The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells
Abstract
Summary. Background: Endothelial dysfunction and oxidative stress are matters of concern in patients with chronic renal failure (CRF). Uremic solutes retained in these patients could be involved in these processes. Notably, the protein-bound uremic solute indoxyl sulfate induces endothelial dysfunction in vitro, and has shown pro-oxidant effects. Objective: To demonstrate that indoxyl sulfate is a potential mediator of oxidative stress in endothelial cells in vitro. Methods: Indoxyl sulfate-induced oxidative stress in human umbilical vein endothelial cells (HUVEC) was studied by measuring reactive oxygen specie (ROS) production by cytofluorimetry, by analyzing the involvement of the pro-oxidative enzymes NAD(P)H oxidase, xanthine oxidase, and NO synthase, and by measuring the levels of the non-enzymatic antioxidant glutathione. Results: We showed that indoxyl sulfate induced a significant production of ROS in HUVEC, with or without human serum albumin. We then investigated the role of pro-oxidative enzymes and measured the levels of the antioxidant glutathione. The NAD(P)H oxidase inhibitors, DPI, and apocynin, inhibited ROS production, whereas inhibitors of xanthine oxidase, NO synthase, and mitochondrial ROS had no effect. Interestingly, indoxyl sulfate strongly decreased the levels of glutathione, one of the most active antioxidant systems of the cell. In addition, the ROS production mediated by indoxyl sulfate was inhibited by the antioxidants vitamin C, vitamin E, and NAC. Conclusion: The uremic solute indoxyl sulfate enhances ROS production, increases NAD(P)H oxidase activity, and decreases glutathione levels in endothelial cells. Thus, indoxyl sulfate induces oxidative stress by modifying the balance between pro- and antioxidant mechanisms in endothelial cells.
Introduction
Endothelial dysfunction and oxidative stress are prominent features in patients with chronic renal failure (CRF) [1–5], but the potential causes are not fully understood. The relation between endothelial dysfunction and oxidative stress in CRF patients [5] suggests that common causative factors or interrelated mechanisms are involved.
Uremic solutes retained in CRF patients are good candidates as specific pathogenic agents inducing oxidative stress and endothelial dysfunction [6–8]. We have shown that indoxyl sulfate, a protein-bound uremic solute [9], induces endothelial dysfunction by inhibiting endothelial proliferation and migration in vitro [10]. Some studies suggest that indoxyl sulfate is also involved in oxidative stress. In hemodialyzed patients, serum levels of indoxyl sulfate are associated with levels of pentosidine, a marker of carbonyl and oxidative stress [11]; in vitro, indoxyl sulfate increases reactive oxygen species (ROS) production in tubular cells [12].
Oxidative stress is commonly considered excessive production of ROS, a family of molecules including free radicals such as superoxide anion (O2−˙), hydroxyl radical (HO˙), nitric oxide (NO˙), and non-free radicals such as hydrogen peroxide (H2O2), peroxinitrite (ONOO−), and hypochlorous acid (HOCl). Increased ROS production results from an imbalance between pro- and antioxidant mechanisms of the cell. Potential sources of ROS include mitochondrial respiration, xanthine oxidase, NAD(P)H oxidase, NO synthases, peroxidases, and other hemoproteins [13]. In addition, endothelial cells possess antioxidant enzymatic and non-enzymatic systems, which act in concert to detoxify ROS produced by free radical reactions. The most active non-enzymatic antioxidant is glutathione, a scavenger for hydrogen peroxide and hydroxyl radical.
In the present study, we investigated whether indoxyl sulfate induces oxidative stress in endothelial cells by studying its effects on ROS production and on pro-oxidant and antioxidant mechanisms.
Materials and Methods
Reagents
Indoxyl sulfate, vitamin C, vitamin E, N-acetyl-l-cysteine (NAC), potassium chloride, allopurinol, N-nitro- l -arginine methyl ester (l -NAME), diphenylene iodonum chloride (DPI), apocynin, rotenone, and 2-thenoyltrifluoroacetone (TTFA) were obtained from Sigma (Saint Quentin Fallavier, France). Methanol was purchased from Carlo Erba (Milano, Italy). EGM-2 medium was from Clonetics Biowhittaker (Verviers, Belgium). Trypsin-EDTA solution, RPMI medium, PBS, and gelatin were from Invitrogen (Cergy-Pontoise, France). Fetal bovine serum (FBS) was from Dominique Dutscher (Brumath, France). Human serum albumin (HSA) 20% solution was purchased from LFB (Courtaboeuf, France). 6-Carboxy-2′,7′-dichlorodihydrofluorescein diacetate, di-AM was purchased from Molecular Probes (Eugene, OR, USA). RNeasy mini-kit and RNase-Free DNase Set were from Qiagen (Courtaboeuf, France). The Stratascript First-Strand Synthesis System and Full velocityTM SYBR Green qPCR Master Mix were obtained from Stratagene Europe (Amsterdam, the Netherlands). All primers were from Invitrogen.
Endothelial cell culture
Human umbilical vein endothelial cells (HUVEC) were obtained from umbilical cord vein by collagenase digestion as previously described [14]. Cells were seeded on gelatin-coated culture plates and grown in EGM-2 medium under standard cell culture conditions (humidified atmosphere, 5% CO2, 37 °C). Cells were then detached with a 0.05% trypsin–0.02% EDTA solution and subcultured to the second passage on gelatin-coated 6-well or 24-well culture plates.
HUVEC were cultured on gelatin-coated culture plates until confluence. Cells were then incubated for 5 h with different doses of indoxyl sulfate (25, 50, 125 and 250 μg mL−1). At these concentrations, indoxyl sulfate did not affect endothelial viability or induce apoptosis [10]. Each test was performed in duplicate. Indoxyl sulfate was diluted at least 1/200 from a stock solution prepared in water.
Measurement of ROS production
For measurement of ROS production, HUVEC cultured in 24-well plates were labeled for 45 min with 10 μm of 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, di-AM (6-carboxy-H2DCF-DA, di-AM), as described [15,16] . Cells were washed with PBS, and treated for 5 h with indoxyl sulfate at concentrations found in CRF patients. Some experiments were performed in the presence of human serum albumin (HSA), at the concentration found in human serum (4 g dL−1). After 5 h, production of ROS by HUVEC was measured. Kinetic study was also performed by measuring the fluorescence intensity every hour during a 5-h period.
In some experiments, HUVEC were incubated for 5 h with indoxyl sulfate in the presence of inhibitors of xanthine oxidase (allopurinol 100 μm), NO synthase (l-NAME 1 mm), and NAD(P)H oxidase (DPI 50 μm, apocynin 50 μm), and of mitochondrial electron transport (rotenone 10 μm, TTFA 10 μm), or in the presence of antioxidants N-acetylcysteine (NAC 10 mm), vitamin E (10 μg mL−1), and vitamin C (200 μm). Indoxyl sulfate and inhibitors or antioxidants were added together.
The production of intracellular ROS was detected by measuring the fluorescence of 6-carboxy-H2DCF-DA-di-AM by the Cytofluor® Series 4000 Fluorescence multi-well plate reader (PerSeptive Biosystems, Framingham, MA, USA). The excitation filter was a 20-nm bandwidth centered at 485 nm and the emission filter was a 25-nm bandwidth centered at 530 nm. Fluorescence intensity reflecting ROS production was expressed in arbitrary units.
In some experiments, ROS production after labeling with 6-carboxy-H2DCF-DA-di-AM was measured by flow cytometry on cells detached with a prewarmed 0.05% trypsin–0.02% EDTA solution for 30 s at 37 °C, resuspended in PBS, and analyzed using an Epics® XL flow cytometer (Beckman-Coulter, Villepinte, France). Analysis was focused on the whole cell population, taken for calculation of mean fluorescence intensity. Mean fluorescence intensity was calculated by the System II™ software (Beckman-Coulter) and expressed in arbitrary units.
Comparative quantification of eNOS and iNOS mRNA levels
mRNA expression of eNOS and iNOS was studied by reverse transcription (RT) and comparative polymerase chain reaction (comparative PCR). HUVEC were incubated for 5 h with indoxyl sulfate at different concentrations (25, 125 and 250 μg mL−1). Total RNA was extracted from HUVEC by an RNeasy mini-kit, according to the manufacturer’s instructions, and DNA was digested with DNase to secure complete DNA removal. Total RNA concentrations were determined by spectrophotometry at 260-nm wavelength. The samples were stored at –80 °C for further RT-PCR. Reverse transcription using random primers was performed on 1 μg of total RNA of each sample using the Stratascript First-Strand Synthesis System, followed by PCR on 20 ng of cDNA using Full velocityTM SYBR Green QPCR Master Mix. The housekeeping genes GAPDH and HPRT give the same results for normalization of the target gene values. The results shown in this study were those normalized with GAPDH. The sequences of primers were the following:
eNOS-R (5′-GTCTTCGTGGTAGCGTTGCT-3′)
eNOS-F (5′-CCTGACAACCCAAGACCTA-3′)
iNOS1-R (5′-ACATCCCCGCAAACATAGAG-3′)
iNOS1-F (5′-AGGAGGAGATGCTGGAGATG-3′)
iNOS2-R (5′-TTCTTCGCCTCGTAAGGAAA-3′)
iNOS2-F (5′-CTCTATGTTTGCGGGGATGT-3′)
GAPDH-R (5′-GAGCTATTGTAATGACCAGTCAACAGGG-3′)
GAPDH-F (5′GTTGCTGTAGCCAAATTCGTTTGT 3′)
HPRT-R (5′GAGCTATTGTAATGACCAGTCAACAGG 3′)
HPRT-F (5′-GGATTATACTGCCTGACCAAGGAAAGC-3′)
All PCR efficiencies were determined with mx3000p software (Stratagene Europe, Amsterdam, the Netherlands) and always comprised between 90% and 110%. The fusion curves were analyzed to assess the specificity of detected fluorescence, and sizes of PCR products were verified by electrophoresis on 1.5% agarose gels containing ethidium bromide.
Glutathione assay
Confluent HUVEC cultured in six-well plates were incubated for 5 h with different doses of indoxyl sulfate (25, 50, 125 and 250 μg mL−1). GSH levels in HUVEC were measured by the dithio nitrobenzen (DTNB) method [17] using a colorimetric assay kit (Glutathione Assay Kit, Cayman Chemical Company, Ann Arbor, MI, USA).
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed with the prism software (GraphPad Software Inc., San Diego, CA, USA). Determination of significant differences was performed by the Student’s paired t-test or by analysis of variance (anova) followed by a Bonferroni test. A P-value lower than 0.05 was considered significant.
Results
Effect of indoxyl sulfate on ROS production in HUVEC
To investigate whether indoxyl sulfate mediates oxidative stress in endothelial cells, we evaluated its effect on intracellular ROS. Indoxyl sulfate significantly increased ROS production in HUVEC by 43% (P < 0.001 vs. control) and 74% (P < 0.001 vs. control), respectively, at 125 μg mL−1 and 250 μg mL−1 (Fig. 1), whereas no significant increase was observed in the presence of the lowest concentrations of indoxyl sulfate. The increase in intracellular ROS in HUVEC induced by indoxyl sulfate at 125 and 250 μg mL−1 was confirmed by flow cytometry (Fig. 2). Hydrogen peroxide, used as a positive control of ROS induction, increased ROS production in HUVEC by 150% (P < 0.001 vs. control). In addition, we verified that potassium salt, present in indoxyl sulfate, had no effect on ROS production (not shown). Kinetic studies showed that indoxyl sulfate at 250 μg mL−1 and 125 μg mL−1 induced significant ROS production in HUVEC after 1 h and 2 h of incubation, respectively (Fig. 3).
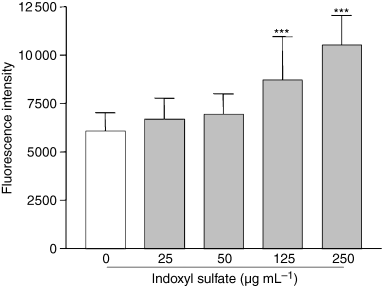
Effect of indoxyl sulfate on ROS production in HUVEC. Endothelial cells were incubated for 5 h in medium without and with different concentrations of indoxyl sulfate, and then ROS production was measured by cytofluorimetry. Data are expressed as mean ± SD of 10 independent experiments. ***P < 0.001 vs. control.
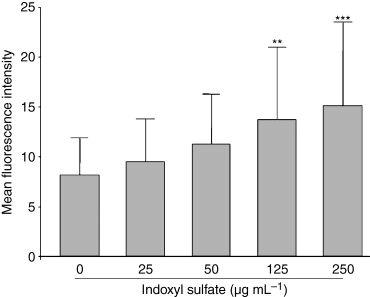
Effect of indoxyl sulfate on ROS production in HUVEC measured by flow cytometry. Results are expressed as mean ± SD of six independent experiments. **P < 0.01 vs. control, ***P < 0.001 vs. control.
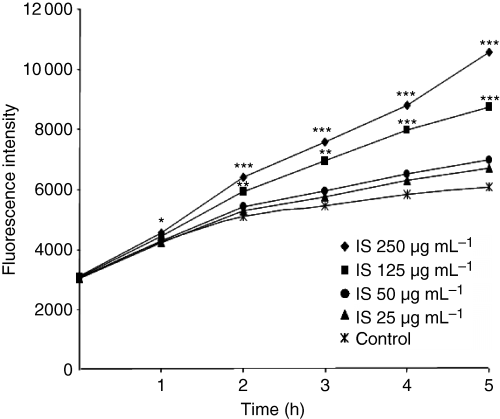
Time course of ROS production by HUVEC induced by indoxyl sulfate. Data are expressed as mean of seven independent experiments. *P < 0.05 vs. control, **P < 0.01 vs. control, ***P < 0.001 vs. control.
Because indoxyl sulfate is protein-bound in human serum, we were concerned that albumin might interfere with the cellular actions of indoxyl sulfate, and we measured ROS production in HUVEC in the presence of HSA. Figure 4 shows that the addition of 4% HSA did not alter the ability of indoxyl sulfate to increase ROS in HUVEC. With and without HSA, indoxyl sulfate-mediated ROS production did not significantly differ.
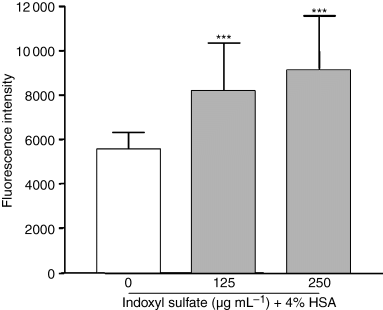
Effect of indoxyl sulfate on ROS production in HUVEC in the presence of albumin. Endothelial cells were incubated for 5 h in medium without and with different concentrations of indoxyl sulfate in the presence of 4% HSA. Then, ROS production was measured by cytofluorimetry. Data are expressed as mean ± SD of 10 independent experiments. ***P < 0.001 vs. control.
Role of pro-oxidative enzymes in indoxyl sulfate-induced ROS production in HUVEC
To study the mechanisms of indoxyl sulfate-mediated ROS production, we examined the potential role of the pro-oxidative enzymes NAD(P)H oxidase and xanthine oxidase, and of mitochondrial electron transport. ROS production in HUVEC was measured after treatment with indoxyl sulfate, in the presence of apocynin or DPI (inhibitors of NAD(P)H oxidase), of allopurinol (xanthine oxidase inhibitor), rotenone and TTFA (inhibitors of mitochondrial electron transport). In the presence of apocynin (Fig. 5), the increase in ROS production induced by indoxyl sulfate was reduced by 71% (P < 0.01 vs. indoxyl sulfate without apocynin). In addition, although its effect was moderate DPI significantly inhibited indoxyl sulfate-induced ROS production in HUVEC (17% reduction, P < 0.05 vs. indoxyl sulfate without DPI), as shown in Fig. 5. Other inhibitors (allopurinol, rotenone, and TTFA) had no effect (Fig. 5).

Effect of inhibitors of pro-oxidative enzymes on indoxyl sulfate-induced ROS production in HUVEC. Apocynin and DPI (diphenylene iodonum chloride): NAD(P)H oxidase inhibitors; allopurinol: xanthine oxidase inhibitor; rotenone and TTFA (2-thenoyltrifluoroacetone): mitochondrial electron transport inhibitors; l-NAME (N-nitro-l-arginine methyl ester): NO synthase inhibitor. Inhibitors were compared with their controls. Data are expressed as mean ± SD of five independent experiments.
Effect of indoxyl sulfate on NO synthases
To study the involvement of NO synthases, we analyzed ROS production in the presence of l-NAME, a NO synthase inhibitor, and examined the effect of indoxyl sulfate on eNOS and iNOS mRNA expression. l -NAME did not modify indoxyl sulfate-induced ROS production (Fig. 5). In addition, the constitutive expression of eNOS mRNA in HUVEC was not altered by indoxyl sulfate (Fig. 6). No expression of iNOS mRNA was detected in HUVEC, even after indoxyl sulfate stimulation (not shown).
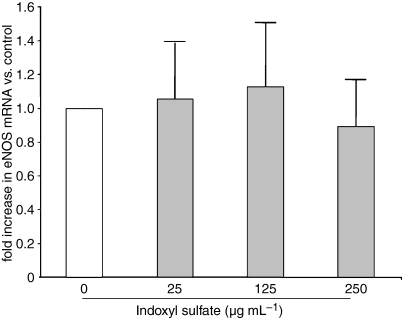
Effect of indoxyl sulfate (IS) on eNOS mRNA in HUVEC. eNOS mRNA was measured in HUVEC after 5 h of incubation with indoxyl sulfate. Data are expressed as mean ± SD of three independent experiments.
Effect of indoxyl sulfate on glutathione levels in HUVEC
We also examined whether indoxyl sulfate could impair an antioxidative mechanism in HUVEC by measuring glutathione (GSH) levels. Indoxyl sulfate at 125 and 250 μg mL−1 decreased endothelial intracellular total glutathione levels by 37% (P < 0.05 vs. control) and 67% (P < 0.001 vs. control), respectively, whereas no significant effect was observed at indoxyl sulfate concentrations of 25 and 50 μg mL−1 (Fig. 7). Hydrogen peroxide, used as a positive control of glutathione depletion, decreased glutathione levels in HUVEC by 78% (P < 0.001 vs. control).
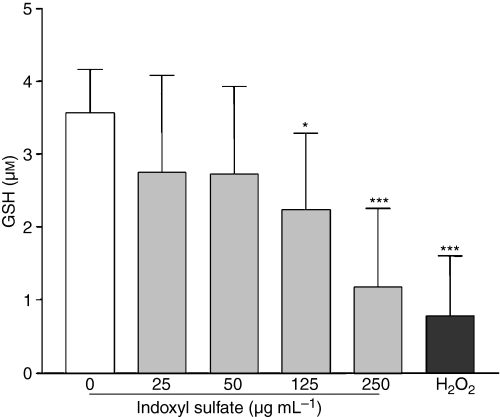
Effect of indoxyl sulfate (IS) on total GSH levels in HUVEC. GSH levels were measured in HUVEC after 5 h of incubation with indoxyl sulfate. Data are expressed as mean ± SD of 11 independent experiments. *P < 0.05 vs. control, ***P < 0.001 vs. control.
Effect of antioxidants on ROS production in HUVEC induced by indoxyl sulfate
We analyzed whether the addition of the antioxidants vitamin C, vitamin E, and NAC could decrease oxidative stress caused by indoxyl sulfate. As shown in Fig. 8, the increase in ROS production induced by indoxyl sulfate was strongly inhibited by vitamin C (90% inhibition, P < 0.001 vs. indoxyl sulfate alone), NAC (65% inhibition, P < 0.01 vs. indoxyl sulfate alone) and, to a lesser extent, by vitamin E (46% inhibition, P < 0.05 vs. indoxyl sulfate alone).
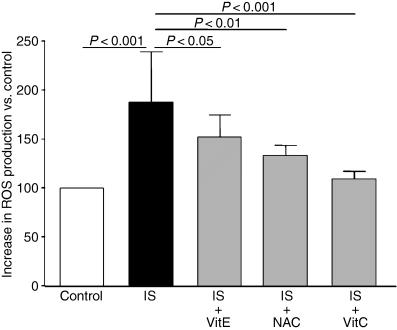
Effect of antioxidants on indoxyl sulfate-induced ROS production in HUVEC. Antioxidants vitamin E (VitE), N-acetyl-l-cysteine (NAC) and vitamin C (VitC) were compared with their controls. Data are expressed as mean ± SD of five independent experiments.
Discussion
Indoxyl sulfate is a protein-bound uremic solute metabolized by the liver from indole produced by the intestinal flora as a metabolite of tryptophan [9]. In CRF patients, serum levels of indoxyl sulfate are increased about fiftyfold compared with healthy subjects, mean and maximal uremic concentrations being around 50 μg mL−1 and 240 μg mL−1, respectively [18]. Approximately 85% of indoxyl sulfate is protein-bound [18,19], and because of its protein-binding, the removal of indoxyl sulfate by dialysis therapy is unsatisfactory. Indeed, only 30% of indoxyl sulfate is removed by hemodialysis, which eliminates over 70% of urea and creatinine [20]. Numerous studies strongly suggest that indoxyl sulfate is involved in glomerular sclerosis and participates in the progression of chronic renal failure [21–24]. In addition, we have previously shown that indoxyl sulfate induces endothelial damage by increasing the production of HUVEC microparticles [1] and inhibiting HUVEC proliferation and migration [10].
In the present study, we have shown that indoxyl sulfate, at concentrations found in uremic patients, increased endothelial ROS production, reflecting an induction of oxidative stress. As oxidative stress results from an imbalance between pro- and antioxidant systems, we first investigated the involvement of pro-oxidative mechanisms in ROS production. We found that ROS production could be decreased in the presence of the NAD(P)H oxidase inhibitors apocynin and, to a lesser extent, DPI. Xanthine oxidase, mitochondrial respiration, and NO synthases seemed not to be involved in increased ROS production. Thus, one of the mechanisms involved in oxidative stress induced by indoxyl sulfate could be the activation of NAD(P)H oxidase.
We were also interested in whether inhibition of antioxidative mechanisms may be involved in indoxyl sulfate-induced oxidative stress. To detoxify ROS, endothelial cells possess non-enzymatic systems and antioxidant enzymes, including superoxide dismutases, catalase, and glutathione peroxidases. The most active non-enzymatic antioxidant is glutathione (GSH), a tripeptide formed by glutamate, glycine, and cysteine, which is a scavenger for hydrogen peroxide and hydroxyl radical. GSH represents a major antioxidant defense mechanism because it provides reducing equivalents for the redox reaction catalyzed by glutathione peroxidase. The action of glutathione peroxidase generates oxidized glutathione (GSSG), a disulfide-linked homodimer. GSSG is reduced back to GSH via the activity of glutathione reductase. In the present study, both GSH and GSSG were measured, and the assay reflects total glutathione. We observed that indoxyl sulfate strongly decreased total glutathione levels in endothelial cells. In addition, NAC, which increases intracellular levels of GSH, inhibited the effect of indoxyl sulfate on ROS production. This effect of indoxyl sulfate on glutathione levels suggests that a decrease in antioxidant defense mechanisms could be involved in oxidative stress induced by this solute.
The protein-binding of indoxyl sulfate in plasma led us to supplement the culture medium with human albumin, in order to decrease its free non-protein bound fraction. As we previously observed [10], the presence of albumin did not modify the effect of indoxyl sulfate on endothelial cells. This finding suggests that the presence of albumin in serum is unlikely to affect the ability of indoxyl sulfate, at concentrations encountered in CRF patients, to induce ROS production in endothelial cells.
In CRF, the balance between pro- and antioxidants is shifted toward an increased oxidative stress. CRF patients present a deficiency in different components of antioxidant defense mechanisms, including reduced levels of vitamin C and vitamin E, and defects in the GSH scavenging system [25]. At the same time, pro-oxidant activity is increased [25]. It has been proposed that increased oxidative stress is a non-traditional cardiovascular risk factor for CRF patients [4]. In addition, CRF patients display endothelium dysfunction [1,2], and oxidative stress and endothelial dysfunction are linked in these patients [5]. One can suppose that common causative agents, not yet clearly defined, are involved. Uremic solutes inducing both oxidative stress and endothelial dysfunction are good candidates as uremia-specific pathogenic agents in the development of atherosclerosis in CRF patients. AGEs prompt intracellular generation of hydrogen peroxide and activation of NAD(P)H oxidase [26], ADMA, and homocysteine increase ROS production [6,7], and homocysteine increases NAD(P)H oxidase expression [7]. Interestingly, these uremic solutes also impair some other endothelial functions involved in the development of atherosclerosis, such as up-regulation of adhesion molecule expression [27], increase in permeability [28], secretion of chemokines [6], or inhibition of proliferation and migration [29]. In the present study, we showed that indoxyl sulfate is another one of the uremic solutes having a pro-oxidative effect on endothelial cells. In addition, in uremic HD patients, serum levels of indoxyl sulfate are associated with levels of pentosidine, a marker of carbonyl and oxidative stress [11], suggesting that indoxyl sulfate is involved in oxidative stress in CRF patients.
We have shown that antioxidants NAC, vitamin E, and vitamin C inhibit the oxidative stress induced by indoxyl sulfate in endothelial cells. These antioxidants are ROS scavengers, and NAC is also a precursor of reduced glutathione [30]. In CRF patients, antioxidants have been used to reduce oxidative stress, and some have displayed beneficial effects [31–33]. Another interesting way to reduce oxidative stress in patients, in addition to therapies with antioxidants, would be to decrease the levels of pro-oxidant uremic solutes, such as indoxyl sulfate.
In conclusion, indoxyl sulfate, at concentrations encountered in CRF patients, induces oxidative stress in endothelial cells by increasing NAD(P)H oxidase activity and decreasing antioxidant defense mechanisms. In addition to its role in the decline of residual renal function, indoxyl sulfate has pro-oxidative effects on endothelial cells, which could be involved in cardiovascular diseases in CRF patients.
Acknowledgement
We thank A. Boyer, C. Scagliarini, R. Giordana, and P. Stelman for technical assistance.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




