Relevance of chloride binding to von Willebrand factor in type 2B von Willebrand disease patients
Type 2B von Willebrand disease (VWD2B) is due to a gain-of-function variant of von Willebrand factor (VWF) characterized by an enhanced interaction with the platelet glycoprotein (Gp)Ib-α [1]. This condition may result in the sequestration of VWF onto the platelet surface, responsible for variable thrombocytopenia [2]. The first report of an increased proteolysis of VWD2B was by Zimmerman et al. [3], who showed the increased intensity of the inner bands of VWF triplet structure in VWD2B patients’ plasma. More recently, Nishio et al. [4] demonstrated that the interaction of the GpIb-α with the VWF A1 domain increased the susceptibility of the adjacent A2 domain to ADAMTS-13, justifying the increased proteolysis in VWD2B. However, only recently De Cristofaro et al. [5] described a specific binding of chloride ions (Cl−) to the VWF A1 domain that reduced the susceptibility of the adjacent A2 domain to ADAMTS-13 activity. Furthermore, the VWD2B variant p.R1306W was found to have a reduced affinity for Cl− and an increased susceptibility to ADAMTS-13 proteolysis [6]. In this study, we extended our investigation to another four VWD2B variants, confirming that all reported mutations reduced their binding to Cl−, and increased their susceptibility to ADAMTS-13 cleavage, although to different degrees. In addition, using the VWF:RCo/Ag ratios, that in VWD2B patients are indicative of the partial loss of high molecular weight multimers (HMWM), we tried to correlate the increased susceptibility to ADAMTS-13 cleavage of these five VWD2B variants with the multimeric pattern in the plasma of patients who carry the corresponding mutations [7].
Site-directed mutagenesis of the expression vector pcDNA3.1SPHA1A2A3V5 [6] allowed us to obtain in HEK 293 cells five distinct VWF A1-A2-A3 domains, each one bearing one of the following VWD2B mutations: p.H1268D, p.R1306W, p.V1316M, p.R1341Q and p.R1341W [6].
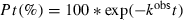
In the titration experiments, performed to evaluate Cl− interaction with the A1-A2-A3 domains, the maximum emission wavelength of all A1-A2-A3 domains was unchanged (338 nm) as a function of NaCl concentration up to 0.1 m, whereas at higher concentrations (> 0.15 m) a little blue shift of about 5 nm was observed. The affinity of Cl− for the A1-A2-A3 domains was calculated from the fluorescence decrease as a function of Cl− concentration, as described [6]. The Cl− affinity for the WT A1-A2-A3 domain was higher than that for the VWD2B A1-A2-A3 variants. The Kd values (mm) were: 41 ± 2 for WT domains; 49.9 ± 2 for p.R1341Q; 92.6 ± 4 for p.R1341W; 108.9 ± 4 for p.V1316M; 112 ± 3 for p.H1268D and 164 ± 6 for p.R1306W (Fig. 1A). All VWF A1-A2-A3 constructs were used to study the effects of Cl− on cleavage by ADAMTS-13 of VWD2B mutations (Fig. 1B). The kcat/Km values (m−1 s−1) were: 7.25 × 104 (WT); 8.8 × 104 (p.R1341Q); 1.2 × 105 (p.R1341W); 1.39 × 105 (p.H1268D); 1.6 × 105 (p.V1316M) and 1.95 × 105 (p.R1306W).

Evaluation of Cl− binding and ADAMTS-13 cleavage of WT and VWD2B A1-A2-A3 domains and their correlation with the VWD2B patients’ plasma multimeric pattern. Panel A: fluorescence titration data of chloride binding to purified WT, p.R1306W and p.R1341W A1-A2-A3 domains of VWF at 25 °C. The continuous lines were drawn to best fit parameter values as detailed in the text. Panel B: RP-HPLC separation of the intact and hydrolyzed VWF A1-A2-A3 domains, under the experimental conditions reported in the text. The arrows show the N-terminal cleaved A1-A2-A3 and the uncleaved A1-A2-A3 peptides separated by acetonitrile gradient in a RP 304 column. Panel C: kinetics of hydrolysis by ADAMTS-13 of WT and mutant A1-A2-A3 domains. The percentage of uncleaved A1-A2-A3 domains as a function of time is shown. The data were fitted to Equation 1. Panel D: the different inhibitory effect of chloride, at 10 and 200 mm, on the proteolysis of WT vs. p.V1316M A1-A2-A3 domains. Of note, the different sensitivity of WT to NaCl compared with the VWD2B variant. Panel E: inverse correlation (R = 0.925, P < 0.006) between the affinity of the A1-A2-A3 molecules for Cl− (KdCl−, horizontal axis) and the specificity constant (kcat/Km, vertical axis) for the ADAMTS-13 proteolysis. Panel F: a linear relation was found (R = 0.884 and P = 0.019) when the values of the VWF:RCo/VWF:Ag ratio of the VWD2B variants [7] were plotted as a function of the corresponding kcat/Km values obtained with the VWF A1-A2-A3 molecules.
All VWD2B A1-A2-A3 constructs were hydrolyzed faster than the WT form (Fig. 1C). In addition, the different inhibitory effect of chloride, at 10 and 200 mm, on the proteolysis of WT vs. p.V1316M A1-A2-A3 domains is reported (Fig. 1D). An inverse correlation was found between the affinity to Cl− of the A1-A2-A3 molecules and their susceptibility to ADAMTS-13 cleavage when the KdCl− values were plotted as a function of the corresponding kcat/Km values measured at 10 mm [Cl−] (Fig. 1E). In addition, a linear relationship was found when the mean value of the VWF:RCo/VWF:Ag ratio of the VWD2B patients carrying these five mutations was plotted as a function of the corresponding kcat/Km values, as shown in Fig. 1F.
In this study we showed that the conformational state of VWF available to interaction with GpIb-α, stabilized by the VWD2B mutations, is also linked to a reduced capacity of the mutated A1 domain to bind the allosteric modulator Cl−. As a consequence of the reduced affinity for Cl− the five VWD2B variants were cleaved by ADAMTS-13 more efficiently than the WT form, even in the absence of interaction with the GpIb-α receptor. As shown in Fig. 1E, these two events appear to be strongly related by an inverse correlation, suggesting that the reduced affinity of the VWD2B variants for the Cl− and their increased susceptibility to ADAMTS-13 proteolysis are thermodynamically linked phenomena. The conformational transitions caused by VWD2B mutations reduce the chloride affinity for the A1 domain and this effect is responsible for the enhanced susceptibility of the adjacent A2 domain to ADAMTS-13 proteolysis. Therefore, we suggest that reduced affinity for Cl−/increased susceptibility to ADAMTS-13 cleavage might be a new common feature of VWD2B variants. The relevance of these finding in VWD2B patients was evaluated by comparing the mean values of the VWF:RCo/VWF:Ag ratio of patients carrying the five mutations, as a function of the corresponding kcat/Km value determined using the mutant A1-A2-A3 molecules. The observed linear relationship shows that the VWD2B variants with the most markedly depleted HMWM in the corresponding patients’ plasma were those associated with the highest kcat/Km values, suggesting the important role of the increased susceptibility to ADAMTS-13 cleavage in the defective HMWM from circulating blood of VWD2B patients.
Acknowledgements
This study was supported by research funding from the Bayer Hemophilia Awards Program to L. Baronciani. Financial support from the Italian Ministry of University and Research (‘PRIN-2007’) is gratefully acknowledged (R. De Cristofaro, F. Peyvandi). The authors thank L. Flaminio Ghilardini for illustration work.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




