilia testing, including: DNA analysis for factor V Leiden and G20210A prothrombin mutation [17,18]; functional assays and/or immunoassays for plasma antithrombin, protein C, and protein S [19]; antiphospholipid antibodies (lupus anticoagulant, anticardiolipin, and anti-β2 glycoprotein I antibodies) [20]; and fasting and post-methionine load homocysteine [21]. For patients who gave consent, DNA was stored, and the JAK2 V617F mutation was searched for in these samples for the purpose of the study.
The JAK2 V617F mutation was tested for in DNA obtained from peripheral blood, with a home-made salting-out procedure [22]. JAK2 V617F status was assessed with an amplificatory refractory mutation system characterized by high sensitivity (0.05% for the mutant allele). Oligonucleotides and PCR conditions are available on request. Samples presenting a band corresponding to the JAK2 V617F mutant allele were tested with a quantitative real-time PCR (qRT-PCR)-based allelic discrimination assay that evaluated the mutant allele burden by application of the standard curve method [22]. The sensitivity of the qRT-PCR-based allelic discrimination assay is approximately 0.2% for mutant alleles [23]. Patients were defined as being positive for the JAK2 V617F mutation in cases of allelic burden of ≥ 1%. This test was performed on DNA obtained from total leukocytes, and therefore did not allow discrimination between heterozygosity and homozygosity for the mutation.
Blood count was considered to be abnormal according to the World Health Organization criteria for the diagnosis of myeloproliferative neoplasms [6].
Statistical analysis
Continuous variables were expressed as medians with minimum and maximum values, and categorical data as counts and percentage. For categorical variables, comparison between groups in a 2 × 2 table was performed with Fisher’s exact test. The annual incidence of myeloproliferative neoplasms was calculated separately for JAK2 V617F-positive and JAK2 V617F-negative patients by dividing the number of events by the total number of patient-years. In order to avoid selection bias, we excluded those patients who already had a myeloproliferative neoplasm at the time of diagnosis of cerebral venous thrombosis. Incidences and 95% confidence intervals (CIs) were calculated under the Poisson distribution assumption. Cumulative survival free from myeloproliferative neoplasms in carriers and non-carriers of the JAK2 V617F mutation was calculated with the Kaplan–Meier method, and comparison between the two groups was performed with the log-rank test. P ≤ 0.05 was chosen as the cut-off for statistical significance. All statistical analyses were performed with the statistical software spss (release 17.0; SPSS, Chicago, IL, USA).
Results
Two hundred and twenty-eight patients with a first cerebral venous thrombosis were referred for thrombophilia screening during the study period. Thirty-seven of them were excluded for the reasons shown in Fig. 1, so that 191 patients entered the study. DNA was not available for testing the JAK2 V617F mutation in 39 patients. Hence, the final study population tested for the JAK2 V617F mutation on blood samples taken at the time of referral to the Thrombosis Center included 152 patients. The median time from cerebral venous thrombosis to blood sampling was 5 months (range 1–12 months).
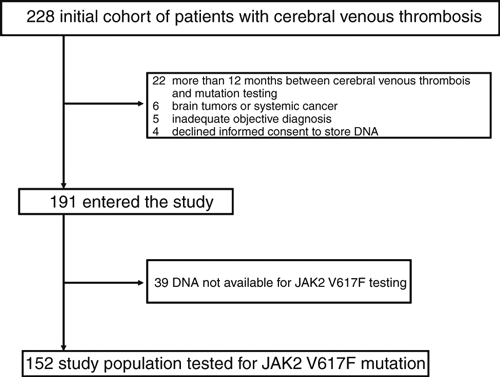
Study flow diagram.
Table 1 shows the general characteristics of the study population. Nearly three-quarters of the patients were women. Cerebral venous thrombosis involved more than one sinus in 75 patients (49.3%), was unprovoked in 41 (27%), and was triggered by two or more risk factors in 14 (9.2%). The most common risk factors for cerebral venous thrombosis were the use of oral contraceptives (74.5% of women) and the presence of thrombophilia (55.9%), particularly that attributable to heterozygosity for the prothrombin G20210A mutation and hyperhomocysteinemia (Table 1). Combined thrombophilia was present in 15 patients (9.9%). Follow-up took place with regular visits at the center for 94 patients (62%) or by telephone contact and questionnaire for the remaining 58 (38%). Seven patients (4.6%) were lost to follow-up, and two died for reasons other than myeloproliferative neoplasms.
| Male/female | 40/112 |
| Age at first cerebral sinus–venous thrombosis (years), median (min.–max.) | 35 (16–79) |
| Age at referral (years), median (min.–max.) | 36 (16–80) |
| Body mass index, median (min.–max.) | 23.6 (14.5–41.5) |
| Site of thrombosis, n (%) | |
| Superior sagittal sinus | 25 (16.4) |
| Lateral sinus | 38 (25) |
| Straight sinus | 4 (2.6) |
| Cavernous sinus | 2 (1.3) |
| Cortical veins | 4 (2.6) |
| Jugular vein | 3 (1.9) |
| Inferior sagittal sinus | 1 (0.6) |
| Combined | 75 (49.3) |
| Risk factors at the time of cerebral sinus–venous thrombosis, n (%)† | |
| Infection | 13 (8.5) |
| Autoimmune or inflammatory disease | 6 (3.9) |
| Trauma | 4 (2.6) |
| Surgery | 11 (7.2) |
| Oral contraceptive use* | 76 (74.5) |
| Pregnancy/puerperium* | 11 (10.8) |
| Thrombophilia, n (%)† | 85 (55.9) |
| Factor V Leiden | 17 (11.2) |
| Prothrombin G20210A mutation | 33 (27.7) |
| Antithrombin, protein C or protein S deficiency | 7 (4.6) |
| Antiphospholipid antibodies | 11 (7.2) |
| Hyperhomocysteinemia | 34 (22.4) |
- *Percentage calculated on 102 women of reproductive age; oral contraceptive use and pregnancy are mutually exclusive. †Some patients had more than one risk factor or combined thrombophilia.
The JAK2 V617F mutation was detected with the amplificatory refractory mutation system in 10 of the 152 patients (6.6%) at the time of cerebral venous thrombosis, and the allelic burden was assessed in eight of them (Table 2). Five patients with the mutation had an abnormal blood count fulfilling the World Health Organization diagnostic criteria of myeloproliferative neoplasms. Bone marrow biopsy allowed the diagnosis of essential thrombocythemia in three patients, and polycythemia vera and an unclassifiable myeloproliferative neoplasm in two patients (Table 2). Another patient with unexplained splenomegaly and normal blood count was diagnosed with primary myelofibrosis by means of bone marrow biopsy. Excluding the six patients with an overt myeloproliferative neoplasm at the time of cerebral venous thrombosis, the prevalence of the JAK2 V617F mutation was 2.7% (four of 146). All patients except the six with overt myeloproliferative neoplasms at the time of cerebral venous thrombosis were followed for a median time of 7.8 years (range 6 months to 21.3 years), giving a total of 1173.5 patient-years. The median follow-up was 2.8 years (range 1.4–3.7 years) for the four patients with the JAK2 V617F mutation and 8.0 years (range 6 months to 21.3 years) for the 142 without. Three patients who had normal blood counts at the time of cerebral venous thrombosis developed essential thrombocythemia during the follow-up period of 2–3.5 years, giving an annual incidence of 0.26% patient-years (95% CI 0.05–0.64). The remaining patient with the JAK2 V617F mutation at the time of cerebral venous thrombosis has normal blood counts and erythropoietin levels after 3 years of follow-up, with a bone marrow biopsy that is not diagnostic of myeloproliferative neoplasm.
| ID no. | Sex | Date of CVT | Age at CVT (years) | Risk factors at the time of CVT | Time elapsed between CVT and diagnosis of MPN (years) | Hb (g dL−1) | Ht (%) | WBC count (× mm3) | Platelets (×103 mm3) | Type of MPN | JAK2 V617F allelic burden at the time of CVT (%)† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | November 1997 | 38 | Oral contraceptives | 3.5 | 12.3 | 38.5 | 5600 | 331 | ET | NA |
| 2 | M | August 1998 | 31 | None | 3 | 13.7 | 41.9 | 6000 | 361 | ET | 10.7 |
| 3 | M | January 1999 | 76 | Protein S deficiency | Concomitant | 16.2 | 48.1 | 5770 | 631 | Unclassifiable | 33.1 |
| 4 | F | February 2000 | 22 | Prothrombin G20210A | Concomitant | 12.2 | 37.0 | 3800 | 366* | ET | 25.8 |
| 5 | M | May 2000 | 28 | Hyperhomocysteinemia | Concomitant | 16.3 | 49.5 | 8200 | 600 | ET | 27 |
| 6 | F | February 2003 | 56 | None | Concomitant | 12.4 | 37.4 | 5200 | 508 | ET | 31.2 |
| 7 | M | June 2004 | 72 | Otitis and ulcerative rectocolitis | Concomitant | 16.1 | 53.0 | 14 000 | 362 | PV | NA |
| 8 | F | January 2007 | 49 | None | Concomitant | 13.0 | 43.0 | 4800 | 207* | PMF | 11.4 |
| 9 | M | November 2008 | 69 | Prothrombin G20210A | 3 | 15.1 | 45.0 | 5590 | 204 | ND | 2.3 |
| 10 | F | October 2009 | 25 | Oral contraceptives and prothrombin G20210A | 2 | 13.3 | 40.7 | 5450 | 334 | ET | 5.9 |
- CVT, cerebral venous thrombosis; ET, essential thrombocythemia; F, female; Hb, hemoglobin; Ht, hematocrit; M, male; MPN, myeloproliferative neoplasm; NA, not assessed; ND, non-diagnostic; PMF, primary myelofibrosis; PV, polycythemia vera; WBC, white blood cell. *Splenomegaly. †The allelic burden was evaluated in DNA obtained from total leukocytes.
In 142 patients (93.4%), all with normal blood counts, the JAK2 V617F mutation was absent at the time of blood sampling within 1 year from cerebral venous thrombosis. They were invited to the center in February and March 2011 to be retested for the JAK2 V617F mutation. Eighteen of them (12.7%) refused to come. In all of the 124 retested patients (87.3%), the JAK2 V617F mutation remained absent. One patient developed unexplained erythrocytosis during the follow-up period (2 years after cerebral venous thrombosis), and phlebotomies were started in order to maintain hematocrit values below 45%. Plasma erythropoietin and red cell mass were normal, and the plasmatic mass was reduced. Bone marrow biopsy was not diagnostic for a myeloproliferative neoplasm. At the onset of erythrocytosis, the JAK2 exon 12 mutation [24], which is associated with polycythemia vera, albeit to a lesser degree than the JAK2 V617F mutation, tested negative. Altogether, myeloproliferative neoplasms were diagnosed in nine of 10 patients (90%) with the JAK2 V617F mutation and in none of 142 without (Fisher’s exact test, P < 0.0001). The myeloproliferative neoplasm-free survival after cerebral venous thrombosis was significantly different in patients with and without the JAK2 V617F mutation (log-rank test χ2: 159 [P < 0.0001]).
Discussion
This study shows that cerebral venous thrombosis may be the first clinical manifestation of a chromosome Philadelphia-negative myeloproliferative neoplasm, such as essential thrombocythemia, polycythemia vera, or primary myelofibrosis. In our cohort of patients with cerebral venous thrombosis, a myeloproliferative neoplasm (mainly essential thrombocythemia) was diagnosed in 6.6% of cases, in 60% of them at the time of cerebral venous thrombosis, and in 40% during the follow-up. The JAK2 V617F mutation was present at the time of cerebral venous thrombosis in all patients with a myeloproliferative neoplasm, including those with normal whole blood counts and no other feature of the disease.
Whether or not cerebral venous thrombosis, like splanchnic vein thrombosis, is associated with myeloproliferative neoplasms is debated. Limited data are available on the association and the usefulness of testing for the JAK2 V617F mutation in patients with cerebral venous thrombosis. The largest retrospective study published so far found only one patient with the JAK2 V617F mutation (and thrombocytosis) among 87 with cerebral venous thrombosis [10]. Three additional studies of smaller sample size [9,12,13] were not able to find the mutation in patients with cerebral venous thrombosis, whereas a fourth study of 48 patients [8,11] found a 6% mutation prevalence, concluding in favor of testing patients with cerebral venous thrombosis and no overt myeloproliferative neoplasms, because they might have a latent disease. We confirm this preliminary observation, the major strength of this study being the follow-up over time of our cohort of patient after their cerebral venous thrombosis. Our main findings are that patients with cerebral venous thrombosis may have a concomitant myeloproliferative neoplasm or develop the disease in the following years if they carry the JAK2 V617F mutation, whereas those with no mutation at the time of cerebral venous thrombosis remain negative at follow-up and have no increased risk of developing myeloproliferative neoplasms. As approximately half of the JAK2 V617F-positive patients had a normal whole blood count at the time of cerebral venous thrombosis, a search for the mutation is recommended to diagnose a latent myeloproliferative neoplasm. The pathogenesis of thrombus formation in patients with myeloproliferative neoplasms involves complex mechanisms of cellular interactions between leukocytes, platelets, and endothelial cells [25]. Having a normal whole blood count in the presence of the JAK2 V617F mutation supports the hypothesis that the increased thrombotic risk associated with myeloproliferative neoplasms is attributable to leukocyte activation more than to the burden of high blood cell count [26].
Some limitations of our study warrant consideration. First, our population of patients is highly selected, because we are a tertiary referral center for thrombophilia screening, and our results do not necessarily apply to all patients with cerebral venous thrombosis. Patients were followed on a yearly basis, but DNA samples were tested retrospectively for the JAK2 V617F mutation. When thrombocytosis or erythrocytosis became manifest, the JAK2 V617 mutation was searched for and a myeloproliferative neoplasm was diagnosed. Then, the JAK2 V617F-positive patients were retested for the mutation in the samples stored at the time of cerebral venous thrombosis, when the blood count was normal and the mutation was already present. The mutation was searched for in DNA extracted from total leukocytes instead of granulocytes, the former method being less sensitive. This may have led to an underestimation of the prevalence of the JAK2 V617F mutation in our series, and did not allow assessment of heterozygosity or homozygosity. Another limitation is that only the JAK2 V617F mutation was tested for, and the possibility that some patients have different mutations in JAK2 or other genes that are more rarely associated with myeloproliferative neoplasms [24] cannot be ruled out, and may be another reason for the possible underestimation of the association between cerebral venous thrombosis and myeloproliferative neoplasms. Finally, for 20% of patients, DNA was not available. Assuming, in the worst scenario, that the JAK2 V617F mutation was absent in these patients, the frequency of the JAK2 V617F mutation in patients with cerebral venous thrombosis would still be substantial (5.2% instead of 6.6%).
What are the clinical implications of an early diagnosis of myeloproliferative neoplasms in patients with cerebral venous thrombosis? These patients are at high risk of thrombosis recurrence, not only because they have a myeloproliferative neoplasm (even if latent), but also because they have already had a cerebral venous thrombosis [27]. They often receive anticoagulant therapy for a limited period of time, depending on the presence of triggering conditions and risk factors for thrombosis [28,29]. When venous thrombosis occurs in patients with a permanent risk factor (e.g. severe thrombophilia or cancer), anticoagulant treatment is continued indefinitely [30]. The same is true for patients with venous thrombosis associated with myeloproliferative neoplasm [27]. Hence, although the optimal duration of anticoagulant therapy in patients with cerebral vein thrombosis who carry the JAK2 V617F mutation is not established, we suggest caution in discontinuing anticoagulation, even if cerebral venous thrombosis occurred in the presence of a transient and no longer present risk factor. The choice of starting cytoreductive therapy, interferon-α or JAK inhibitors should be tailored on an individual basis [7,31].
In conclusion, a myeloproliferative neoplasm should be suspected in a population of selected patients with cerebral venous thrombosis, such as those referred to a tertiary care center. Patients with cerebral venous thrombosis can carry the JAK2 V617F mutation independently of the blood count. The probability of having a myeloproliferative neoplasm concomitant with thrombosis or in the follow-up period is very high in patients with the mutation.
Addendum
S. M. Passamonti and I. Martinelli: had the original idea for the study; S. M. Passamonti and F. Gianniello: collected data; E. Biguzzi, F. Franchi, and D. Pietra: performed laboratory tests; P. Bucciarelli and S. M. Passamonti: performed statistical analysis; I. Martinelli: wrote the manuscript; M. Cazzola and P. M. Mannucci: performed critical revision of the manuscript for important intellectual content. The manuscript was critically revised and approved by all authors; the corresponding author had full access to all data, and had final responsibility for the decision to submit for publication.
Acknowledgements
Studies performed at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo and University of Pavia were supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan, Italy) and Fondazione Cariplo (Milan, Italy). In particular, M. Cazzola acknowledges funding from the AIRC Special Program ‘Molecular Clinical Oncology five per mille’.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




