Oxidative stress-mediated platelet CD40 ligand upregulation in patients with hypercholesterolemia: effect of atorvastatin
Abstract
Summary. Objectives: We speculated that in patients with hypercholesterolemia CD40L overexpression could depend on low-density lipoprotein (LDL)-induced enhanced intraplatelet formation of O2·− and statin could reduce platelet CD40L via interference with platelet O2·− production. Background: CD40L is a protein with inflammatory and thrombotic properties. CD40L is upregulated in platelets from hypercholesterolemic (HC) patients but the underlying mechanism is unclear. Methods: Collagen-induced platelet CD40L and platelet O2·− expression were investigated in 40 HC patients and 40 healthy subjects. HC patients were then randomized to either a diet (n = 20) (group A) or atorvastatin 10 mg day (n = 20) (group B); the above variables were measured at baseline and after 3 and 30 days of treatment. O2·− and CD40L were also measured in vitro in LDL-treated platelets with or without nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor or atorvastatin added. Results: Compared with controls, HC patients showed higher values of platelet CD40L (P < 0.001) and O2·− (P < 0.001). Platelet CD40L was significantly correlated with O2·− (P < 0.001). The interventional trial showed no changes in group A and a significant and parallel decrease in platelet CD40L (P < 0.001) and O2·− (P < 0.001) in group B. In vitro studies demonstrated that LDL-induced platelet CD40L and GP IIb/IIIa (PAC1 binding) activation via the NADPH oxidase pathway. CD40L upregulation was counteracted by atorvastatin in a dose-dependent fashion. Conclusions: This study suggests that in patients with hypercholesterolemia platelet CD40L is upregulated via NADPH oxidase-dependent O2·− generation. Atorvastatin downregulated CD40L with an oxidative stress-mediated mechanism likely involving platelet NADPH oxidase, an effect that seemed to be independent of its cholesterol-lowering action.
Introduction
CD40 ligand (CD40L), a member of the tumor-necrosis factor family, is a transmembrane protein found on the cells of the immune system as well as on endothelial cells, smooth muscle cells, macrophages and platelets [1]. Upon interaction with its receptor CD40, CD40L exerts an inflammatory and prothrombotic activity, including an increased expression of matrix metalloproteinases, chemokines, cytokines and tissue factor [1]. The role of CD40L in the pathogenesis of atherosclerosis is confirmed by the fact that administration of an antibody against CD40L to hyperlipidemic mice reduced the atherosclerotic lesion [2]. CD40L is expressed by platelets upon stimulation with common agonists [3]; it is then cleaved from platelets over a period of minutes to hours, thus generating a soluble form (sCD40L) [4]. It has been calculated that more than 95% of circulating sCD40L is of platelet origin [5]. Elevated plasma levels of sCD40L have been found in patients with acute coronary syndrome and in patients at risk of cardiovascular events [6–8]. There is also converging evidence that patients with hypercholesterolemia have enhanced levels of sCD40L, suggesting that this protein may represent an important promoter of atherosclerotic complications occurring in this setting [9–11]. As sCD40L stems essentially from platelet CD40L, patients with hypercholesterolemia could be expected to show an upregulation of platelet CD40L. Consistent with this suggestion, we found that in patients with hypercholesterolemia platelet CD40L was unregulated and significantly correlated with sCD40L [10].
These data suggest a role for low-density lipoprotein (LDL) in enhancing platelet CD40L expression but the underlying mechanism is unclear. We have previously shown that oxygen-free radicals are implicated in upregulating CD40L as shown by the fact that in patients with hereditary deficiency of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is one of the most important sources of O2·− [12], platelet CD40L is downregulated [13]. These data would imply that in case of overproduction of oxygen-free radicals platelet CD40L could be upregulated. It is of interest that experimental and clinical studies indicated that cholesterol is a stimulus for production of oxygen-free radicals and related compounds [14–16]. In particular, incubation of LDL with platelets enhanced the formation of superoxide anion (O2·−) [16], a reactive oxygen species implicated in the expression of platelet CD40L [13]. Taken together these data would lead to speculation that in patients with hypercholesterolemia CD40L overexpression could depend on LDL-induced enhanced intraplatelet formation of O2·− but this hypothesis has never been explored.
To analyze this issue we investigated the behavior of platelet O2·− and CD40L in patients with hypercholesterolemia and the role of NADPH oxidase, which is one of the most important cellular sources of O2·− [12]. We then investigated if atorvastatin, which is known to inhibit NADPH oxidase [17], reduced platelet CD40L via interference with platelet O2·− production. For this purpose, we analyzed if short-term treatment with atorvastatin, that is known to reduce platelet CD40L with a mechanism independent of lipid-lowering effect [11], inhibited both platelet O2·− and CD40L. Finally we performed in vitro experiments to see if: (i) LDL enhances platelet CD40L via NADPH oxidase-dependent activation; and (ii) atorvastatin downregulates platelet CD40L by inhibiting platelet NADPH oxidase activation.
Methods
Clinical study
The clinical study was divided in two parts. In the first part of the study, we compared 40 patients with polygenic hypercholesterolemia (20 males, 20 females; mean age, 54.0 ± 4.9 years) and 40 sex- and age-matched normocholesterolemic healthy subjects (HS) (20 males and 20 females; mean age 52.5 ± 5.1 years). Both patients and controls were recruited from the same geographic area and followed a typical Mediterranean diet. None of the patients had clinical evidence of cardiovascular disease (as shown by clinical history, physical examination, ECG), diabetes mellitus or hypertension. Patients with hypercholesterolemia had not taken any lipid-lowering agents or antiplatelet drugs in the previous 30 days. Lipid profile, platelet CD40L expression and O2·− production were measured in patients and controls.
In the second part of the study, we tested the hypothesis that atorvastatin could influence platelet CD40L and O2·− with a mechanism independent of its cholesterol-lowering effect. Thus only hypercholesterolemic (HC) patients were randomized (by a procedure based on a random numeric sequence) to either a diet (American Heart Association step I diet) for group A (n = 20, 10 males, 10 females) or atorvastatin 10 mg/day for group B (n = 20, 10 males, 10 females). Most of these patients participated in a previous study where short-term effect of atorvastatin on platelet function and clotting activation was investigated [11]. Lipid profile, CD40L platelet expression and platelet O2·− were measured at baseline and after 3 and 30 days of treatment. All assays were performed blind.
Blood samples mixed with 0.13 mol/L of sodium citrate (ratio 9:1) were obtained between 08:00 and 09:00 hours from patients and healthy volunteers who had fasted for 12 h and had provided their informed consent to participate in the study; an aliquot of serum was used to measure lipid profile. The study protocol was approved by the ethics committee of our university.
Lipid profile
Serum levels of total cholesterol and triglycerides were determined using an enzyme-based method. High-density lipoprotein (HDL) cholesterol was measured after phosphotungstic acid/MgCl2 precipitation of fresh plasma. LDL cholesterol was calculated using the Friedewald formula (LDL cholesterol = total cholesterol – cholesterol HDL – Triglycerides/5).
Platelet isolation from whole blood
Blood samples were drawn between 08:00 and 09:00 hours without stasis from an antecubital vein with a 21-gauge needle from patients on a 12-h fast, and mixed with 0.13 mol/L sodium citrate (ratio 9:1). Washed platelets were prepared as previously described [18].
Analysis of platelet O2·− production
O2·− production was measured in a platelet suspension stimulated with collagen (6 μg/mL) using the lucigenin (5 μm) chemiluminescence method as previously reported [19] and expressed as a stimulation index (SI = mean level of stimulated platelet luminescence/average level of luminescence in unstimulated platelets).
Flow cytometric analysis of CD40L expression
CD40L expression on platelet membrane in basal conditions and after collagen (6 μg/mL) was analyzed using specific fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies (mAb) (Anti-CD40L Ab; Beckman Coulter, Fullerton, CA, USA). In all assays, an irrelevant isotype-matched antibody was used as a negative control.Twenty microliters of mAb was added to 200 μL of platelet suspension (2 × 108/mL) previously fixed with (2%) paraformaldehyde [0.1% bovine serum albumin (BSA)] and incubated for 60 min at 4 °C. The unbound mAb was removed by addition of 0.1% BSA phosphate buffer saline (PBS) and centrifugation at 300 × g for 3 min (twice). Fluorescence intensity was analyzed on an Epics XL-MCL Cytometer (Beckman Coulter, Fullerton, CA, USA) equipped with an argon laser at 488 mm. For every histogram, 50 000 platelets were counted to determine the proportion of positive platelets. Antibody reactivity was reported in arbitrary units obtained by multiplying the number of positive events resulting from platelets stimulation by the mean values of the fluorescence observed when the specific mAb was used, and by correcting the values obtained in unstimulated samples treated with the same antibody.
Analysis of sCD40L
Blood samples mixed with 0.13 m sodium citrate (ratio 9:1) samples were immediately centrifuged at 300 × g for 20 min at 4 °C to separate plasma from platelets, and the supernatant was collected and stored at −80 °C until measurement. Plasma levels of sCD40L were measured with a commercial immunoassay (Quantikine CD40 ligand; R&D Systems, Inc., Minneapolis, MN, USA). Intra-assay and inter-assay coefficients of variation were 7% and 9%, respectively.
In vitro study
Platelet CD40L expression, sCD40L release and O2·− formation by LDL
Human platelets taken from HS (n = 10, five males, five females, mean age 53.0 ± 3.8 years) were washed and suspended in tyrode buffer (2 × 108/mL). Platelet suspensions were incubated (30 min at 37 °C) with and without native LDL-cholesterol (100 μg/mL), and the NADPH oxidase inhibitor apocynin (100 μm) [20], a LOX1 receptor blocking peptide (LOX1-BP) (10 μg/mL), nitro-l-arginine methyl ester (l-NAME) (300 μm) or atorvastatin (0.1–10 μm) before collagen (6 μg/mL) stimulation. Atorvastatin alone did not affect platelet viability as assessed using the ipotonic shock response test (not shown) [21]. Platelet suspension was treated as described above to detect CD40L expression O2·− production. sCD40L was detected in the supernatant after sample centrifugation as described above.
Flow cytometry analysis of PAC-1
PAC-1 is an antibody that recognizes an epitome on the glycoprotein (GP) IIb/IIIa of activated platelets, at/or near the platelet fibrinogen receptor.
PAC-1 binding on platelets membrane was analyzed using the specific FITC-labeled mAb antiPAC-1. All assays included samples to which an irrelevant isotype-matched antibody (FITC-labeled IgM) was added.
Platelet suspension (200 μL, 2 × 108/mL) was incubated for 30 min at 37 °C with or without LDL-cholesterol (100 μg/mL) and LOX1-BP (10 μg/mL) or apocynin (100 μm). The suspension was then stimulated with collagen (6 μg/mL) (5 min at 37 °C) and platelets were fixed with (2%) paraformaldehyde (0.1% BSA) for 60 min at room temperature. The suspension was treated with mAb (20 μL) for 60 min at 4 °C. The unbound mAb was removed by centrifugation at 300 × g for 3 min (twice) after the addition of PBS (0.1% bovine serum albumin). Fluorescence intensity was analyzed on an Epics XL-MCL Cytometer (Coulter Electronics) equipped with an argon laser at 488 mm. For every histogram, 50 000 platelets were counted to evaluate the percentage of positive platelets. Antibody reactivity was reported as mean fluorescence intensity [22].
Platelet NADPH oxidase activity
Measurement of platelets NADPH oxidase activity was performed in platelet homogenates according to Seno et al. [23]. Washed platelets were incubated (30 min at 37 °C) with native LDL-cholesterol (100 μg/mL) and then suspended in homogenate buffer containing 50 mm Tris–HCl (ph 7.4), 1.0 mm ethylenediaminetetraacetic acid, 2.0 mm leupeptin and 2.0 mm pepsatin A, and then homogenized. Platelet homogenates were incubated for 10 min at 37 °C with 25 μm nicotinamide adenine dinucleotide phosphate (NADPH) with or without atorvastatin added (0.1–10 μm). The assay solution contained 400 μL Tyrode buffer and 5 μm lucigenin. After preincubation at 37 °C for 3 min, the reaction was started by adding 100 μL of platelet homogenates in the presence or less of arachidonic acid (AA) 0.5 mm. The chemiluminescent signal was expressed as counts per minute (cpm) for an average of 10 min and corrected by protein concentration (cpm per mg). Protein concentrations were determined by the method of Lowry [24].
Statistical analysis
Based on the assumption that a short-term (3 days) treatment with atorvastatin would reduce platelet CD40L expression and O2·− by 25%, we postulated that the study sample should consist of at least 15 patients in each group (alpha = 0.05 and 1-beta = 0.92). Comparisons between groups were carried out using the analysis of variance (anova one-way and repeated measures) [25], and were replicated as appropriate using non-parametric tests such as Wilcoxon’s and Kolmogorov–Smirnov (Z) tests in case of non-homogenous variances verified by Levene’s test. Independence of categorical variables was tested using the chi-squared test. manova with Bonferroni’s test was used for multiple comparisons. The correlation analysis was carried out using Pearson’s test.
To identify the significant predictors of CD40L we performed multiple linear regression analysis (by stepwise selection method) including, as independent variables, all those related to CD40L by a P-value < 0.20 on Pearson’s linear regression test. Data are presented as mean ± SD. Statistical significance was defined at level P < 0.05.The statistical analysis was performed using the spss 13.0 software for Windows (SPSS Inc., Chicago, IL, USA).
Results
Collagen- induced O2·− and platelet CD40L in HS and HC patients
Platelet O2·− and CD40L were investigated by adding platelets with collagen, that is a reliable stimulus for the platelet production of both molecules [13]. Table 1 shows the clinical characteristics of patients and controls. Compared with controls, patients with hypercholesterolemia had enhanced production of platelet O2·− and CD40L (Fig. 1A,B). Platelet CD40L significantly correlated with platelet O2·− [R = 0.722 in HS, P < 0.001; R = 0.654 in HC patients, P < 0.001; overall correlation: R = 0.779, P < 0.001]. LDL cholesterol significantly correlated with platelet CD40L [R = 0.618 in HS, P < 0.001; R = 0.622 in HC patients, P < 0.001; overall correlation: R = 0.539; P < 0.001] and platelet O2·− [R = 0.568 in HS, P < 0.001; R = 0.324 in HC patients, P < 0.001; overall correlation: R = 0.535; P < 0.001].
| (A) | Hypercolesterolemic | Healthy subjects | P |
|---|---|---|---|
| Age (years) | 54.0 ± 4.9 | 52.5 ± 5.1 | NS |
| Gender | 20 males, 20 females | 20 males, 20 females | NS |
| Study participants | |||
| Smokers | 5 | 3 | NS |
| Glycemia (mg/dL) | 85.1 ± 11.3 | 83.8 ± 12.3 | NS |
| Systolic blood pressure (mmHg) | 127.5 ± 9.7 | 124.0 ± 11.5 | NS |
| Dyastolic blood pressure (mmHg) | 75.3 ± 9.0 | 74.7 ± 9.8 | NS |
| Total cholesterol (mg/dL) | 270.8 ± 22.2 | 162.0 ± 24.1 | < 0.001 |
| LDL-cholesterol (mg/dL) | 187.5 ± 11.6 | 97.6 ± 13.8 | < 0.001 |
| Triglycerides (mg/dL) | 102.7 ± 20.7 | 72.5 ± 15.0 | < 0.001 |
| (B) | Diet | Atorvastatin | P |
| Age (years) | 53.5 ± 5.6 | 54.6 ± 4.1 | NS |
| Gender | 10 males, 10 females | 10 males, 10 females | NS |
| HC patients | |||
| Smokers | 3 | 2 | NS |
| Glycemia (mg/dL) | 83.4 ± 11.5 | 86.9 ± 11.1 | NS |
| Systolic blood pressure (mmHg) | 129.5 ± 9.4 | 125.5 ± 9.8 | NS |
| Dyastolic blood pressure (mmHg) | 77.7 ± 8.1 | 73.0 ± 9.5 | NS |
| Total cholesterol | |||
| Baseline (mg/dL) | 272.8 ± 22.3 | 268.9 ± 22.3 | NS |
| After 3 days (mg/dL) | 273.2 ± 24.0 | 260.9 ± 23.9 | NS |
| After 30 days (mg/dL) | 254.1 ± 20.5* | 203.7 ± 21.9† | < 0.001 |
- LDL, low-density lipoprotein; NS, non-significant.
- Results are given as mean ± SD; statistical analysis was performed by ANOVA one-way for continuous variable and by chi-squared test for categorical variables. NS.
- *P = 0.01 vs. 3 days.
- † P < 0.001 vs. 3 days.
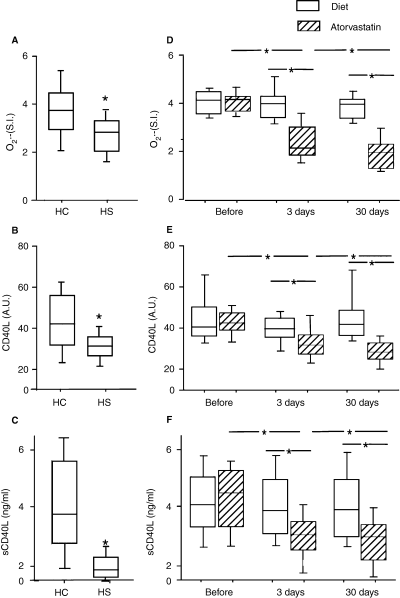
Collagen (6 μg mL−1) induced platelet O2·− production (A) and CD40 ligand expression (CD40L) (B) in hypercholesterolemic patients (HC) (n = 40) and healthy subjects (HS) (n = 40). Soluble CD40L (sCD40L) in HC (n = 40) and healthy subjects (HS) (n = 40) (C). O2·− production (D), platelet (CD40L) (E) and soluble (sCD40L) CD40 ligand (F) expression in group A (diet ) (n = 20) and group B (diet + atorvastatin 10 mg day−1) (n = 20) patients before and after 3 and 30 days of treatment. Repeated measures anova: *P < 0.001 HC vs. HS. Results are given as mean ± SD. Box plots depict median and 95% confidence intervals; whiskers represent minimum and maximum values.
In order to establish the significant predictors of CD40L among HC patients, we performed a multiple linear regression analysis, including as independent variables those linearly associated with CD40L (Table 2); this analysis showed that LDL cholesterol (B:0. 284; E.S.: 0.081; standardized coefficient : 0.318; P = 0.001), platelet O2·− (B: 7.516; E.S.: 1.18; standardized coefficient : 0.491; P < 0.001), and triglycerides (B:0. 145; E.S.: 0.043; standardized coefficient : 0.288; P = 0.002) were significant predictors for 89% of the total variability of CD40L.
| Variables | CD40L |
| Age | |
| R | −0.100 |
| P | 0.539 |
| Gender | |
| R | 0.008 |
| P | 0.960 |
| Platelet O2·− | |
| R | 0.809* |
| P | < 0.001* |
| Total cholesterol | |
| R | 0.762* |
| P | < 0.001* |
| LDL cholesterol | |
| R | 0.789* |
| P | < 0.001* |
| HDL cholesterol | |
| R | 0.454* |
| P | 0.003* |
| Triglycerides | |
| R | 0.733* |
| P | < 0.001* |
| Fasting glucose | |
| R | −0.041 |
| P | 0.801 |
| Systolic blood pressure | |
| R | 0.236 |
| P | 0.398 |
| Diastolic blood pressure | |
| R | 0.155 |
| P | 0.339 |
- Selected as independent variables for multiple linear regression analysis. LDL, low-density lipoprotein; HDL, high-density lipoprotein.
As such correlations led us to speculate that cholesterol could enhance platelet CD40L via an oxidative stress-mediated mechanism, we performed in vitro experiments to explore this hypothesis. Incubation of collagen-stimulated platelets with apocynin resulted in a significant decrease of both platelet O2·− and CD40L; an opposite effect was observed in platelets incubated with L-NAME (Fig. 2A,B). We then investigated if cholesterol has prooxidant property and demonstrated that LDL enhanced platelet O2·− formation, an effect that seemed to be dependent upon NADPH oxidase activation as its inhibitor significantly reduced LDL-induced platelet O2·− overproduction (Fig. 2B). Incubation of LDL-treated platelets with apocynin also revealed a functional interplay between platelet O2·− and CD40L as the inhibition of platelet O2·− was associated with CD40L downregulation (Fig. 2A,B). An opposite effect was detected with L-NAME, which further increased LDL-induced platelet O2·− and CD40L overexpression (Fig. 2A,B). Conversely, a competitive peptide for the LOX1 receptor modulated LDL-induced platelet activation as it significantly inhibited both platelet O2·− and CD40L expression (Fig. 2A,B).
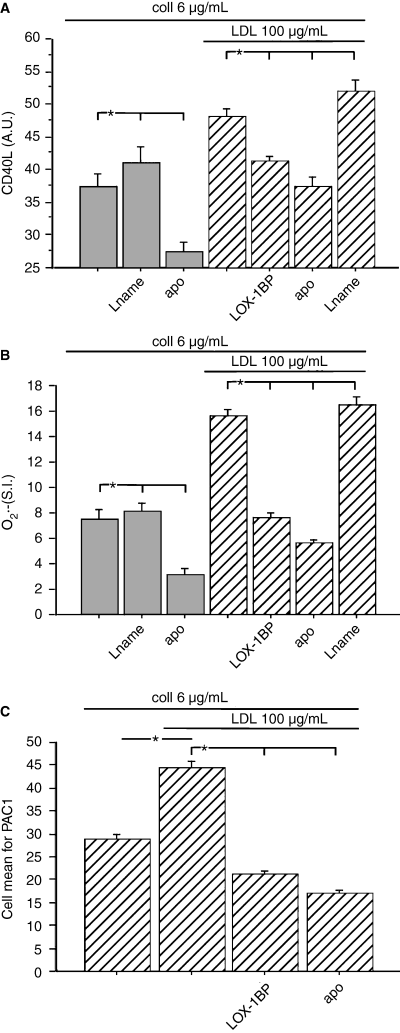
Collagen-induced platelet CD40L expression (A), O2·− production (B) and GP IIb/IIIa activation (by PAC1 binding) (C) in untreated or LDL-cholesterol (100 μg/mL)-treated platelets. Samples were added or not to apocynin (APO) (100 μm), nitro-l-arginine methyl ester (l-NAME) (300 μm) or the LOX1 receptor blocking peptide (LOX1-BP) (10 μg/mL) (healthy subjects, n = 10, five males, five females; mean age 53 years). Results are given as mean ± SD. *P < 0.001
Further support for the role of LDL in enhancing platelet activation was provided by experiments exploring the effect of LDL on the behaviour of GP IIb/IIIa, that, in fact, was activated in LDL-treated platelets compared with the control (Fig. 2C); inhibition of GP IIb/IIIa activation was observed in platelets treated with apocynin or an inhibitor of LOX1 (Fig. 2C)
sCD40L in HC and HS
Compared with controls, patients with hypercholesterolemia had enhanced sCD40L plasma levels (1.8 ± 0.7 vs. 4.3 ± 1.6 ng/mL, P < 0.001) (Fig. 1C). sCD40L significantly correlated with platelet CD40L [R = 0.637 in HS, P < 0.001; R = 0.472 in HC patients, P = 0.002; overall correlation: R = 0.709, P < 0.001] and platelet O2·− [R = 0.676 in HS, P < 0.001; R = 0.542 in HC patients, P < 0.001; overall correlation: R = 0.751, P < 0.001] and LDL cholesterol [R = 0.625 in HS, P < 0.001; R = 0.402 in HC patients, P = 0.03; overall correlation: R = 0762; P < 0.001].
Effect of atorvastatin on platelet, soluble CD40L and O2·− in HC patients
At baseline, patients randomized to a diet alone (group A) and those randomized to a diet plus atorvastatin 10 mg day−1 (group B) had similar levels of platelet CD40L (45.1 ± 13.0 vs. 44.1 ± 15 AU), sCD40L (3.99 ± 1.27 vs. 4.60 ± 1.21 ng/mL) and platelet O2·− (3.8 ± 0.5 vs. 3.7 ± 0.7 SI).
In group A (n = 20), no changes in platelet O2·−, platelet CD40L and sCD40L were detected after 3 and 30 days (Fig. 1D–F). Conversely, in group B (n = 20), a progressive decrease in platelet O2·−, platelet CD40L and sCD40L was observed after the follow-up periods (Fig. 1D–F).
In group B, before-after treatment changes at 3 days between platelet CD40L and platelet O2·− (R = 0.67, P < 0.001) were significantly correlated.
During the follow-up, in both groups, serum cholesterol significantly decreased after 30 days but the decrement was significantly higher in group B than group A (Table 1).
In vitro effect of atorvastatin on O2·− and CD40L in LDL-treated platelets
Incubation of platelets with atorvastatin elicited a significant decrease in CD40L expression, sCD40L and platelet O2·− formation in LDL-treated platelets (P < 0.001) (Fig. 3A–C). This effect was dependent on the concentration of atorvastatin, as demonstrated by dose-response curves (CD40L R = 0.93, P < 0.001) (O2·−R = 0.90, P < 0.001).

CD40 ligand (CD40L) expression (A), sCD40L (B) and O2·− production (C) in LDL-treated collagen-stimulated platelets added to 0.1, 1 or 10 μm of atorvastatin. Effect of atorvastatin on arachidonic acid-induced nicotinamide adenine dinucleotide phosphate oxidase activation (D). Platelets were taken from healthy subjects (n = 10, five males, five females; mean age 53 years). Repeated measures anova:*P < 0.001. Results are given as mean ± SD. AA, arachidonic acid.
In order to analyze if atorvastatin reduced platelet O2·− via inhibition of NADPH oxidase, platelet O2·− formation was measured in the presence or less of NADPH, the substrate of the enzyme. In this experiment, AA was used as a platelet agonist because a previous study showed that among platelet agonists AA is a strong stimulus of NADPH oxidase [19]. We observed that incubation of AA-stimulated platelets with NADPH significantly enhanced platelet O2·− formation, that, however, was markedly inhibited by atorvastatin in a dose-dependent manner (Fig. 3D).
Discussion
This study provides evidence that in HC patients platelet overproduction of O2·− and CD40L upregulation coexist and suggests that, in hypercholesterolemia, platelet CD40L overexpression may be mediated by enhanced intraplatelet production of O2·−
Platelet O2·− and CD40L in patients with hypercholesterolemia
In a previous study we showed that in HC patients platelets over express CD40L but the underlying mechanism was not investigated [11]. Oxidative stress, in particular platelet production of O2·−, has a key role in platelet CD40L expression. Thus, in patients with hereditary deficiency of gp91phox, the central core of NADPH oxidase, platelet CD40L is downregulated [13], suggesting that platelet production of O2·− is implicated in the expression of CD40L [13]. A close relationship between platelet CD40L and O2·− was also detected in HC patients, in whom platelet O2·− and CD40L upregulation coexisted and was significantly correlated. As a previous report showed that LDL enhanced platelet O2·− production [16], we hypothesized that cholesterol could upregulate platelet CD40L via platelet O2·− overproduction. Consistent with this hypothesis, we found that LDL enhanced platelet O2·− production and an inhibitor of NADPH oxidase almost completely abolished such an effect. Among the cytosolic and membrane subunits of NADPH oxidase [26] gp 91phox has a crucial role in producing platelet O2·− [13], therefore it could be tempting to speculate that LDL upregulates this subunit but further study is required to support such hypothesis.
Owing to the relationship between platelet O2·− and CD40L, it is also arguable that NADPH oxidase-generating O2·− could be a mechanism through which LDL upregulates platelet CD40L.
Upon stimulation platelets produce not only O2·− but also NO, a molecule with vasodilating and antiplatelet effects [27,28]. The interplay between O2·− and NO is relevant in the context of platelet activation as O2·− rapidly inactivates NO so reducing its antiplatelet effect [27,28]. Such interplay seems to play a role also in our experimental model as inhibition of platelet NO synthase was associated with enhanced oxidative stress and CD40L upregulation in both untreated and LDL-treated platelets. This provides indirect evidence of the key role played by redox status in modulating LDL-induced platelet activation but the exact mechanism through which cholesterol influences intracellular signaling responsible for modulating oxidative stress and redox status requires further investigation.
Overexpression of CD40L by LDL may have potential implications for platelet activation as CD40L has a crucial role in platelet-dependent thrombosis via binding to GP IIb/IIIa [29]. The interplay between CD40L and GP IIb/IIIa has been recently corroborated by Chakrabarty et al. [30], who demonstrated a crucial role of CD40L in enhancing reactive oxidant species formation by activated platelets, an effect that was abrogated by inhibiting GP IIb/IIIa activation.
Atorvastatin, oxidative stress AND CD40L
As these data suggested that in patients with hypercholesterolemia NADPH oxidase could be upregulated, we decided to further corroborate this hypothesis by performing in vitro and in vivo studies with a drug category that has been shown to inhibit NADPH oxidase activity. Previous studies showed, in fact, that statins lower the expression of the NADPH oxidase subunits gp22phox and nox1, and prevent p21RAC isoprenilation that is involved in the NADPH oxidase activation [17].
In vitro study provided evidence of a direct inhibition of NADPH oxidase and in turn of CD40L. It should be noted that such effects were achieved with atorvastatin concentrations as low as 0.1 μmol/L, which may be achieved in the peripheral circulation of subjects treated with 5–20 mg/day atorvastatin [31].
The clinical study corroborated the in vitro experiments as early as after 3 days of atorvastatin treatment: lipid profile was unchanged while a simultaneous and parallel reduction of platelet O2·− and CD40L and a significant decrease of sCD40L were observed. This excludes that the antioxidant effect of atorvastatin is mediated by its lipid lowering action and indirectly supports the hypothesis that in patients with hypercholesterolemia NADPH oxidase-dependent O2·− generation has a pivotal role in regulating platelet CD40L expression. However, after 30 days of treatment, a further decrease in CD40L was seen coincidentally with a significant decrease in serum cholesterol. This finding, which is in accordance with previous report showing that other statins inhibit sCD40L coincidentally the lipid-lowering effect [32], suggests that at least two mechanisms may cooperate in the downregulation of CD40L, one being potentially related to the antioxidant and the other to the lipid-lowering effect of statins. The specific role of these two putative statins’ properties on the reduction of CD40L expression should be investigated in the future.
Limitations of the study
This study has some limitations that deserve consideration. While the study suggests that LDL enhances NADPH oxidase-generating platelet O2·−, the exact mechanism through which LDL increases platelet oxidative stress has not been investigated. In a previous study, we observed that activation of platelet AA metabolism was implicated in LDL-induced O2·− formation; thus, incubation of a LDL-treated platelet with an inhibitor of phospholipase A2 significantly inhibited O2·− formation [16]. These data are consistent with other experiments indicating a crucial role for AA in activating NADPH oxidase [19]. Another possibility is that the increase of oxidative stress is mediated by CD40L, which is a powerful intracellular stimulus for oxidative stress (10,30). This would imply the existence of a positive feedback between oxidative stress and CD40L expression but this hypothesis requires further investigation.
A central role for NADPH oxidase activation emerges also in experiments with atorvastatin but it is unclear if this occurs through a direct inhibition of the enzyme or via interference with the platelet pathway upstream NADPH oxidase activation.
Conclusion
In conclusion, this study shows that in patients with hypercholesterolemia platelet CD40L upregulation is dependent upon NADPH oxidase-dependent O2·− generation. Downregulation of platelet CD40L via inhibition of O2·−-dependent NADPH oxidase activation provides a novel target of statin’s antioxidant effect, which could further contribute to retard the progression of atherosclerotic disease observed with this drug category.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




