A common 253-kb deletion involving VWF and TMEM16B in German and Italian patients with severe von Willebrand disease type 3
Summary.
Background: Severe von Willebrand disease (VWD) type 3 is caused by large deletions, insertions, small truncating mutations, splice site mutations and missense mutations of the VWF gene, respectively. Large deletions have been regarded as being a rare cause of VWD type 3. Complete gene deletions have only been identified in Italian and German patients to date. However, their extent and breakpoints have not been determined yet.
Objectives: To identify the breakpoints of complete VWF deletions in patients with VWD type 3.
Patients/methods: Five index patients with large deletions from two unrelated German and three Italian families were investigated by polymerase chain reaction (PCR) and primer walking. Haplotypes were composed of eight deletion flanking markers.
Results: After initial characterization of a homozygous 253,246 bp deletion (Δ253 k) in a German patient, with the centromeric breakpoint located between CD9 and VWF and the telomeric breakpoint in intron 3 of TMEM16B, respectively, we identified the same Δ253 k in an additional two homozygous and two compound-heterozygous patients, and in their heterozygous parents. All patients share the same deletion-associated marker haplotype. The genomic structure of the breakpoint regions favors DNA double-strand breaks followed by non-homologous end-joining repair as an underlying molecular mechanism rather than a homologous recombination event.
Conclusions: Our results suggest a single genetic origin of Δ253 k. Homozygosity for Δ253 k in non-consanguineous families from two different countries may indicate a higher incidence of large deletions in VWD type 3 than previously thought. The availability of a Δ253k-specific assay allows simple and rapid detection of even heterozygous patients and carriers.
Introduction
von Willebrand disease (VWD) is caused by quantitative and/or qualitative defects of von Willebrand factor (VWF) a large protein, playing a key role in hemostasis [1]. VWD type 3 (MIM #277 480) is defined as the clinically most severe form of the disease with virtual absence of VWF and paralleled by a severe decrease of factor VIII (FVIII) levels as a result of the loss of its protection by VWF. The molecular defects causing VWD type 3 published to date are large deletions, and insertions, small truncating mutations, splicing defects, recombination events between the gene and its pseudogene at chromosome 22 [2] and missense mutations of the gene (VWF mutation database: http://www.vwf.group.shef.ac.uk/). Large deletions have been regarded as being a rare cause of the complete lack of VWF [3]. Most of them reported to date are partial deletions of heterogeneous size and location [3–7]. The most recent report of a partial 61-kb deletion between VWF introns 5 and 16 identified in a Chinese family suggests an Alu-mediated mechanism [7]. Besides their occurrence in patients with VWD type 3, a large in-frame deletion with its breakpoints in intron 25 and 34 has been identified in a patient with a variant VWD type 2A [8].
Complete deletions of VWF have only been identified in Italian and German patients to date [3,9–11]. The deletions in both Italian and German patients, correlated with the presence of alloantibodies that presumably developed subsequent to VWF replacement therapy (9, 10, this study). The publications on the Italian patients [9,10] were the first to report molecular defects of VWF at all. However, their extent and their breakpoints have not been determined until now. We applied polymerase chain reaction (PCR) and primer walking to identify the 5′ and 3′ deletion breakpoints of a previously studied homozygous German patient with VWD type 3 [3] and established a deletion-specific assay which also allowed simple and rapid detection of heterozygous patients and carriers.
Patients
We investigated two unrelated index patients with VWD type 3 from Germany (patients F1-3 and F2-3) and three apparently unrelated index patients from Italy (patients F3-3, F4-3 and F5-3). The German patient F1-3 and the Italian patients F3-3 and F4-3, respectively, have been described in previous reports on complete VWF deletions [3,9,10]. Patient F1-3 (identical to patient 0803 in [3]) suffered terminal renal failure presumably caused by chronic glomerulonephritis. However, a biopsy was not performed to avoid the risk of bleeding. He received a renal transplant in 1982, a re-transplant in 1986 [12], and a 3rd transplant in 2001 after subsequent graft failure. The patient’s mother F1-2 had normal laboratory parameters and no history of bleeding; the father was not available for study. The German patient F2-3 with VWD type 3 and frequent bleeding has not been communicated before. The patient’s mother had normal VWF parameters and no bleeding history. The unaffected father displayed slightly reduced VWF. The Italian patients F3-3 and F4-3 are the index patients of the previously published families G and C, respectively [9,10]. The mother of patient F3-3 (F3-2) reported a history of recurrent epistaxis and menorrhagia with normal levels of FVIII and borderline VWF parameters; the father (F3-1) had a negative bleeding history and normal FVIII and VWF levels. In the case of patient F4-3, only the mother was available for the study. She reported a history of menorrhagia with normal levels of FVIII and VWF.
The Italian patient F5-3 is the index patient of family A described in previous reports with a clinically milder form of VWD and measurable VWF:Ag of 2 IU dL−1 being a compound heterozygote for the missense mutation 8012G>A in exon 49 (C2671Y) and a large, presumably complete deletion [11,13].
Phenotypic and genotypic data are summarized in Table 1. Informed consent was obtained from all patients and probands and the study was in accordance with the amended version of the declaration of Helsinki (Tokyo, 2004) and the respective institutional guidelines of the participating institutions.
| Id | VWF:Ag U dL−1 | VWF:RCo U dL−1 | FVIII:C U dL−1 | inh | mut 1 | mut 2 | Ref. |
|---|---|---|---|---|---|---|---|
| F1-2 | nd | 96 | 100 | nd | Δ253K | wt | 3 |
| F1-3 | <1 | <5 | 3 | + | Δ253K | Δ253K | 3 |
| F2-1 | 35 | 70 | 105 | nd | Δ253K | wt | † |
| F2-2 | 105 | 102 | 175 | nd | 3605delC* | wt | † |
| F2-3 | <1 | <5 | 2 | + | Δ253K | 3605delC* | † |
| F3-1 | 59 | 60 | 116 | nd | Δ253K | wt | 9, 10 |
| F3-2 | 44 | 49 | 110 | nd | Δ253K | wt | 9, 10 |
| F3-3 | <1 | <3 | 1 | + | Δ253K | Δ253K | 9, 10 |
| F4-2 | 56 | 54 | 121 | nd | Δ253K | wt | 9, 10 |
| F4-3 | <1 | <3 | 2 | + | Δ253K | Δ253K | 9, 10 |
| F5-1 | 47 | 46 | 58 | nd | C2671Y | wt | 11 |
| F5-2 | 40 | 40 | 80 | nd | Δ253K | wt | 11 |
| F5-3 | 2 | <3 | 10 | – | Δ253K | C2671Y | 11 |
- Id, family and individual no; inh, presence or absence of alloantibodies; mut, mutation; ref., references; *frameshift mutation, starting at codon 1202, predicting a nonsense mutation at codon 1214 [1202 (fsX12)]; †this study.
Methods
Diagnosis of VWD has previously been made by the contributing expert institutions according to their standard diagnostic tests. The presence of alloantibodies has been suspected because of a poor response to VWF replacement and was confirmed by a modified Bethesda method [14,15] using VWF:Ag [16], ristocetin cofactor (VWF:RCo, [17]) and more recently VWF:collagen binding (VWF:CB, [18]) as parameters, respectively.
Mutation screening
Genomic DNA was extracted from peripheral blood cells by standard techniques in the different institutions. The genotype of all index patients except patient 2 was known from previous studies [3,9–11]. Therefore, only the DNA of the compound heterozygous patient 2 had still to be screened for mutations by PCR and direct sequencing of all 51 coding exons and their flanking intronic regions as previously described [2].
Deletion mapping strategy
Previous results from Southern Blot studies suggested complete deletions of the VWF gene in patients F1-3, F3-3 and F4-3 [3,9,10]. Later we confirmed these findings by negative results of PCR reactions attempting to amplify the patients’VWF exons 2 and 52 (Schneppenheim unpublished). In our current study, we extended the region of interest towards the VWF adjacent centromeric CD9 gene which is transcribed in the opposite direction compared with VWF and the adjacent telomeric TMEM16B gene. A set of PCR primers covering approximately 61 kb 5′ of the VWF coding sequence and 386 kb extending from the VWF 3′ UTR into the TMEM16B gene were chosen from the published sequence (genbank accession no. NT009759). The sequence NT009759 refers to the complementary strand with respect to VWF; however, nucleotide numbering in this paper refers to the original sequence. Positions of the different primer pairs are given in Fig. 1. After sufficiently narrowing the putative deletion breakpoint regions, we finally selected a suitable PCR primer pair in order to obtain a product presumably harboring the breakpoints of the deleted sequence. A maximum size of 3.1 kb was assumed for this final PCR product. PCR reactions were carried out in the T-Gradient Cycler (Biometra, Goettingen, Germany). Direct sequencing was performed by means of the ‘ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit’ on an ABI Prism 310 or an ABI 377 (ABI, Foster City, CA, USA) using the respective PCR primers. Primer sequences and conditions can be provided on request.
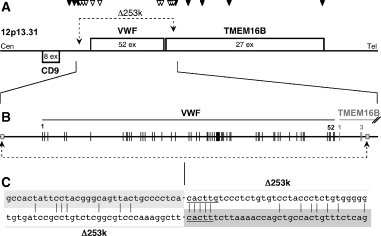
Deletion of the VWF gene and functional disruption of the TMEM16B gene. (A) Genomic location on 12p13.31. Three genes are located in this vicinity. CD9 is transcribed in the direction of the centromere, while VWF and TMEM16B are transcribed in the direction of the telomere. CD9, a 228-amino-acid protein is encoded by 8 exons. von Willebrand factor (VWF) is encoded by 51 of 52 exons with the first exon being untranslated, resulting in a pre-pro-protein of 2.813 amino acids. TMEM16B is encoded by 27 exons resulting in a protein of 999 amino acids. Arrow heads mark the positions of polymerase chain reaction primer pairs used for the deletion mapping: filled arrow heads, primer pairs in the non-deleted region; unfilled arrowheads, primer pairs in the deleted region. (B) Genomic structure of VWF and part of TMEM16B. The recombination sites are located between CD9 and VWF, and within intron 3 of the TMEM16B gene. (C) Sequences of both recombination sites reveal no significant homology, suggesting that homologous recombination is an unlikely molecular mechanism to explain the deletion event. By contrast, the presence of the mini-direct-repeat sequence 5′-CACTT-3′ (underlined) directly at the genomic fusion site indicates a possible Non-Homologous End-Joining-mediated mechanism as the molecular basis of Δ253 k. Light gray and dark gray: genomic fusion; upper sequence: original sequence upstream of the VWF gene: lower sequence: original sequence of TMEM16B intron 3; vertical lines: identical nucleotides between both sequences; underlined: mini-direct repeat; overall identity is 19/67 = 28% (much to low to favor a homologous recombination mechanism).
Deletion specific assay
Initially we analyzed the DNA of the German patient F1-3 with the homozygous complete VWF deletion. After identification of the deletion breakpoints by primer walking and by direct sequencing of the PCR product spanning the deletion, we constructed deletion-specific PCR primers that allowed the amplification of a product including both breakpoints in the presence of the deletion and another set of primers that allowed amplification in the absence of the deletion only. The two primer sets that shared the antisense primer (common antisense primer 5′-AGA TTT CAG AGG CGT TCT AAA ACT CAC TC-3′, starting at nt 6130 794; sense wild-type specific primer 5′-AAG AAC CGA AGT CCC AGG AGA AAG GAA AG-3′, starting at nt 6130 516; sense deletion specific primer 5′-GGA AAG TGG GAT GGC GAC AGA GCC TGA G-3′, starting at nt 5877 322) were employed in a duplex PCR of 35 cycles at 61 °C annealing temperature and a final concentration of 2.5 mm MgCl2 using Taq polymerase provided by Invitrogen (Karlsruhe, Germany). This assay allowed the simultaneous identification of the wild-type sequence (279 bp), the homozygous deletion (228 bp) and the heterozygous state (both fragments), respectively, and was subsequently used to identify further patients and carriers with the deletion. Deletion-specific PCR products from additional index patients were sequenced to confirm identity of the deletion in all patients.
Gene dosage analysis
Although our results suggested homozygosity, compound heterozygosity for the identified deletion and a possibly even larger deletion cannot be excluded by our assay if both parents are not available for analysis. We therefore established a duplex PCR with the deletion specific primers and a reference primer pair for gene dosage analysis. In case of an identical deletion on both chromosomes, the deletion-specific PCR product should correlate with a normal gene dosage, whereas compound heterozygosity for two different deletions should be represented by a half normal concentration of the deletion-specific PCR product in relation to the reference (SMARCB1 exon 3), respectively. PCR products of all homozygous patients compared with all heterozygotes and a normal individual as control were subsequently analyzed by denaturing high-performance liquid chromatography (DHPLC) on a WAVE analyzer (Transgenomic Inc., Omaha, NE, USA). At a column oven temperature of 50 °C, 20 μL of sample was injected into a preheated C18 reversed phase column based on non-porous poly (styrene-divinylbenzene) particles (DNA-Sep®; Transgenomic Inc.). Samples were eluted by a mixture of buffer A [0.1 m triethylammonium acetate (TEAA)] and buffer B (0.1 m TEAA, 25% acetonitrile) with a linear gradient of 40–72% buffer B with a 2% point increase per minute.
Haplotype analysis
To establish the genetic background of Δ253 k, we carried out haplotype analysis by means of SNPs flanking both sides of the deleted region in close proximity chosen from the NCBI single nucleotide polymorphism (SNPs) database (http://www.ncbi.nlm.nih.gov/projects/SNP; last accessed 6 March 2007) (Table 2). SNPs were analyzed by PCR and direct sequencing.
| No | refSNP ID | nt | Distance | SNP | H | delhap |
|---|---|---|---|---|---|---|
| 1 | rs7956209 | 6132 921 | 2297 | C/T | 0.534 | T |
| 2 | rs10774403 | 6131 572 | 948 | C/T | 0.509 | T |
| 3 | rs12321808 | 6131 219 | 595 | A/G | 0.517 | A |
| 4 | rs1860363 | 6130 794 | 170 | C/T | 0.517 | C |
| 5 | rs10466913 | 5876 756 | 623 | A/G | 0.417 | G |
| 6 | rs12308742 | 5857 946 | 19,433 | A/T | 0.696 | T |
| 7 | rs4764563 | 5855 331 | 22,048 | A/G | 0.473 | A |
| 8 | – | 5855 271 | 22,108 | +A/−A | nd | -A |
- SNPs 1-4 are located in the centromeric flanking region of the deletion, SNPs 5-8 in the telomeric flanking TMEM16B gene. No. 8 refers to an informative A insertion/deletion polymorphism identified during this analysis. Distance in rows 1-4 refers to the distance in bp between the centromeric SNPs and the centromeric deletion breakpoint (nt 6130 624), distance in rows 5-8 refers to the distance in bp between the telomeric breakpoint (nt 5877 379) and the telomeric SNPs, respectively; H, published frequency of heterozygosity in the European population [20]; ND, not determined; delhap, deletion-associated haplotype.
In silico analysis of the breakpoint regions
The areas of interest flanking the deletion breakpoints were analyzed using the RepeatMasker web server to identify repetitive DNA elements located at or nearby the observed recombination sites (http://www.repeatmasker.org) [14].
Results
Deletion mapping
Analysis of the homozygous German patient by deletion mapping identified the breakpoints of an approximately 253-kb (exactly 253,246 bp) large deletion (Δ253 k) spanning the genomic region between CD9 and the 5′ UTR of VWF to the large intron 3 of TMEM16B a member of the TMEM16 family, encoding an eight transmembrane domain protein comprising nine human homologues (Fig. 1).
Deletion-specific PCR
By means of a duplex PCR applying deletion-specific and wild-type specific primers, we could also unequivocally identify compound-heterozygous patients and unaffected heterozygous carriers of the deletion (Fig. 2). Subsequently, we found identical deletions in another German patient with an inhibitor who is compound heterozygous for Δ253 k and the single base deletion 3605delC in exon 27, in both homozygous Italian index patients of families F3 and F4 who were previously published in the first papers on VWF molecular defects ( families G and C, respectively, [9,10]) and in another previously reported Italian patient (F5-3) who is compound heterozygous for Δ253 k and the cysteine mutation C2671Y (index patient of family A [11,13]). In all patients, with the exception of the compound-heterozygous Italian patient with the mutation C2671Y, alloantibodies against VWF have been detected compromising the therapeutic options.
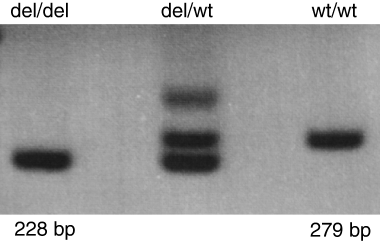
Deletion specific polymerase chain reaction (PCR). Agarose gel electrophoresis of the deletion-specific (del/del, size 228 bp) and the wild-type-specific (wt/wt, size 279 bp) PCR product and the heterozygous electrophoretic pattern (del/wt). The third band in lane 2, seen in all heterozygous individuals, is probably the result of heteroduplex formation.
Gene dosage analysis
To exclude compound heterozygosity for Δ253 k and a possibly even larger deletion in seemingly homozygous patients, we also carried out gene dosage analysis by DHPLC for the deletion-specific allele in comparison to a reference. A normal gene dosage in patients obtained after PCR with the deletion-specific primers in comparison to a heterozygous carrier (half normal gene dosage) confirmed the suspected homozygosity in the respective patients (Fig. 3).
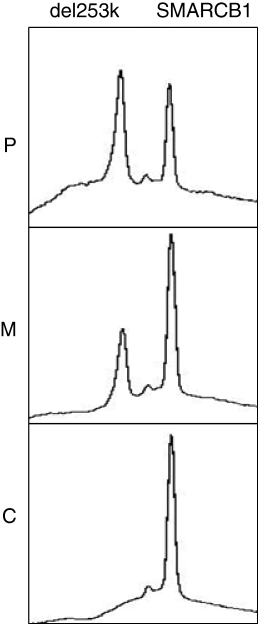
Gene dosage analysis using denaturing high-performance liquid chromatography. An identical deletion on both chromosomes of apparently homozygous patients is suggested by the normal concentration of the deletion-specific PCR product compared with a reference peak (ref. = SMARCB1 exon 3). P, patient F1-3; F1-2, heterozygous mother of F1-3; C, normal control without deletion.
Haplotype analysis
Haplotype analysis comprising seven SNPs and a single A deletion/insertion polymorphism flanking the deleted region in close proximity revealed that the deletion-associated haploytpe is probably identical in all families from Germany and Italy (Table 2, Fig. 4).
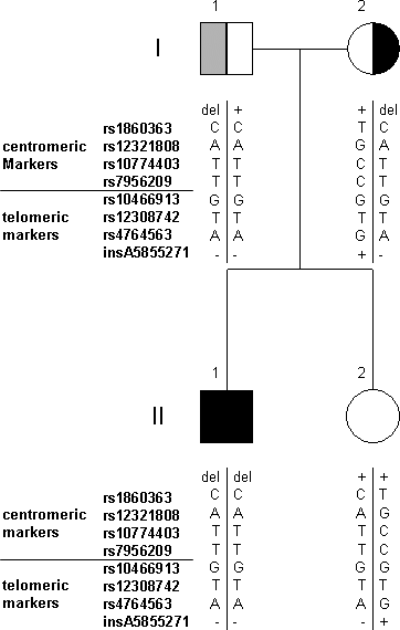
Pedigree of the German family F1 with haplotypes of polymorphic markers flanking the deletion breakpoints. The father (F1-1) was not available for analysis. His haplotypes were derived from those of the other family members. del, presence of the deletion, +, presence of the normal sequence.
In silico breakpoint analysis
An illegitimate recombination event occurred 42 700 bp upstream of the first VWF exon and involved sequences 35 053 bp downstream of the VWF TGA stop codon. The analysis for the presence of repetitive DNA elements revealed that the recombination site 5′ to VWF is located between a MER58C and an AluY repetitive DNA element that are located 220 and 325 nucleotides upstream and downstream of the recombination site, respectively. Thus, it can be assumed that they were not involved in the recombination process. The 3′- recombination site is localized precisely at nucleotide -1 of an AluY repetitive DNA element but does not involve sequences of the repetitive DNA element. Therefore, it can be assumed that this genetic event was not necessarily driven by a homologous recombination but rather by DNA damage (DNA double-strand breaks) and a subsequent DNA repair mechanism. In fact, a 5 nucleotide long mini-direct repeat (CACTT) can be identified directly at the genomic fusion site. This indicates a non-homologous-end-joining (NHEJ)-dependent DNA repair mechanism (Fig. 1).
Discussion
Using deletion mapping and a deletion-specific PCR assay, we could identify and characterize the same large deletion of the VWF gene and the TMEM16B gene in a German patient with severe VWD type 3 and subsequently in four additional patients of German and Italian descent, respectively. All index patients were from obviously unrelated non-consanguineous families.
In silico analysis of the genomic regions flanking the deletion breakpoints did not reveal repetitive elements that would facilitate repeated homologous recombination events resulting in the same subsequent deletion. This and the identical breakpoints in all investigated patients from Italy and Germany suggest a common genetic background shared by the Italian and German families rather than the same repeated molecular event. This is further emphasized by the identical haplotypes of eight polymorphic markers in close vicinity to the deletion breakpoints, although the Δ253k-associated haplotype might be common.
The deletion involves two genes, VWF and TMEM16B. Whereas the VWF deletion-associated phenotype is clearly defined by clinical symptoms and laboratory parameters and corresponds to severe VWD type 3, nothing is known about a possible TMEM16B deletion-related phenotype. TMEM16B belongs to family 16 of transmembrane (TMEM) proteins currently comprising nine members. In general, the function of the TMEM16 family proteins is largely unknown. Some of them are upregulated in certain cancers suggesting their involvement (reviewed in [19]), and TMEM16E is mutated in gnathodiaphyseal dysplasia, a disorder characterized by frequent bone fractures and osseous lesions of the jaw with autosomal dominant inheritance [20]. However, there is no information on the possible role of TMEM16B (M. Katoh, National Cancer Center Research Institute, Japan, personal communication). All of our investigated patients appeared normal apart from their bleeding disorder, for example no additional obvious phenotype was observed in our patients that could be attributed to the lack of TMEM16B. However, alternative splicing that could potentially rescue a putative TMEM16B-deficient phenotype cannot completely be excluded, as an alternative splicing variant lacking part of the 5′ region of the gene has been observed (M. Katoh, pers. comm.).
What all homozygous patients have in common is the presence of alloantibodies. However, as seen from one example in our cohort (patient F2-3) and from other reports, development of alloantibodies is not confined to patients with complete homozygous or compound-heterozygous deletions but is also seen in association with partial deletions of VWF and particular truncating point mutations [5,21–23]. Therefore, it seems unlikely that the deletion of TMEM16B contributed to the generation of alloantibodies in our patients.
Up to now, large deletions of VWF had only been reported in single families. Our report is the first on a common gross deletion in patients with VWD. Its occurrence in homozygous patients from non-consanguineous families and even from different countries presumably on the same genetic background (identical haplotypes) suggests a higher incidence of large deletions and in particular of Δ253 k than previously thought.
There is a very tight correlation between homozygosity for Δ253 k and the development of alloantibodies. Using our deletion-specific assay as a simple and rapid detection method for homozygous and compound-heterozygous individuals with Δ253 k, we can prospectively identify patients at risk of developing inhibitory alloantibodies which will be useful for future management of these patients.
Acknowledgements
We wish to thank R. Eisert for contributing additional clinical data of patient F1-03 and A. Goodeve for editing the manuscript. The expert technical assistance of S. Schneppenheim, B. Weber and T. Obser is gratefully acknowledged. This study was supported in part by a research grant from the Federal Ministry for Education and Research funding the National Genome Research Net (NGFN2) ‘Cardiovascular diseases’ (grant no. NHK-S17T22) and by the Society of Thrombosis and Hemostasis (GTH) both to R. Schneppenheim.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




