High peak reflux velocity in the proximal deep veins is a strong predictor of advanced post-thrombotic sequelae
Abstract
Summary. Background: The presence of reflux in the femoral vein (FV) and popliteal vein (POPV) after acute deep vein thrombosis (DVT) is considered to contribute to the development of advanced post-thrombotic syndrome (PTS). However, a quantification of reflux has yet to be determined. The purpose of study was to determine the indicative parameters reflecting the progression of PTS. Methods: Venous abnormalities were evaluated in 131 limbs out of 130 patients who completed a six-year follow-up after an acute DVT. Clinical manifestations were categorized according to the clinical, etiologic, anatomic, and pathophysiologic (CEAP) classification, and the patients were divided into two groups at a six-year follow-up point: group I, C0–3Es,As,d,p,Pr,o, early chronic venous insufficiency (CVI); group II, C4–6Es,As,d,p,Pr,o, advanced CVI. Venous segments were examined whether they were occluded or recanalized. The reflux parameters assessed were the diameter (cm), the reflux time (RT; s), the peak reflux velocity (PRV; cm s−1), and total refluxed volume, and these parameters were assessed especially in the FV and POPV at the two-year (early phase) and subsequent six-year (late phase) follow-up points after DVT. Results: There were 98 limbs in group I and 33 in group II. The frequency of venous reflux was significantly higher in group II (<0.0001). In contrast, the proportion of occlusion did not differ between the groups (P = 0.138). The proportions of FV and POPV incompetence were significantly higher in group II (P < 0.0001 and P < 0.0001, respectively). In these veins, the RT did not improve the discrimination power between the two groups. In contrast, the PRV had significant discrimination power in these veins at both the two- and six-year follow-up points. In the superficial venous system, there were no significant differences in any of the determined parameters between the groups. In group II, 19 patients (58%), who had early symptoms of CVI at the two-year follow-up point, subsequently developed advanced symptoms of PTS. After calculating a suitable cutoff point using receiver operating characteristic curves analysis at the two-year follow-up point, multivariable analysis showed that a PRV of >25.4 cm s−1 in the POPV was the strongest independent predictor of advanced CVI [odds ratio (OR) 60.32; 95% confidence interval (95CI) 43.1–1238.97, P < 0.0001]. Similarly, in the FV, a PRV of >24.5 cm s−1 was found to be a strong predictor of advanced CVI (OR 25.77, 95CI 10.56–331.12, P < 0.0001). Conclusions: These findings suggest that the presence of a high PRV in the proximal deep veins is an independent predictor of advanced symptoms of PTS.
Introduction
The initial treatment of deep vein thrombosis (DVT) is directed at the prevention of thrombus propagation, the development of pulmonary embolism, and minimizing the recurrence. In contrast, the prevention of late post-thrombotic syndrome (PTS) has emerged because of increasing awareness of its impact on the quality of life for patients. Previous studies have demonstrated that PTS can occur many years after a venous thromboembolism [1,2]. In a 13-year follow-up study, Eichlisberger et al. [3] have demonstrated, using a phlebographic study, that 39% of patients showed chronic venous insufficiency (CVI) and 10% developed chronic venous leg ulcers. However, PTS is difficult to predict in the acute phase of DVT.
The risk factors that affect the development of PTS are not fully recognized. Venous hypertension caused by venous obstruction, gravitational reflux by valve incompetence, or a combination of the two is considered to be the major contributor of PTS [4,5]. In the late phase, the presence of reflux in the femoral vein (FV) and the popliteal vein (POPV) is considered to contribute to the development of PTS. However, a quantification of reflux in these patients has yet to be determined.
In this study, venous abnormalities were evaluated in patients six years after DVT. The presence of venous obstruction and reflux was evaluated using duplex scanning. Furthermore, quantitative venous reflux parameters were also evaluated especially in the FV and POPV. The purpose of this study was to compare the different duplex-derived parameters between patients with relatively early symptoms of CVI and those with advanced symptoms of CVI, and to determine the indicative parameters reflecting the progression of PTS.
Materials and methods
Patients
From May 1999 to May 2006, venous abnormalities in relation to the severity of the PTS were evaluated in 131 limbs out of a consecutive 130 patients who completed a six-year follow-up after an acute DVT. These patients were part of a long-term follow-up study since the initial onset of DVT. Patients in this study were placed on a regular follow-up, at least twice a year, and clinical signs and symptoms, and venous abnormalities were screened. There were 71 males and 59 females, ranging in age from 21 to 91 years (mean, 61 years). The presence of DVT was diagnosed by venous duplex scanning. After initial diagnosis, all patients were treated with intravenous unfractionated heparin for 5–14 days during the acute phase adjusted to maintain the activated partial thromboplastin time at 1.5–2.5 times control [8], followed by oral warfarin for at least eight weeks with an International Normalized Ratio (INR) level of 2–3. All patients were encouraged to ambulate, and graduated thigh-high compression stockings were applied immediately upon making diagnosis [9]. Patients with initial isolated calf DVT were excluded from this study. Patients with recurrent DVT during the follow-up period were excluded because venous reflux parameters change after reobstruction. Patients with arterial insufficiency, identified on the basis of an ankle-brachial pressure index of <0.9, were excluded from the study. Also, patients with poor compliance of the use of compression stockings were also excluded from the study. This study was approved by the institutional review board. Informed consent was obtained from all participants.
Clinical assessment
The clinical manifestations of these patients were categorized according to the clinical, etiologic, anatomic, and pathophysiologic (CEAP) classification of reporting standards in venous disease supported by the North American Chapter of the Society for Vascular Surgery and the International Society for Cardiovascular Surgery [10,11]. All of the patients’ lesions were classifiable into no signs of venous disease (CEAP C0), telangiectases or reticular veins (C1), varicose veins (C2), edema (C3), changes in the skin and subcutaneous tissue (C4a,b), or chronic ulcers (C5 and C6). Etiologic classification included secondary venous dysfunction (Es). The anatomic sites of the venous reflux were included as superficial (As), deep (Ad), and perforating (Ap) veins. Clinical signs of the symptoms may result from reflux (Pr), obstruction (Po), or both (Pr,o). Thus, they were divided into two groups at the six-year follow-up point: group I (C0–3Es,As,d,p,Pr,o, relatively early stage of CVI) and group II (C4–6Es,As,d,p,Pr,o, advanced CVI).
Venous duplex scans
The presence of venous occlusion was diagnosed with duplex scans. A color duplex scanner (LOGIQ 500MD; GE Medical Systems, Milwaukee, WI, USA) with a 5–10 MHz transducer was used. Initially, each patient was placed supine in a reverse Trendelenburg position at 15 °. Venous duplex scanning began at the common femoral vein (CFV), and moved to the FV at the adductor canal. Afterwards, the patient was placed in a prone position with the knee flexed at 30 °, and the residual POPV was evaluated [12]. The diagnosis of DVT was based on both non-compressibility of the vein on B-mode, and no spontaneous flow on color Doppler imaging. Venous segments were assessed to determine whether they were occluded, partially occluded, or totally recanalized. Venous segments were considered as occluded if they showed incompressibility and absence of flow with distal augmentation. Partial occlusion was defined by partial compressibility of the vein and diminished cephalad flow by distal augmentation. Complete recanalization was determined by complete compressibility of the vein and spontaneous cephalad flow on color Doppler imaging.
The venous reflux was evaluated as described by van Bemmelen et al. [13]. Reflux time (RT) was measured in the superficial and deep venous systems, with the patient in the upright position with his/her weight supported on the contralateral leg. This consisted of sequential evaluation of the CFV, FV, POPV, sapheno–femoral junction (SFJ), greater saphenous vein (GSV), and sapheno–popliteal junction (SPJ). For evaluation of the CFV, FV, SFJ, and GSV, a pneumatic thigh cuff (Hokanson, Bellevue, WA, USA) was attached to the thigh, inflated to 80 mmHg and then rapidly deflated. For evaluation of the POPV and SPJ, a cuff was applied to the calf, inflated to 100 mmHg and then rapidly deflated. Calf perforating veins were examined with the patients in the sitting position, with the foot resting on a stool; distal calf compression was undertaken. The main duplex-derived parameters assessed were the diameter (cm), the RT (s), the peak reflux velocity (PRV; cm s−1), the mean reflux velocity (MRV; cm s−1) and the total refluxed volume (TRV; ml) calculated using the equation: total refluxed volume (ml) = MRV × area (r2) × RT. The vessel cross-sectional area was calculated from the diameter, assuming a circular vessel shape. An RT of >0.5 s was considered to be incompetent. Venous reflux parameters were assessed especially in the FV and POPV at the two-year (early phase) and subsequent six-year (late phase) follow-up points after DVT.
Statistical analysis
All data were analyzed using StatView for Windows (Version 5.0, SAS Institute Inc., Cary, NC, USA). Wilcoxon's non-parametric rank sum test was used to estimate the differences between numerical data, and chi-squared contingency table analysis was used to evaluate the differences between proportions. Receiver operator characteristics (ROC) curves (MedCalc software, Mariakerke, Belgium) were used to assess the predictive power of the two different groups. Also, the odds ratio (OR) and 95% confidence intervals (CI) were calculated using multiple logistic regression analysis. Continuous data were expressed as mean ± standard deviation (SD). Statistical significance was defined as P < 0.05.
Results
The base line characteristics of the study patients are shown in Tables 1 and 2. There were 98 limbs from 97 patients in group I (C0–3Es,As,d,p,Pr,o) and 33 limbs from 33 patients in group II (C4–6Es,As,d,p,Pr,o). In group I, the mean age was higher than in group II, but there was no significant difference between the groups (P = 0.285). There was no significant difference in the proportion of male patients between the two groups (P = 0.993). The proportion of patients with no venous abnormalities was found to be significantly higher in group I (P < 0.0001). The proportion of occlusion did not differ between the groups (P = 0.138). In contrast, reflux was significantly predominant in group II (P < 0.0001). The frequency of axial reflux was also significantly higher in group II (P < 0.0001).
| Class | Signs and symptoms | Limbs (n) |
|---|---|---|
| C0 | No visible or palpable signs of venous disease | 65 |
| C1 | Telangiectasis or reticular veins | 7 |
| C2 | Varicose veins; distinguished from reticular veins by a diameter of 3 mm or more | 12 |
| C3 | Edema | 15 |
| C4 | Changes in skin and subcutaneous tissue secondary to chronic venous disease | 22 |
| C4a | Pigmentation or eczema | 15 |
| C4b | Lipodermatosclerosis or atropic blanche | 7 |
| C5 | Healed venous ulcer | 9 |
| C6 | Active venous ulcer | 2 |
| Group I (C0–3Es,As,d,p,Pr,o) n = 97 patients | Group II (C4–6Es,As,d,p,Pr,o) n = 33 patients | P-value | |
|---|---|---|---|
| Mean age (year) | 61.9 ± 16.5 | 57.1 ± 21.5 | 0.285 |
| Gender (% male) | 53 (54.6) | 18 (54.5) | 0.993 |
| Distribution of venous abnormalities | n = 98 limbs | n = 33 limbs | |
| No abnormality (%) | 47 (48.0) | 1 (3.0%) | <0.0001 |
| Occlusion (%) | 16 (16.3) | 2 (6.1) | 0.138 |
| Reflux (%) | 23 (23.5) | 20 (60.6) | <0.0001 |
| Occlusion + reflux (%) | 12 (12.2) | 10 (30.3) | 0.014 |
| Axial reflux (%) | 10 (10.2) | 16 (48.5) | <0.0001 |
The distribution of venous reflux is shown in Table 3. There was no difference in the proportion of limbs with isolated superficial and deep vein incompetence between the two groups (P = 0.198 and 0.175, respectively). In contrast, the proportions of limbs with isolated deep vein insufficiency, superficial combined with deep venous insufficiency, deep combined with perforator vein insufficiency and overall superficial, deep, and perforator vein incompetence were all significantly higher in group II (P = 0.001, P = 0.008, P = 0.020, P = 0.015, P < 0.0001, and P < 0.012, respectively). The most common pattern of venous insufficiency was reflux in the deep vein in both groups (38% and 88%, respectively). In deep veins, the proportions of CFV, FV and POPV incompetence were significantly higher in group II (P = 0.0017, P < 0.0001, and P < 0.0001, respectively). In superficial venous system, there were significant differences in the proportions of SFJ and GSV reflux between the two groups (P = 0.038 and 0.030, respectively).
| Group I (C0−3Es,As,d,p,Pr,o) n = 98 limbs | Group II (C4−6Es,As,d,p,Pr,o) n = 33 limbs | P-value | |
|---|---|---|---|
| Distribution of venous reflux | |||
| Superficial alone (%) | 2 (2.0) | 0 (0) | 0.408 |
| Deep alone (%) | 29 (29.6) | 18 (54.5) | 0.001 |
| Superficial + deep (%) | 4 (4.1) | 6 (18.2) | 0.008 |
| Superficial + deep + perforator (%) | 2 (2.0) | 2 (6.1) | 0.246 |
| Deep + perforator (%) | 1 (1.0) | 3 (9.1) | 0.020 |
| Overall superficial (%) | 8 (8.2) | 8 (24.2) | 0.015 |
| Overall deep (%) | 36 (36.7) | 29 (87.9) | <0.0001 |
| Overall perforator (%) | 3 (3.1) | 5 (15.2) | 0.012 |
| Anatomic distribution of deep vein reflux | |||
| Common femoral vein (%) | 10 (10.2) | 11 (33.3) | 0.0017 |
| Femoral vein (%) | 12 (12.2) | 18 (54.5) | <0.0001 |
| Popliteal vein (%) | 33 (33.7) | 24 (72.7) | <0.0001 |
| Anatomic distribution of superficial venous reflux | |||
| Sapheno–femoral junction (%) | 6 (6.1) | 6 (18.2) | 0.038 |
| Greater saphenous vein (%) | 4 (4.1) | 5 (15.2) | 0.030 |
| Sapheno–popliteal junction (%) | 6 (6.1) | 5 (15.2) | 0.106 |
Table 4 shows the duplex-derived venous reflux parameters in the FV at two- and six-year follow-up points between groups I and II. There were similar tendencies in venous reflux parameters between the early and late phases after DVT. There were no significant differences in the RT between the groups (P = 0.814 and 0.209, respectively). In contrast, the PRV had significant discrimination power in both phases (P = 0.002 and 0.002, respectively). Similarly, in group II, the values in the MRV were significantly higher than in group I (P = 0.003 and 0.002, respectively).
| Parameters | Group I 12 limbs | Group II 18 limbs | P-value |
|---|---|---|---|
| FV (two-year) | |||
| Diameter (cm) | 0.87 ± 0.19 | 0.70 ± 0.10 | 0.038 |
| Reflux time (s) | 2.28 ± 0.60 | 2.30 ± 0.88 | 0.814 |
| Peak reflux velocity (cm s−1) | 12.8 ± 6.2 | 33.9 ± 8.2 | 0.002 |
| Mean reflux velocity (cm s−1) | 5.6 ± 2.2 | 11.5 ± 5.8 | 0.003 |
| Total refluxed volume (mL) | 11.2 ± 8.1 | 13.6 ± 8.1 | 0.480 |
| FV (six-year) | |||
| Diameter (cm) | 0.88 ± 0.19 | 0.69 ± 0.11 | 0.034 |
| Reflux time (s) | 2.23 ± 0.61 | 2.04 ± 0.78 | 0.209 |
| Peak reflux velocity (cm s−1) | 14.3 ± 5.8 | 38.3 ± 9.2 | 0.002 |
| Mean reflux velocity (cm s−1) | 6.1 ± 2.1 | 13.5 ± 6.6 | 0.002 |
| Total refluxed volume (mL) | 11.8 ± 7.8 | 14.3 ± 9.4 | 0.433 |
Table 5 shows the duplex-derived venous reflux parameters in the POPV at two- and six-year follow-up points between groups I and II. Like the FV, similar tendencies were found in venous reflux parameters between the early and late phases. There were no significant differences in the RT between the groups (P = 0.568 and 0.732, respectively). In contrast, the values in the PRV were significantly higher in group II (P < 0.0001 and <0.0001, respectively). Similarly, in group II, the values in the MRV were significantly higher than in group I (P < 0.0001 and <0.0001, respectively). There were significant increases in the TRV in group II (P = 0.0003 and 0.019, respectively).
| Parameters | Group I 33 limbs | Group II 24 limbs | P-value |
|---|---|---|---|
| POPV (two-year) | |||
| Diameter (cm) | 0.80 ± 0.13 | 0.74 ± 0.15 | 0.010 |
| Reflux time (s) | 2.83 ± 2.59 | 2.68 ± 1.37 | 0.568 |
| Peak reflux velocity (cm s−1) | 16.3 ± 5.5 | 41.4 ± 9.5 | <0.0001 |
| Mean reflux velocity (cm s−1) | 4.6 ± 2.3 | 12.0 ± 5.1 | <0.0001 |
| Total refluxed volume (mL) | 9.6 ± 9.1 | 20.2 ± 17.3 | 0.0003 |
| POPV (six-year) | |||
| Diameter (cm) | 0.85 ± 0.11 | 0.75 ± 0.16 | 0.016 |
| Reflux time (s) | 2.85 ± 2.49 | 2.64 ± 1.35 | 0.732 |
| Peak reflux velocity (cm s−1) | 18.1 ± 6.6 | 43.6 ± 9.3 | <0.0001 |
| Mean reflux velocity (cm s−1) | 5.2 ± 2.4 | 12.2 ± 4.7 | <0.0001 |
| Total refluxed volume (mL) | 11.4 ± 10.2 | 20.8 ± 17.5 | 0.019 |
Table 6 shows the duplex-derived venous reflux parameters in the superficial venous system at the six-year follow-up point. At the SFJ, the PRV and MPV were found to be much higher in group II. Similarly, in the GSV, the PRV and MPV showed much higher values in group II. However, there was no significant discrimination power in the superficial venous reflux.
| Parameters | Group I (C0–3Es,As,Pr) | Group II (C4–6Es,As,Pr) | P-value |
|---|---|---|---|
| SFJ | Six limbs | Six limbs | |
| Diameter (cm) | 0.65 ± 0.11 | 0.60 ± 0.08 | 0.465 |
| Reflux time (s) | 3.61 ± 2.72 | 4.41 ± 3.50 | >0.999 |
| Peak reflux velocity (cm s−1) | 17.3 ± 11.5 | 23.5 ± 16.7 | 0.068 |
| Mean reflux velocity (cm s−1) | 6.9 ± 5.1 | 9.0 ± 6.3 | 0.273 |
| Total refluxed volume (mL) | 12.1 ± 13.5 | 8.8 ± 4.6 | >0.999 |
| GSV | Four limbs | Five limbs | |
| Diameter (cm) | 0.51 ± 0.08 | 0.44 ± 0.02 | 0.144 |
| Reflux time (s) | 2.91 ± 0.73 | 2.71 ± 0.41 | 0.715 |
| Peak reflux velocity (cm s−1) | 22.5 ± 14.7 | 27.3 ± 10.2 | 0.593 |
| Mean reflux velocity (cm s−1) | 5.3 ± 3.7 | 11.4 ± 3.2 | 0.068 |
| Total refluxed volume (mL) | 6.4 ± 5.7 | 7.3 ± 1.9 | >0.999 |
| SPJ | Six limbs | Five limbs | |
| Diameter (cm) | 0.52 ± 0.14 | 0.48 ± 0.13 | 0.225 |
| Reflux time (s) | 2.30 ± 0.55 | 2.96 ± 1.28 | 0.345 |
| Peak reflux velocity (cm s−1) | 24.2 ± 9.3 | 24.9 ± 12.5 | 0.686 |
| Mean reflux velocity (cm s−1) | 8.3 ± 3.2 | 6.9 ± 4.0 | 0.138 |
| Total refluxed volume (mL) | 8.3 ± 6.5 | 10.8 ± 5.1 | 0.686 |
Table 7 shows the CEAP clinical manifestation in group I at the two- and six-year follow-up points. At the two-year follow-up point, patients showed advanced symptoms of PTS. However, in group II, 19 patients (58%), who had early symptoms of CVI at the two-year follow-up point, subsequently developed advanced symptoms of PTS (Table 8).
| Class | Two-year follow-up point (limbs) | Six-year follow-up point (limbs) |
|---|---|---|
| C0 | 66 | 65 |
| C1 | 9 | 7 |
| C2 | 8 | 12 |
| C3 | 15 | 15 |
| C4 | 0 | 0 |
| C4a | 0 | 0 |
| C4b | 0 | 0 |
| C5 | 0 | 0 |
| C6 | 0 | 0 |
| Class | Two-year follow-up point (limbs) | Six-year follow-up point (limbs) |
|---|---|---|
| C0 | 1 | 0 |
| C1 | 2 | 0 |
| C2 | 5 | 0 |
| C3 | 11 | 0 |
| C4 | 11 | 22 |
| C4a | 4 | 15 |
| C4b | 7 | 7 |
| C5 | 2 | 9 |
| C6 | 1 | 2 |
Using reflux parameters as a unit of analysis, separate ROC curves analysis was generated for limbs with FV and POPV reflux at the two-year follow-up point. Also, the most suitable cutoff point with highest accuracy, minimal false negative and false positive results for discrimination of the two groups was then identified. In the FV, ≤0.65 cm was optimal for the vein diameter, ≤1.98 s for the RT, >24.5 cm s−1 for the PRV, >8.2 cm s−1 for the MRV, and >5.0 mL for the TRV. Similarly, in the POPV, ≤0.81 cm was optimal for the vein diameter, ≤2.97 s for the RT, >25.4 cm s−1 for the PRV, >5.7 cm s−1 for the MRV, and >10.9 mL for the TRV. Of these, ROC curves analysis confirmed that the PRV was a predictor of advanced CVI in the FV (area under the curve 0.95, 95% CI 0.81–0.99, P = 0.0001). Also, the PRV of >24.5 cm s−1 had a sensitivity of 94% and a specificity of 92% for the prediction of advanced symptoms. Similarly, in the POPV, the PRV of >25.4 cm s−1 was a good predictor of severe PTS (area under the curve 0.98, 95% CI 0.90–0.99, P = 0.0001) with a sensitivity of 96% and a specificity of 97% (Fig. 1). The MRV was also an important predictor for PTS in the FV (area under the curve 0.82, 95% CI 0.63–0.93, P = 0.0001). Also, the MRV of >8.2 cm s−1 had a sensitivity of 56% and a specificity of 100%. In the POPV, the MRV of >5.7 cm s−1 was a good predictor of severe PTS (area under the curve 0.95, 95% CI 0.85–0.99, P = 0.0001) with a sensitivity of 96% and a specificity of 82% (Fig. 2).
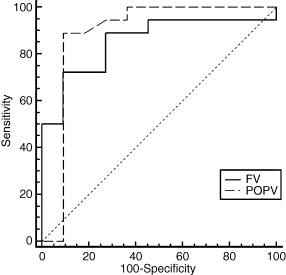
Receiver operating characteristics curves analysis showing usefulness of the peak reflux velocity (PRV) in predicting advanced chronic venous insufficiency. The solid line denotes the femoral vein (area under the curve 0.95, 95% confidence interval 0.8–0.99, P = 0.0001) and the dashed line denotes the popliteal vein (area under the curve 0.98, 95% confidence interval 0.90–0.99, P = 0.0001).
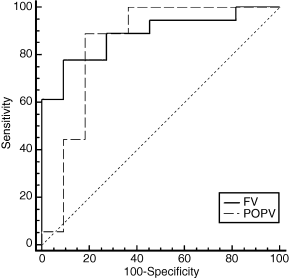
Receiver operating characteristics curves analysis showing usefulness of the mean reflux velocity (MRV) in predicting advanced chronic venous insufficiency. The solid line denotes the femoral vein (area under the curve 0.82, 95% confidence interval 0.63–0.93, P = 0.0001) and the dashed line denotes the popliteal vein (area under the curve 0.95, 95% confidence interval 0.85–0.99, P = 0.0001).
Table 9 shows the association of advanced CVI with venous reflux parameters with an optimal cutoff point. Using multivariable analysis, the PRV of >25.4 cm s−1 in the POPV was the strongest predictor of advanced CVI (OR 60.32, 95% CI 43.1–1238.97, P < 0.0001) followed by the MRV of >5.7 cm s−1 in the POPV (OR 42.61, 95% CI 14.03–118.23, P < 0.0001) at the two-year follow-up point. The calculated TRV in the POPV also had a high OR (OR 14.51, 95% CI 2.68–30.34, P = 0.0001). In the FV, the PRV of >24.5 cm s−1 was a strong predictor of advanced post-thrombotic sequelae (OR 25.77, 95% CI 10.56–331.12, P < 0.0001).
| Variables | Cutoff point | Odds ratio (95% confidence interval) | P-value |
|---|---|---|---|
| FV | |||
| Diameter (cm) | ≤0.65 | 6.35 (1.16–103.97) | 0.012 |
| Reflux time (s) | ≤1.98 | 2.65 (0.67–23.7) | 0.104 |
| Peak reflux velocity (cm s−1) | >24.5 | 25.77 (10.56–331.12) | <0.0001 |
| Mean reflux velocity (cm s−1) | >8.2 | 7.82 (1.45–130.3) | 0.005 |
| Total refluxed volume (mL) | >5.0 | 0.37 (0.14–2.85) | 0.541 |
| POPV | |||
| Diameter (cm) | ≤0.81 | 9.34 (1.75–19.56) | 0.002 |
| Reflux time (s) | ≤2.97 | 0.17 (0.24–2.55) | 0.678 |
| Peak reflux velocity (cm s−1) | >25.4 | 60.32 (43.7–1238.97) | <0.0001 |
| Mean reflux velocity (cm s−1) | >5.7 | 42.61 (14.03–118.23) | <0.0001 |
| Total refluxed volume (mL) | >10.9 | 14.51 (2.68–30.34) | 0.0001 |
Discussion
PTS is considered to be a common late complication of an acute DVT, but the factors that affect the development of PTS are not fully understood. PTS will become apparent within the first two years after an acute DVT [1,14–16]. Previous studies have shown that proximal DVT is the strongest independent risk factor of PTS (OR 2.1, 95%CI 1.3–3.7) [17], and residual venous obstruction or reflux in the axial deep venous system, or a combination of the two, could be the predisposing factors for PTS. Johnson et al. [6] found that only 3% of limbs with PTS were normal, 18% had reflux alone, 15% had obstruction alone, and 65% had both reflux and obstruction; they concluded that a combination of residual reflux and residual partial or a complete obstruction in the major deep veins is more likely to be associated with the development of PTS. In contrast, Killewich et al. [4] showed that reflux in the deep veins was the main contributor to the severity of PTS. In patients with deep vein reflux, the highest prevalence of valve incompetence was found in the FV and POPV [18]. Haenen et al. [19] also demonstrated that advanced CVI was found in the FV and POPV with reflux, and reflux in the proximal deep veins significantly contributed to the development of PTS. These reports suggest that residual abnormalities in the proximal deep veins play a major role in the development of late PTS. In the current study, complete or partial obstruction or a combination of obstruction and reflux did not contribute to the development of PTS, and reflux in the FV and POPV was the main contributor to advanced CVI.
Other investigators have shown the primary importance of superficial venous reflux in the development of symptoms of PTS. The venous pressure in the superficial veins might increase after the perforating veins become insufficient, resulting in a gradually developing insufficiency of the superficial veins [20]. Labropoulos et al. [21] found that the incidence of ulceration increased with an increased extent of reflux in the presence of superficial venous insufficiency and that 47% of patients with symptomatic PTS had superficial and deep venous insufficiency. They also reported that the absence of superficial venous reflux was associated with a low incidence of leg ulceration, even in the presence of deep vein insufficiency. Similarly, Haenen et al. [22] studied venous reflux and calf muscle dysfunction in relation to the severity of PTS, and found that 64% of the patients with severe signs of PTS had a combination of superficial and deep reflux. These studies suggest that superficial venous insufficiency is an increasing risk factor for advanced CVI. We have also found a notable increase in the frequency of superficial venous insufficiency in limbs with advanced CVI. However, the proportion of overall superficial reflux was markedly lower than in these studies, and the frequency of isolated superficial and deep venous insufficiency did not differ among the classes. The frequency of superficial and perforating vein insufficiency increased when reflux in the deep vein was combined. Moreover, we did not find any significant discrimination power in the superficial venous reflux.
Quantification of venous reflux is the key to a better understanding of CVI, and several investigators have attempted to quantify venous reflux and associate clinical presentation with RT predominantly in patients with primary valvular insufficiency, as an RT of ≥0.5 s has been used as the gold standard for the evaluation of venous reflux [23–25]. Welch et al. [26] carried out a segmental evaluation of venous reflux and found significant prolongation of reflux in the deep system. They concluded that the deep vein system plays an important role in the progression of CVI. Weingarten et al. [27] calculated the total RT and found that the mean total RT was significantly longer in limbs with ulceration than in those without, and that the RT of >9.96 s was predictive of venous ulceration. Rodriguez et al. [28] and Welch et al. [29] also studied the association of the RT with the magnitude of refluxed volume, and concluded that the total RT was moderately associated with the TRV, although the individual RT was not associated with the magnitude of refluxed volume. However, recent studies have shown that the RT does not correlate with the magnitude of reflux, or may not be a useful variable. Masuda and Kistner [30] found that a peak velocity of ≥30 cm s−1 was associated with venographic incompetence. Yamaki et al. [31,32] attempted to quantify the degree of reflux with clinical manifestations in patients with isolated superficial venous insufficiency, and found that a peak reflux velocity of ≥30 cm s−1 was associated with venous ulcers. Later, they found that the PRV was a better parameter than the RT for the discrimination of clinical severity in both superficial and deep vein insufficiency [33]. They showed that patients with advanced CVI had a notably higher PRV with a shorter RT. These reports have shown that the PRV is a better discriminator than the RT.
Reflux parameters have also been examined in patients with PTS as well as primary valvular insufficiency. Danielsson et al. [34] studied 401 patients with CVI (302 with primary venous insufficiency and 99 with secondary venous insufficiency) and found that the PRV was significantly higher in legs with clinical class C4–C6 disease, compared with those with C0–C3 disease. However, the difference for the RT did not reach significance, although there was a clear trend for the RT compared with legs without skin changes. Furthermore, they found that the presence of axial reflux in the deep veins contributes significantly to an increased prevalence of advanced CVI, whereas the presence of axial GSV reflux did not indicate an increased prevalence of skin changes or ulcer. Similarly, Neglén et al. [35] studied 244 (178 with primary venous insufficiency and 66 with secondary venous insufficiency) limbs with various clinical classes of CVI. He examined limbs with axial deep and superficial reflux, and found that the PRV was significantly higher in legs with clinical class C4–C6 (76 cm s−1) compared with those with C0–C3 (76 cm s−1 vs. 39 cm s−1, P = 0.0002). The RT, however, did not discriminate the two subset of limbs (8.1 s vs. 7.3 s, P = 0.6699). They concluded that the RT cannot be used to quantify the severity of reflux and that the PRV appeared to better reflect the magnitude of venous incompetence. However, these reports included both PTS and primary valvular insufficiency. To our knowledge, this is the first report on detailed venous reflux parameters in patients with PTS alone.
In this study, we fail to discriminate between early and advanced CVI using the RT. The RT should only be used to detect reflux in patients with post-thrombotic disease as well as those with primary valvular insufficiency. In contrast, a PRV of >25.4 cm s−1 in the POPV was found to be the strongest predictor of advanced symptoms of PTS. Similarly, in the FV, a PRV of >24.5 cm s−1 was a strong predictor of advanced CVI. The MRV was also a good contributor to the severity of PTS using multiple logistic regression analysis. Because the PRV is easy to measure, and less prone to error with the present technique, it may be the preferred technique [35].
In conclusion, patients with advanced PTS frequently have axial reflux in the proximal deep veins. Reflux in the proximal deep veins is an important contributor to severe symptoms of PTS. However, an increased prevalence of superficial venous reflux does not indicate an increased prevalence of advanced CVI. The RT measured by duplex scanning is used to detect reflux, but is a poor predictor of advanced PTS. In contrast, a PRV of >25.4 cm s−1 in the POPV and a PRV of >24.5 cm s−1 in the FV, determined by ROC curves analysis, are good indicators of advanced CVI. These findings suggest that the presence of a high PRV in the deep proximal veins is an independent predictor of advanced PTS.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




